Introduction
Hepatitis B virus (HBV) is a hepatotropic enveloped
DNA virus, which causes transient and chronic hepatitis B in humans
(1). HBV infection has become a
primary public health issue that results in liver cirrhosis and the
development of hepatocellular carcinoma (2). In China, early in 2012, ~170 million
people are chronically infected with HBV (3). However, effective therapy for patients
with HBV is limited owing to a long disease course and easy
relapse.
At present, two types of drug are utilized for HBV
treatment, including nucleoside analogues and interferon (IFN)-α)
and its derivatives (4). IFNs are
cytokines that exhibit anti-proliferative, antiviral and
immunomodulatory activities (5,6).
Furthermore, IFN-α serves a key role in the inhibition of viral
replication (7). IFN-γ mediates
various critical functions by regulating pro-inflammatory,
anti-viral and anti-tumor responses (8,9).
However, IFNs possess a variety of shortcomings that limit their
widespread application, including low response rates, liver
decompensation, easy recurrence and numerous side effects,
including fever and headache (10).
Therefore, enhancing the efficacy of IFN is of vital
importance.
The transgenic Dunaliella salina (TDS) system
has been widely used as a novel bioreactor for expressing exogenous
genes (11–13) and has many advantages, including fast
growth, low production cost, easy culture, easy transgenic
manipulation and a large-scale production of exogenous proteins
(13–15). More importantly, the exogenous
proteins may also be easily purified to meet the demands of safety
and efficiency (9).
HepG2.2.15 cells are derived from the human
hepatoblastoma cell line HepG2 and are characterized by exhibiting
stable HBV expression and replication within the culture system
(16). HepG2.2.15 has been
frequently used as a cellular source capable of producing HBV in
previous studies (17,18).
The multifunctional cytokine, thymosin α1 (TA1) is a
polypeptide hormone with multiple bioactivities that is being
clinically trialed for the treatment of HBV and hepatitis C virus
(19,20). The present study designed a novel
fusion interferon (IFN-TA1) combining IFN-α/IFN-γ with TA1 in a TDS
system. The aim was to assess the anti-HBV effect of IFN-TA1 in TDS
in vitro and in vivo. The HepG2.2.15 cell line and
DHBV-infected duck model were utilized to evaluate the anti-HBV
activity of IFN-TA1 in TDS.
Materials and methods
Human tissues
Human liver tissues from 13 patients (6 male, 7
female; age, 31–46) with liver hemangioma receiving liver surgery
were obtained from September 2011 to May 2014 in the First
Affiliated Hospital of Zhengzhou University (Zhengzhou, China).
Patients were included in the present study if they exhibited
normal biochemical indexes and had no history of hypertension,
diabetes, fatty liver disease and other chronic diseases, including
liver cirrhosis. Patients were excluded if they received
hepatotoxic drugs, smoked and consumed alcohol in the first 3
months prior to surgery. The use of human tissue was approved by
the ethics committee of the First Affiliated Hospital of Zhengzhou
University (Zhengzhou, China) and all patients gave informed
consent prior to enrollment in the present study.
Transformation of IFN-α/IFN-γ fusion
gene and TA1 in TDS
The D. salina strain. UTEX-1644, was obtained
from the Algae Culture Collection at the University of Texas
(Austin, USA) and were grown in modified PKS medium (NaCl 87.7 g/l;
MgS04 with 7H20, 1.2 g/l; CaCl2, 0.022 g/l;
KN03 1.0 g/l; KH2P04, 0.054 g/l,
ferric salt solution 2.0 ml/l including Na2; EDTA with
2H2 0 0.74 g/l; FeCl3, 6H20 0.216
g/l) with a 12-h light-dark cycle under a light intensity of 50
mmol photon m−2s−1 at 26°C for 24 h (21). To amplify the IFN-α, IFN-γ and TA1
gene, the total RNA of human liver tissues was isolated using
Trizol (Sigma, St. Louis, MO, USA). The IFN-α/IFN-γ fusion gene was
generated using splicing by overlap-polymerase chain reaction (PCR)
(22). The IFN-α/IFN-γ fusion gene
and TA1 gene were then inserted into pUΩ-GUS using restriction
enzyme HaeIII (New England BioLabs, Inc., Ipswich, MA, USA) and T4
DNA ligase (New England BioLabs, Inc., Ipswich, MA, USA) to
generate pUΩ-IFN-α/IFN-γ and pUΩ-TA1 novel vectors, respectively.
