Introduction
Cerebral apoplexy (also known as stroke or
cerebrovascular accident) is an acute medical condition whose major
clinical manifestations are brain ischemia and hemorrhagic injury
(1,2). Cerebral apoplexy is associated with a
higher mortality and morbidity than other types of brain injury,
and is mainly divided into hemorrhagic cerebral apoplexy
(intracerebral hemorrhage and subarachnoid hemorrhage) and ischemic
cerebral apoplexy (cerebral infarction, cerebral thrombosis)
(3,4). Studies have indicated that cerebral
swelling is of particular concern following cerebral apoplexy, as
it is a major cause of mortality and disability in affected
patients (5–7). Cerebral apoplexy, which may be one of
the potential complications of cerebral infarction, is a
potentially fatal condition that has serious consequences even
after successful treatment of cerebral infarction (8). Therefore, it is important to identify
potential targets for the treatment of cerebral apoplexy, e.g., by
exploring additional underlying molecular mechanisms to expand the
network of known molecular interactions and thereby provide a new
horizon for the development of therapies for human cerebral
apoplexy.
Cerebral apoplexy is followed by acute and prolonged
inflammatory responses characterized by increased plasma
inflammatory cytokine levels and leukocytes (9). Inflammation has an important role in
the progression of cerebral apoplexy and has been reported to
mediate damage as a potential therapeutic target in acute ischemic
cerebral apoplexy (10). Evidence
for the epidemiological association of inflammatory markers has
accrued in patients with cerebral apoplexy (11). Current and future therapeutic
strategies to target inflammation to resolve inflammatory responses
in patients with cerebral apoplexy have been reviewed (12). Another review has provided an
overview of the impact of systemic inflammation on the
susceptibility to cerebral apoplexy and on patient outcome,
outlined the potential mechanisms underlying its impact on ischemic
brain injury and highlighted strategies for cerebral apoplexy
prevention, therapy and prognosis (13). However, to the best of our knowledge,
no previous study has performed any systemic investigations on
inflammatory cytokines and cells in patients with cerebral apoplexy
in an intensive care unit (ICU) setting.
In the present study, the serum levels of
inflammatory cytokines and plasma concentrations of lymphocytes as
well as the expression of inflammatory genes were investigated in
patients with cerebral apoplexy in an ICU setting. The present
study highlights the importance of inflammatory responses in the
evaluation of the risk of cerebral apoplexy and suggests that
anti-inflammatory interventions may be beneficial for the treatment
of patients with cerebral apoplexy.
Materials and methods
Ethics statement
This study was approved by Ethics Committee of
Sichuan Provincial People's Hospital (Chengdu, China). A total of
85 patients (mean age, 35.6 years; range, 23–50 years; female:male,
41:44) with cerebral apoplexy at the ICU and 68 healthy individuals
(mean age, 37.8 years; range, 24–52 years; female:male, 32:36) were
recruited for analysis of inflammatory cells and factors. This
study was performed in Sichuan Provincial People's Hospital between
May 2014 and July 2016. Patients and healthy volunteers provided
written informed consent.
Flow cytometry
Peripheral blood was drawn from patients with
cerebral hemorrhage in the ICU and total leukocytes were extracted
using a Human Leukocyte Extraction kit (cat. no. AM1933M;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Serum levels of lymphocytes, plasmacytes, neutrophils, monocytes,
macrophages and mast cells in patients with cerebral apoplexy or
healthy volunteers were analyzed by flow cytometry using
CellTracker™ Green BODIPY™ Dye (cat. no.
C2102; Thermo Fisher Scientific, Inc.) as described previously
(14).
ELISA
In the present study, commercialized Human ELISA
Kits from Thermo Fisher Scientific, Inc. were used to assess TNF-α
(cat. no. BMS2034TEN), IL-4 (cat. no. BMS225-2TEN), IL-6 (cat. no.
BMS213-2TEN), IL-8 (cat. no. KHC0083), IL-10 (cat. no. KHC0102),
IL-1β (cat. no. KHC0019) and IL-17A (cat. no. BMS2017TEN) in the
peripheral blood of patients with cerebral apoplexy at the ICU. The
ELISAs were performed according to the manufacturer's protocols.
The absorbance of the plates was measured at 570 nm using an ELISA
reader and finally converted to the concentrations of TNF-α, IL-4,
IL-6, IL-8, IL-10, IL-1β and IL-17A.
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR) assay
A total of 15 ml peripheral venous blood was
obtained from patients with cerebral apoplexy. Human peripheral
blood mononuclear cells (hPMCs) were separated by density gradient
centrifugation (CsCl). Total RNA was extracted from in hPMCs cells
using RNAzol, RNase-free DNase was used to digest total RNA at 37°C
for 15 min, and the RNeasy kit was then applied to purify RNA and
adjust its concentration to 1 µg/µl. A total of 2 µg RNA was used
as a template to synthetize complementary (c)DNA by reacting it
with reverse transcriptase at 37°C for 120 min, at 99°C for 4 min
and at 4°C for 3 min using High Capacity cDNA Reverse Transcription
kit (cat. no. 4368814; Applied Biosystems; Thermo Fisher
Scientific, Inc.) according to manufacturer's protocol.
Subsequently, PCR was performed to amplify the cDNA of tumor
necrosis factor (TNF)-α, interleukin (IL)-4, IL-6, IL-8, IL-10,
IL-1β and IL-17A with the primers (Invitrogen; Thermo Fisher
Scientific, Inc.) listed in Table I
to determine the transcription level of mRNA, and β-actin was used
as the housekeeping gene of the internal control group. The
reaction mixture was as follows: 2 µl cDNA synthesized from the RT
reaction, 5 pmol of each primer, 25 µl of SYBR Green Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and 23 µl
water in a total volume of 50 µl. The reaction conditions were
performed as follows: 95°C for 10 min, and 35 cycles of 95°C for 20
sec and 58°C for 1 min. Subsequently, agarose electrophoresis with
1% ethidium bromide was adopted to assess the PCR-amplified
products. Relative mRNA expression changes were calculated using
the 2−ΔΔCq method (15).
The results are expressed as the fold change compared with the
control.
 | Table I.Sequences of primers used for
polymerase chain reaction. |
Table I.
Sequences of primers used for
polymerase chain reaction.
| Gene name | Sequence |
|---|
| TNF-α | Forward,
5′-GGCGATTACAGACACAACT-3′ |
|
| Reverse,
5′-TCCAGACTTCCTTGAGACA-3′ |
| IL-4 | Forward,
5′-CCTCTGTTCTTCCTGCTAG-3′ |
|
| Reverse,
5′-CTCTGGTTGGCTTCCTTC-3′ |
| IL-6 | Forward,
5′-GTGAGGAACAAGCCAGAG-3′ |
|
| Reverse,
5′-TGACCAGAAGAAGGAATGC-3′ |
| IL-8 | Forward,
5′-TGGCATCTTCACTGATTCTTG-3′ |
|
| Reverse,
5′-TCAGTGCATAAAGACATACTCC-3′ |
| IL-10 | Forward,
5′-GCCCAGCCCACCTCCACTCC-3′ |
|
| Reverse,
5′-TGGGCTACGTGACCTATGAC-3′ |
| IL-1β | Forward,
5′-GTGCTGACGCTAACTGACC-3′ |
|
| Reverse,
5′-GCACCCATGGCAGAAGGAGGAG-3′ |
| IL-17A | Forward,
5′-ATGCACAGCCACCGCGACTT-3′ |
|
| Reverse,
5′-CTTCATGACTGCCTCCAAGTAG-3′ |
| β-actin | Forward,
5′-AGCCTTCTCCATGGTCGTGA-3′ |
|
| Reverse,
5′-CGGAGTCAACGGATTTGGTC-3′ |
Statistical analysis
Values are expressed as the mean ± standard
deviation. All data were analyzed with SPSS 17.0 (IBM Corp.,
Armonk, NY, USA). Comparisons between two groups were performed
using Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of patients with
cerebral apoplexy
A total of 85 patients with cerebral apoplexy and 68
healthy individuals were recruited for the present clinical study.
The mean of age was 35.6 and 37.8 years in cerebral apoplexy
patients and healthy individuals, respectively. The numbers of male
and female cerebral apoplexy patients and healthy individuals were
approximately equal. The characteristics of the patients with
cerebral apoplexy are summarized in Table II.
 | Table II.Characteristics of patients with
cerebral apoplexy at the intensive care unit and the control
group. |
Table II.
Characteristics of patients with
cerebral apoplexy at the intensive care unit and the control
group.