Subsequently, these vectors were co-transformed into D.
salina cells using the glass bead method (21). The individual positive TDS colonies
were selected using 3 mg/l of phosphinothricin (Hoechst-Roussel AG,
Frankfurt, Germany) at 26°C for 5 days and used for further study
after 24 h.
Cell culture
HepG2.2.15 cells purchased from American Type
Culture Collection (ATCC, Manassas, VA, USA) were incubated in
Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.), 100 IU/ml penicillin
and streptomycin, 380 mg/l antibiotic G-418 sulfate (Promega
Corportation, Madison, WI, USA) and 1% L-glutamine at 37°C in 5%
CO2.
Cell viability assay
Cells were incubated in a 96-well plate at a density
of 5×104 cells per 100 µl at 37°C for 24 h. The cells
were then treated with 1,000 IU/ml IFN-α or various concentrations
of TDS (0.1, 0.2, 0.4, 0.8, 1.6 and 3.2 mg/ml) at 37°C for 5 days.
Untreated cells were utilized as a control. Following treatment,
cell viability was measured using an MTT assay as described
previously (23). Based on the cell
cytotoxicity detected by the MTT assay, the concentrations of TDS
(0.4, 0.8 and 1.6 mg/ml) were selected for the following
experiments in considerations of the lower toxicity. Furthermore, a
treatment index (TI) was used to assess the clinical application
prospect of the drug (24): TI
<1, toxic, ineffective; TI=1-2, effective, with some toxicity;
TI >2, greater effectiveness, with low toxicity.
HBV surface antigen (HBsAg) and HBV
early antigen (HBeAg) assay
Viral proteins in the culture medium, HBsAg and
HBeAg, from the cells treated with different concentrations of TDS
(0.1, 0.2, 0.4, 0.8, 1.6 and 3.2 mg/ml) were measured by using
HBeAg (cat. no. KA3288) and HBsAg (cat. no. KA0286) ELISA kits
(Abnova, Taipei City, Taiwan) according to the manufacturers'
protocols.
Quantification of HBV DNA
HBV DNA was detected in HepG2.2.15 cells treated
with IFN-α or TDS (0.8, 1.6 and 3.2 mg/ml) using quantitative PCR.
Total DNA was extracted from the cell supernatant using the TIANamp
Virus DNA/RNA kit (Tiangen Biotech Co., Ltd., Beijing, China) and
Wizard® Genomic DNA Purification kit (Promega
Corporation). The quantification of HBV DNA copies was performed
using the SYBR green premix reagent (Takara Biotechnology Co.,
Ltd., Dalian, China). Total DNA (2 µg) was used as the template for
each quantitative PCR assay. PCR was performed using an ABI 7900
real-time PCR detector (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The thermocycling conditions were as follows:
93°C for 2 min, 10 cycles at 93°C for 45 sec and 55°C for 60 sec,
and 30 cycles at 93°C for 30 sec and 55°C for 45 sec. GAPDH served
as a control gene. The primers utilized for HBV DNA fragment
amplification were as follows: Forward primer,
5′-CCTCTTCATCCTGCTGCT-3′ and reverse primer,
5′-AACTGAAAGCCAAACAGTG-3′. GAPDH primers: Forward primer,
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse primer,
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′. Assays were repeated in triplicate
and mean quantification cycle values were used to calculate the
levels of HBV-DNA using the 2−ΔΔCq method (25). The inhibitory rate was calculated
using the formula: Inhibitory rate (%)=[DNA copy (C) control-C
sample]/C control ×100.
Animals and drug treatment
A total of 3 day-old ducklings (weight, 40–50 g)
were purchased from Zhejiang Academy of Agricultural Sciences
(Zhejiang, China). The sex of ducklings was not distinguished as
prior studies have demonstrated that this does not affect results
(26,27). All ducks received ad libitum
access to standard diet and water, and housed under controlled
conditions (temperature, 28–30°C; humidity 56–70%; and a 24-h light
cycle) (26,27). After adaptive maintenance for 3 days,
0.5 ml of blood was obtained from each duck for analysis. Only
DHBV-positive ducklings were used for the subsequent experiments.