| Parameter | n (%) |
|---|
| Patients | 85 (100) |
|
Males | 43 (51) |
|
Females | 42 (49) |
| Mean
age | 35.6 |
| Healthy controls | 68 (100) |
|
Males | 33 (49) |
|
Females | 35 (51) |
| Mean
age | 37.8 |
Analysis of inflammatory cells in
serum of patients with cerebral apoplexy
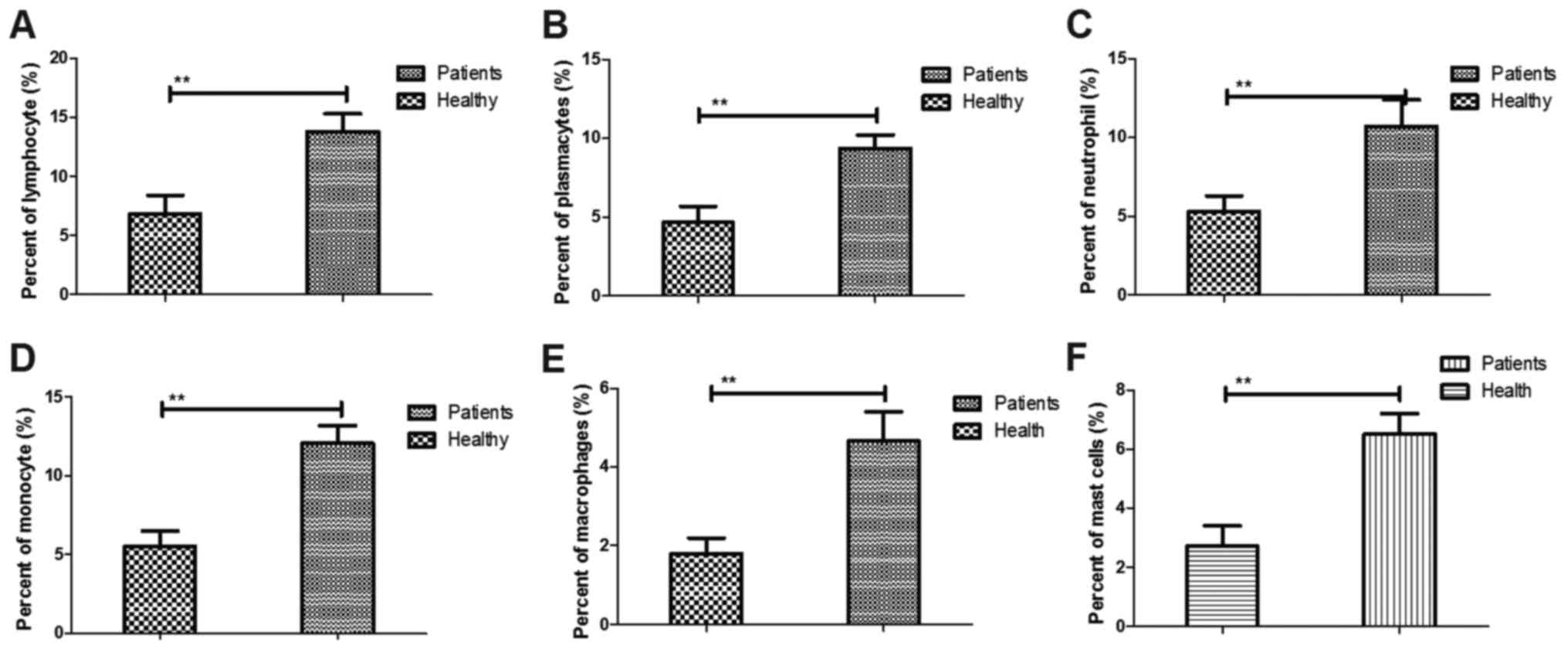
The changes of inflammatory cells were analyzed in
the serum of patients with cerebral apoplexy. It was demonstrated
that the plasma concentration of lymphocytes, plasmacytes,
neutrophils and monocytes was increased in the patients with
cerebral apoplexy compared with that in healthy individuals
(Fig. 1A-D). Furthermore, the
percentage of macrophages and mast cells was increased in patients
with cerebral apoplexy compared with that in healthy individuals
(Fig. 1E and F). Taken together,
these outcomes suggest that inflammatory cells were upregulated in
patients with cerebral apoplexy compared with those in healthy
individuals.