Animal handling protocols were approved by the Animal Ethics
Committees of The First Affiliated Hospital of Zhengzhou University
(Zhengzhou, China).
Ducks infected with congenital DHBV were randomly
divided into 5 groups (each n=6): An IFN-α group treated with IFN-α
(4,000 IU/injections, 500 µl/day; Sigma); 3 groups which received
TDS via gastric perfusion at different concentrations (5, 10 and 20
g/kg TDS, respectively); and a control group treated with normal
saline. The respective treatments were administered once daily for
21 consecutive days. Blood was drawn from all ducks prior to
treatment (T0), following 7 (T7), 14 (T14) and 21 (T21) days of
treatment and following withdrawal of the drug after 5 days (P5).
The serum samples were separated using centrifugation at 7,000 × g
for 15 min at 4°C and stored at −80°C. The levels of DHBV DNA in
the serum were detected by quantitative PCR using the SYBR Green
real-time PCR Master Mix (Takara Biotechnology Co., Ltd.) with the
following primers: Forward primer, 5′-GATACTGGAGCCCAAACC-3′ and
reverse primer 5′-GGCAGAGGAGGAAGTCAT-3′. GAPDH served as a control
gene, forward primer, 5′-CACAGCCACACACGAAGACA-3′ and reverse
primer, 5′-CCTTAGCCAGCCCCAGTAGA-3′. The thermocycling conditions
were as follows: 95°C for 1 min, 40 cycles including 95°C for 5
sec, 56°C for 5 sec and 72°C for 25 sec and 40°C for 10 sec. The
level of HBV-DNA was calculated using the 2−ΔΔCq method
(25).
Histological analysis
Following 21 days of drug treatment, ducks were
immediately anesthetized with sodium pentobarbital (Sigma;
intraperitoneal injection; 150 mg/kg) and subsequently sacrificed
by exsanguination. Liver specimens were collected from the ducks
and separated into two sections (1×1 cm). One was fixed with 4%
buffered formalin and embedded in paraffin for 24 h at room
temperature. The samples were then stained with hematoxylin and
eosin (H&E) for 15 min at room temperature and examined under
an optical microscope.
The second section was also fixed with 4% buffered
formalin for 24 h at room temperature and embedded in paraffin for
24 h at room temperature. Then the samples were stained with orcein
for 30 min at 37°C and examined under an optical microscope to
detect HBsAg.
Statistical analysis
Data are presented as the mean ± standard deviation
and each experiment was performed in triplicate. Data were analyzed
using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA). Statistically
significant differences between two groups were detected using
Student's t-test and the comparison of multiple groups was
performed using one-way analysis of variance followed by a post-hoc
Tukey's test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of TDS treatment on cell
viability and HBV antigen secretion in HepG2.2.15 cells in
vitro
The effect of the drug treatment on cell viability
and HBV antigen secretion in HepG2.215 cells in vitro was
assessed. As presented in Fig. 1A,
TDS had a marked inhibitory effect on cell viability, suggesting
that TDS produced a cytotoxic effect on HepG2.2.15 cells. In
addition, treatment with TDS (0.4, 0.8, 1.6 and 3.2 mg/ml) resulted
in a significant reduction of HBsAg (Fig. 1B) and HBeAg secretion (Fig. 1C). The TI values of TDS for HBsAg and
HBeAg were 2.96 and 3.07 respectively in HepG2.2.15 cells,
indicating that TDS was effective and exhibited low toxicity
(Fig. 1D). Furthermore, the TI
values of TDS for HBsAg and HBeAg were significantly higher than
that of IFN-α, which suggests that TDS may be more effective in
clinical application.