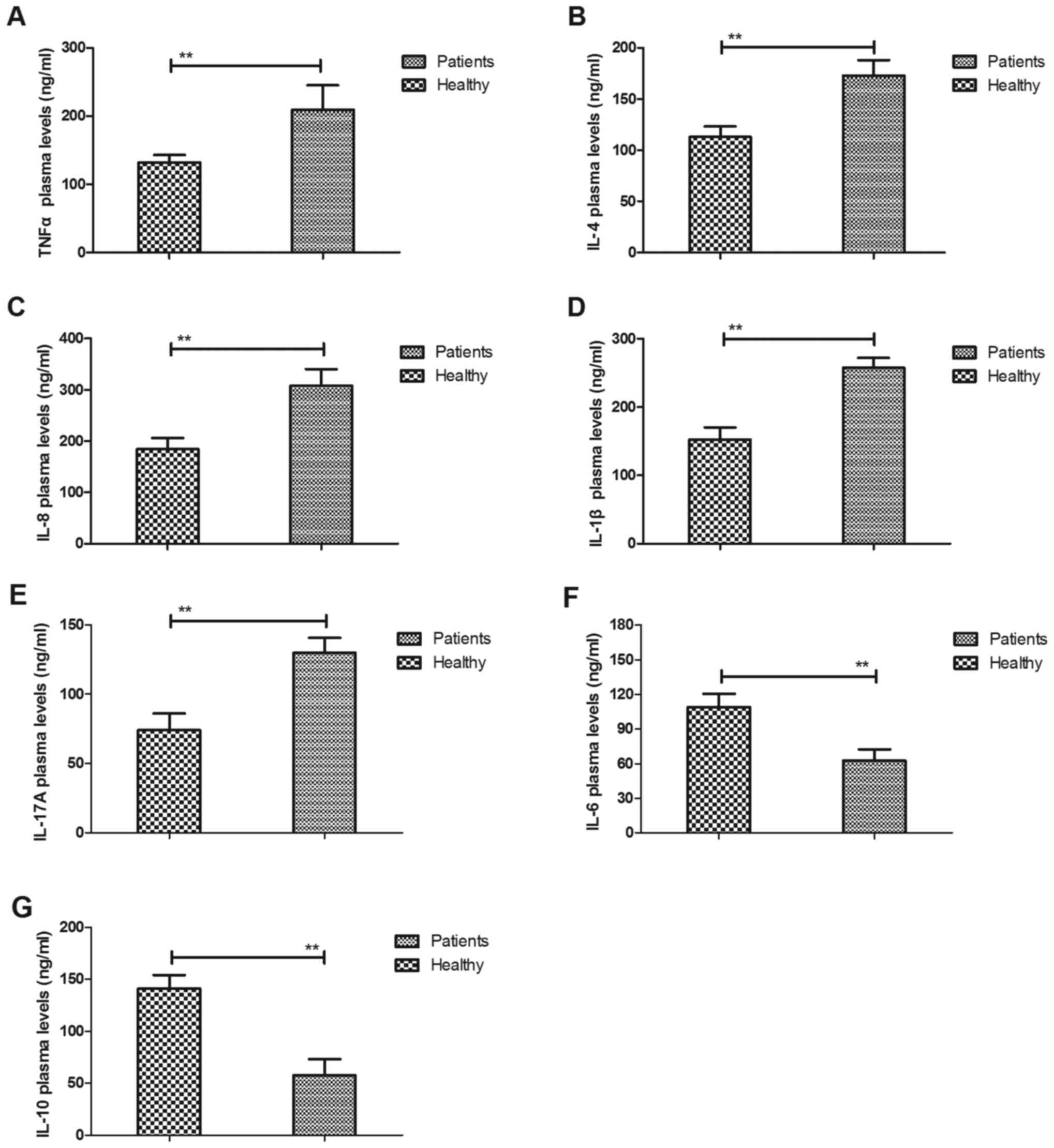
Analysis of inflammatory cytokines in
patients with cerebral apoplexy
To detect the association between inflammation and
cerebral apoplexy, the plasma levels of inflammatory factors were
measured in patients with cerebral apoplexy in the ICU and compared
with those in healthy volunteers as control. It was observed that
the serum levels of TNF-α, IL-4, IL-8, IL-1β and IL-17A were
upregulated in patients with cerebral apoplexy compared with those
in healthy individuals (Fig. 2A-E).
It was also demonstrated that the plasma levels of IL-6 and IL-10
were downregulated in patients with cerebral apoplexy in the ICU
compared with those in healthy individuals (Fig. 2F and G). These results suggest that
inflammatory cytokines in those patients was increased, while
anti-inflammatory cytokines were decreased in patients with
cerebral apoplexy.
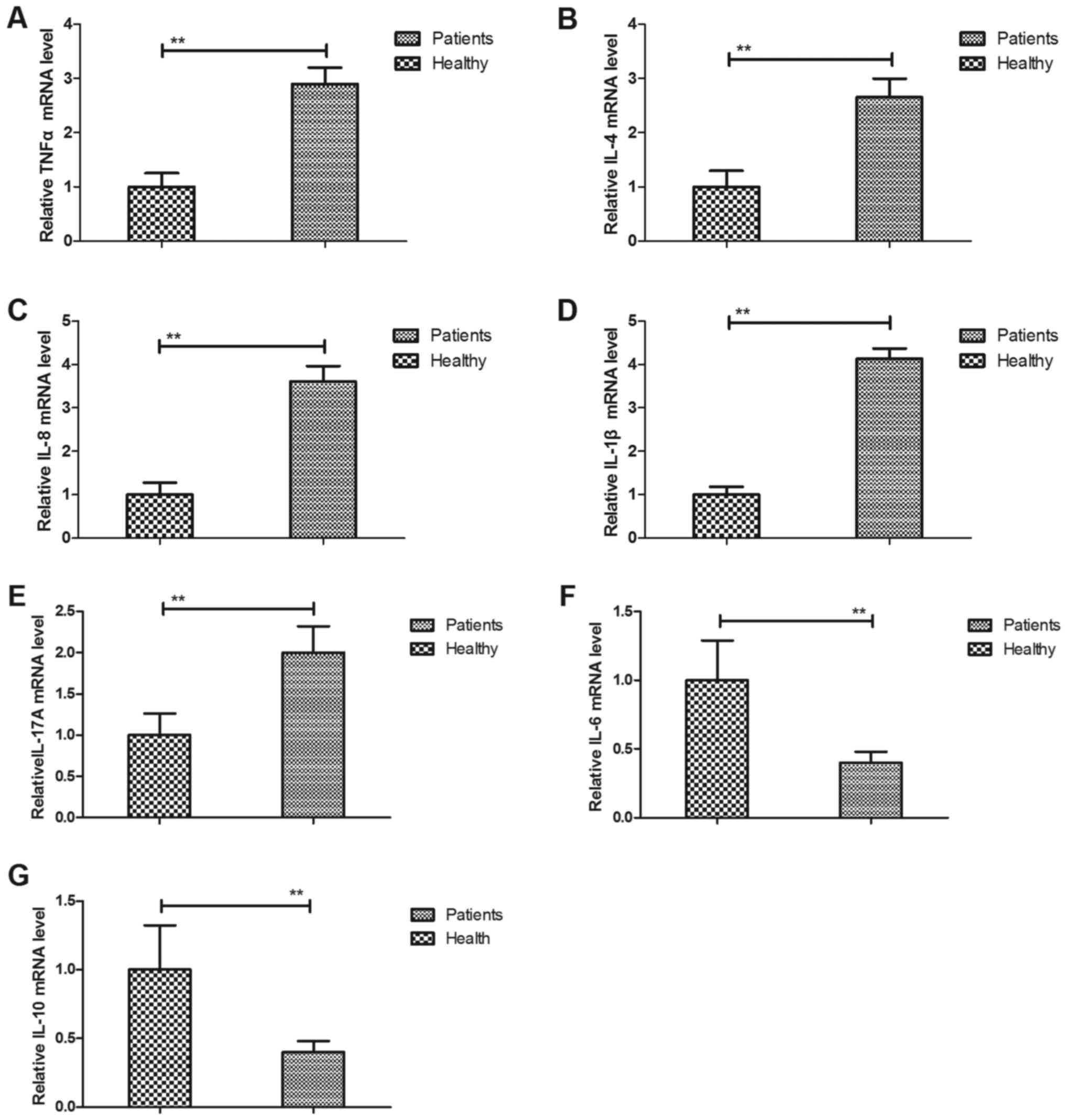
Analysis of inflammatory gene
expression in hPMCs of patients with cerebral apoplexy
Previous studies have suggested that the expression
levels of inflammatory cytokines are correlated with the severity
of patients with cerebral apoplexy (16,17). The
present study analyzed the expression of inflammatory genes in
hPMCs of patients with cerebral apoplexy. The results indicated
that the gene expression levels of TNF-α, IL-4, IL-8, IL-1β and
IL-17A were upregulated in patients with cerebral apoplexy compared
with those in healthy individuals (Fig.
3A-E). The results also demonstrated that the gene expression
levels of IL-6 and IL-10 were downregulated in patients with
cerebral apoplexy in the ICU compared with those in healthy
individuals (Fig. 3F and G). These
and the above results indicate that in cerebral apoplexy, hPMCs
produce inflammatory cytokines, which are then secreted into the
serum, while anti-inflammatory cytokine production is
downregulated.
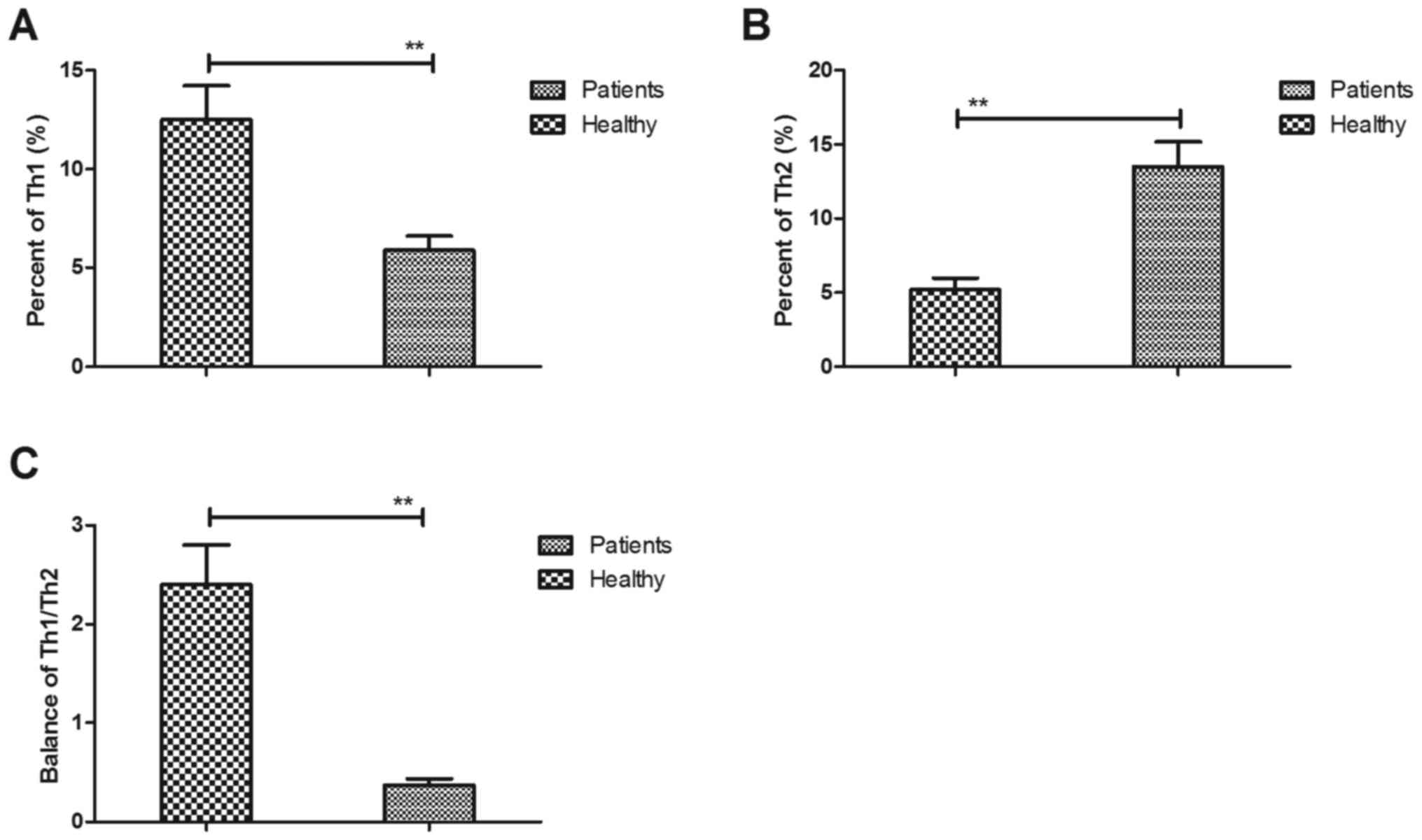
Analysis of the imbalance of T helper
cell type 1 (Th1)/Th2 cytokines in patients with cerebral
apoplexy
The imbalance of Th1/Th2 cytokines is a crucial
indicator and has an essential role in the pathology of cerebral
apoplexy in the ICU according to a previous study (18). In the present study, Th1 and Th2
cytokines were therefore quantified in patients with cerebral
apoplexy in the ICU. The results demonstrated that patients with
cerebral apoplexy presented with lower serum levels of Th1
cytokines compared with those in healthy individuals (Fig. 4A). However, higher serum levels of
Th2 cytokines were observed compared with those healthy individuals
(Fig. 4B). It was revealed that the
balance of Th1/Th2 cytokines was disturbed in patients with
cerebral apoplexy compared with that in healthy volunteers
(Fig. 4C). Collectively, these
results suggest that the balance of Th1/Th2 cytokines is disturbed
in patients with cerebral apoplexy.
Discussion
Inflammatory factors are secreted by inflammatory
cells and have been reported to be associated with the morbidity
and severity of cerebral apoplexy (19,20). The
present study attempted to investigate changes in the inflammatory
cytokines and cells in patients with cerebral apoplexy. Previous
studies have analyzed the inflammation markers C-reactive protein,
IL-6, IL-10, intercellular adhesion molecule-1, vascular cell
adhesion molecule-1, matrix metalloproteinase-9 and cellular
fibronectin, and predicted the recurrence risk of vascular disease
post-cerebral apoplexy (16,21). The present study confirmed the
previous results, i.e., that inflammatory cells are upregulated in
patients with cerebral apoplexy compared with those in healthy
individuals. Furthermore, the balance of Th1/Th2 cytokines was
disturbed in patients with cerebral apoplexy, which may be utilized
for the prevention or prognosis prediction of cerebral
apoplexy.
Inflammatory cytokines are regarded as potential
therapeutic targets in the treatment of cerebral apoplexy,
cardiovascular and cerebrovascular diseases (22). A study has indicated that a
polymorphism in the IL-1 receptor antagonist gene variable number
tandem repeat is associated with ischemic cerebral apoplexy in a
Chinese Uyghur population (23). The
present results indicated that the serum levels of IL-1β and the
associated gene expression in hPMCs were upregulated in patients
with cerebral apoplexy. Sumbria et al (24) suggested that biologic TNF inhibitors
may be re-engineered for blood-brain barrier penetration, which
indicates that the immunoglobulin G-TNF receptor fusion protein may
be regarded as a therapeutic agent after delayed intravenous
administration in experimental cerebral apoplexy. In addition, the
results in the current study suggested that IL-4 has a
neurodegenerative role in the lesion-protection process. In acute
cerebral apoplexy, TNF-α and IL-8 were increased and modulation of
these cytokines by antiplatelet agents was demonstrated to be
beneficial in affected patients (25). In addition, immunomodulatory effects
of bone marrow stromal cells on IL-17-mediated ischemic cerebral
apoplexy were identified in the pathophysiological process of
cerebral infarction, and these results may help to understand the
roles of cytokines in cerebral infarction (26). The present study indicated that the
serum levels of TNF-α, IL-4, IL-8, IL-1β and IL-17A were
upregulated in patients with cerebral apoplexy compared with those
in healthy individuals, which may be utilized as an approach for
the treatment of patients with cerebral apoplexy.
IL-6 is a predictive biomarker for cerebral
apoplexy-associated infection and risk of mortality in the elderly
after ischemic cerebral apoplexy (27). The present results indicated that the
serum levels of IL-6 were lower in patients with cerebral apoplexy.
A previous study indicated that the anti-inflammatory IL-10 is
upregulated in both hemispheres after experimental ischemic
cerebral apoplexy (28). The results
of the present study indicate that the gene expression levels in
hPMCs and the plasma concentration of IL-6 and IL-10 and were
downregulated in patients with cerebral apoplexy in the ICU
compared with those in healthy individuals. Attenuating
inflammatory responses has been reported to be beneficial in the
treatment of cerebral apoplexy (29,30).
In conclusion, the present results indicate that the
gene expression and secretion of inflammatory cytokines is
upregulated in patients with cerebral apoplexy. Of note,
inflammatory cells were upregulated and the balance of Th1/Th2
cytokines was disturbed in patients with cerebral apoplexy compared
with that in healthy individuals, which may provide a potential
anti-inflammation treatment approach for patients with cerebral
apoplexy.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW performed the majority of the experiments. ZH,
SY, CL, HY and DW performed the experiments and analyzed the data.
FG designed experiments in the current study.
Ethical approval and consent to
participate
This study was approved by Ethics Committee of
Sichuan Provincial People's Hospital (Chengdu, China). All patients
and healthy volunteers provided written informed consent.
Consent for publication
All patients have provided written informed consent
for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jeon BC, Park YS, Oh HS, Kim YS and Chun
BK: Pituitary apoplexy complicated by chemical meningitis and
cerebral infarction. J Korean Med Sci. 22:1085–1089. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dev R, Singh SK, Sharma MC, Khetan P and
Chugh A: Post traumatic pituitary apoplexy with contiguous intra
cerebral hematoma operated through endonasal route-a case report.
Pituitary. 10:291–294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ben-Nakhi A, Muttikkal TJ, Chavan VN,
Al-Turkomani AY and Gupta R: Pituitary apoplexy: A rare cause of
cerebral infarction. A case report. Neuroradiol J. 21:661–665.
2008.
|
|
4
|
Ahmed SK and Semple PL: Cerebral ischaemia
in pituitary apoplexy. Acta Neurochir (Wien). 150:1193–1196. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cerase A, Tarantino A, Muzii VF, Vittori C
and Venturi C: Vasospasm and cerebral infarction from pituitary
apoplexy. A case report. Neuroradiol J. 23:321–324. 2010.
|
|
6
|
López Hernández N, García Escrivá A, Moltó
Jordá JM and García Barragán N: Massive cerebral infarction
secondary to apoplexy due to pituitary adenoma. Neurologia.
23:248–255. 2008.(In Spanish). PubMed/NCBI
|
|
7
|
Das NK, Behari S and Banerji D: Pituitary
apoplexy associated with acute cerebral infarct. J Clin Neurosci.
15:1418–1420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang C, Feng F, Zhu Y, Wang R and Xing B:
Cerebral infarction caused by pituitary apoplexy: Case report and
review of literature. Turk Neurosurg. 24:782–787. 2014.PubMed/NCBI
|
|
9
|
de Silva DA, Woon FP, Gan HY, Cameron J,
Kingwell B, Koh TH, Chen C, Chang HM and Wong MC: Arterial
stiffness, metabolic syndrome and inflammation amongst Asian
ischaemic stroke patients. Eur J Neurol. 15:872–875. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chamorro A and Planas AM:
Inflammation-mediated damage as a potential therapeutic target in
acute ischemic stroke. Ernst Schering Res Found Workshop. pp.
185–204. 2004, PubMed/NCBI
|
|
11
|
Chamorro A: Role of inflammation in stroke
and atherothrombosis. Cerebrovasc Dis. 17(Suppl 3): S1–S5. 2004.
View Article : Google Scholar
|
|
12
|
Zhang W and Stanimirovic D: Current and
future therapeutic strategies to target inflammation in stroke.
Curr Drug Targets Inflamm Allergy. 1:151–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McColl BW, Allan SM and Rothwell NJ:
Systemic infection, inflammation and acute ischemic stroke.
Neuroscience. 158:1049–1061. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Filipova J, Rihova L, Vsianska P, Kufova
Z, Kryukova E, Kryukov F and Hajek R: Flow cytometry in
immunoglobulin light chain amyloidosis: Short review. Leuk Res, Jul
13, 2015 (Epub ahead of print).
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaplan RC, McGinn AP, Baird AE, Hendrix
SL, Kooperberg C, Lynch J, Rosenbaum DM, Johnson KC, Strickler HD
and Wassertheil-Smoller S: Inflammation and hemostasis biomarkers
for predicting stroke in postmenopausal women: The women's health
initiative observational study. J Stroke Cerebrovasc Dis.
17:344–355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McColl BW, Rothwell NJ and Allan SM:
Systemic inflammation alters the kinetics of cerebrovascular tight
junction disruption after experimental stroke in mice. J Neurosci.
28:9451–9462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Theodorou GL, Marousi S, Ellul J, Mougiou
A, Theodori E, Mouzaki A and Karakantza M: T helper 1 (Th1)/Th2
cytokine expression shift of peripheral blood CD4+ and CD8+ T cells
in patients at the post-acute phase of stroke. Clin Exp Immunol.
152:456–463. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Caso JR, Pradillo JM, Hurtado O, Lorenzo
P, Moro MA and Lizasoain I: Toll-like receptor 4 is involved in
brain damage and inflammation after experimental stroke.
Circulation. 115:1599–1608. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lip GY, Patel JV, Hughes E and Hart RG:
High-sensitivity C-reactive protein and soluble CD40 ligand as
indices of inflammation and platelet activation in 880 patients
with nonvalvular atrial fibrillation: Relationship to stroke risk
factors, stroke risk stratification schema, and prognosis. Stroke.
38:1229–1237. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Castillo J, Alvarez-Sabin J, Martinez-Vila
E, Montaner J, Sobrino T and Vivancos J; MITICO Study
Investigators: Inflammation markers and prediction of post-stroke
vascular disease recurrence: The MITICO study. J Neurol.
256:217–224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nguyen H, Aum D, Mashkouri S, Rao G, Vega
Gonzales-Portillo JD, Reyes S and Borlongan CV: Growth factor
therapy sequesters inflammation in affording neuroprotection in
cerebrovascular diseases. Expert Rev Neurother. 16:915–926. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tong Y, Han J, Guan X, Lu Z, Miao X, Ye J,
Hou SY, Zhang Y, Geng Y, Li Y, et al: Association of IL-1 receptor
antagonist gene VNTR polymorphism with ischemic stroke in the
Chinese Uyghur population. Biochem Genet. 51:698–706. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sumbria RK, Boado RJ and Pardridge WM:
Brain protection from stroke with intravenous TNFα decoy
receptor-Trojan horse fusion protein. J Cereb Blood Flow Metab.
32:1933–1938. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Al-Bahrani A, Taha S, Shaath H and Bakhiet
M: TNF-alpha and IL-8 in acute stroke and the modulation of these
cytokines by antiplatelet agents. Curr Neurovasc Res. 4:31–37.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma S, Zhong D, Chen H, Zheng Y, Sun Y, Luo
J, Li H, Li G and Yin Y: The immunomodulatory effect of bone marrow
stromal cells (BMSCs) on interleukin (IL)-23/IL-17-mediated
ischemic stroke in mice. J Neuroimmunol. 257:28–35. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kwan J, Horsfield G, Bryant T, Gawne-Cain
M, Durward G, Byrne CD and Englyst NA: IL-6 is a predictive
biomarker for stroke associated infection and future mortality in
the elderly after an ischemic stroke. Exp Gerontol. 48:960–965.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fouda AY, Kozak A, Alhusban A, Switzer JA
and Fagan SC: Anti-inflammatory IL-10 is upregulated in both
hemispheres after experimental ischemic stroke: Hypertension blunts
the response. Exp Transl Stroke Med. 5:122013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sieber MW, Jaenisch N, Brehm M, Guenther
M, Linnartz-Gerlach B, Neumann H, Witte OW and Frahm C: Attenuated
inflammatory response in triggering receptor expressed on myeloid
cells 2 (TREM2) knock-out mice following stroke. PLoS One.
8:e529822013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jin R, Yang G and Li G: Inflammatory
mechanisms in ischemic stroke: Role of inflammatory cells. J Leukoc
Biol. 87:779–789. 2010. View Article : Google Scholar : PubMed/NCBI
|