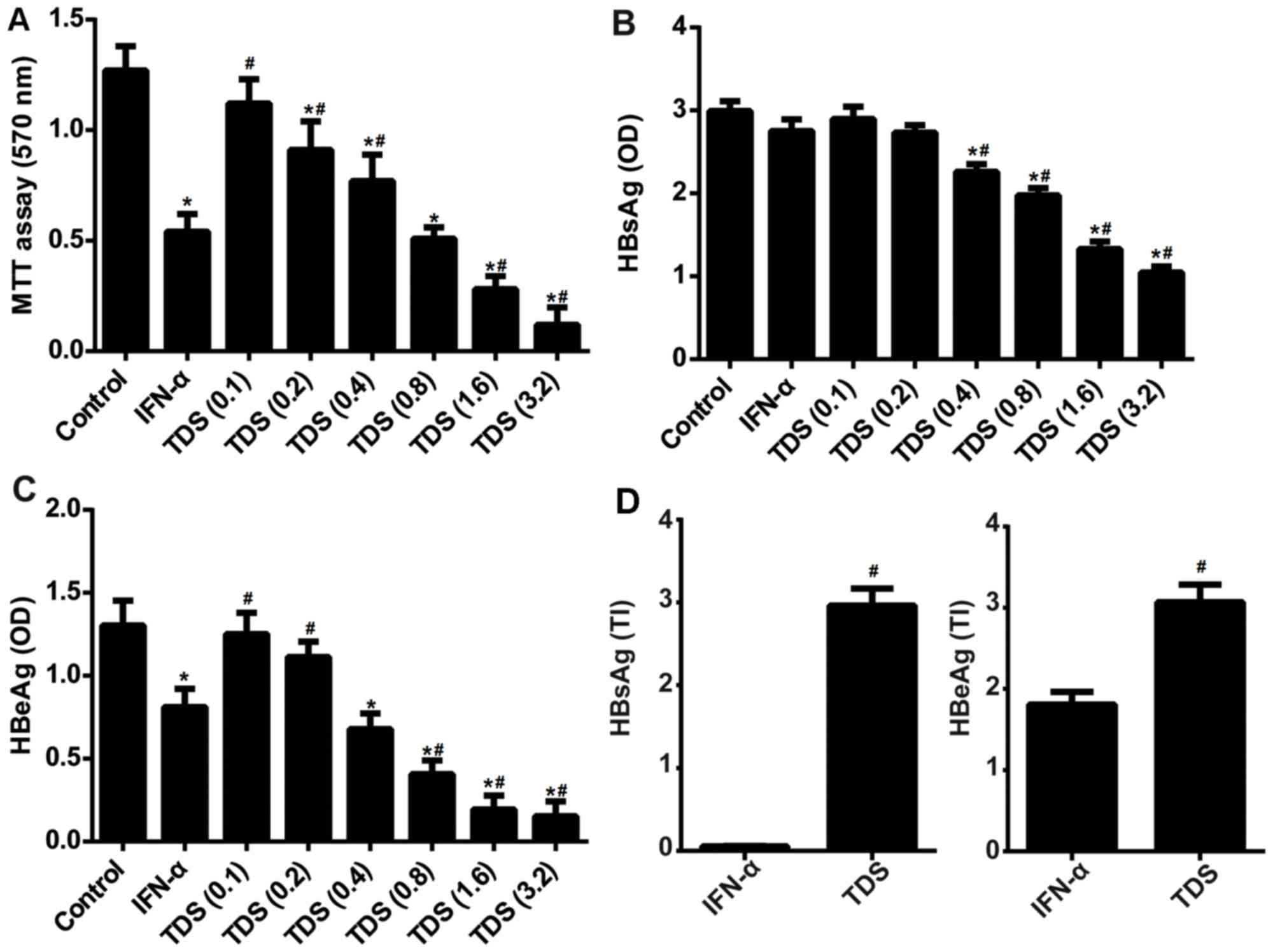 | Figure 1.TDS treatment suppressed cell
viability and HBV antigen secretion, but promoted TI in HepG2.2.15
cells. Cells were treated with 1,000 IU/ml IFN-α or different
concentrations of TDS (0.1, 0.2, 0.4, 0.8, 1.6 and 3.2 mg/ml) for 5
days. (A) Cell viability was measured using an MTT assay. (B) HBsAg
and (C) HBeAg levels in the culture supernatants. (D) The TI values
for HBsAg and HBeAg following TDS and IFN-α treatment in HepG2.2.15
cells. *P<0.05 vs. the control, #P<0.05 vs. IFN-α
group. HBV, hepatitis B virus; TI, treatment index; IFN-α,
interferon-α; TDS, transgenic Dunaliella salina; HBsAG, HBV
surface antigen; HBeAg, HBV early antigen; OD, optical density. |
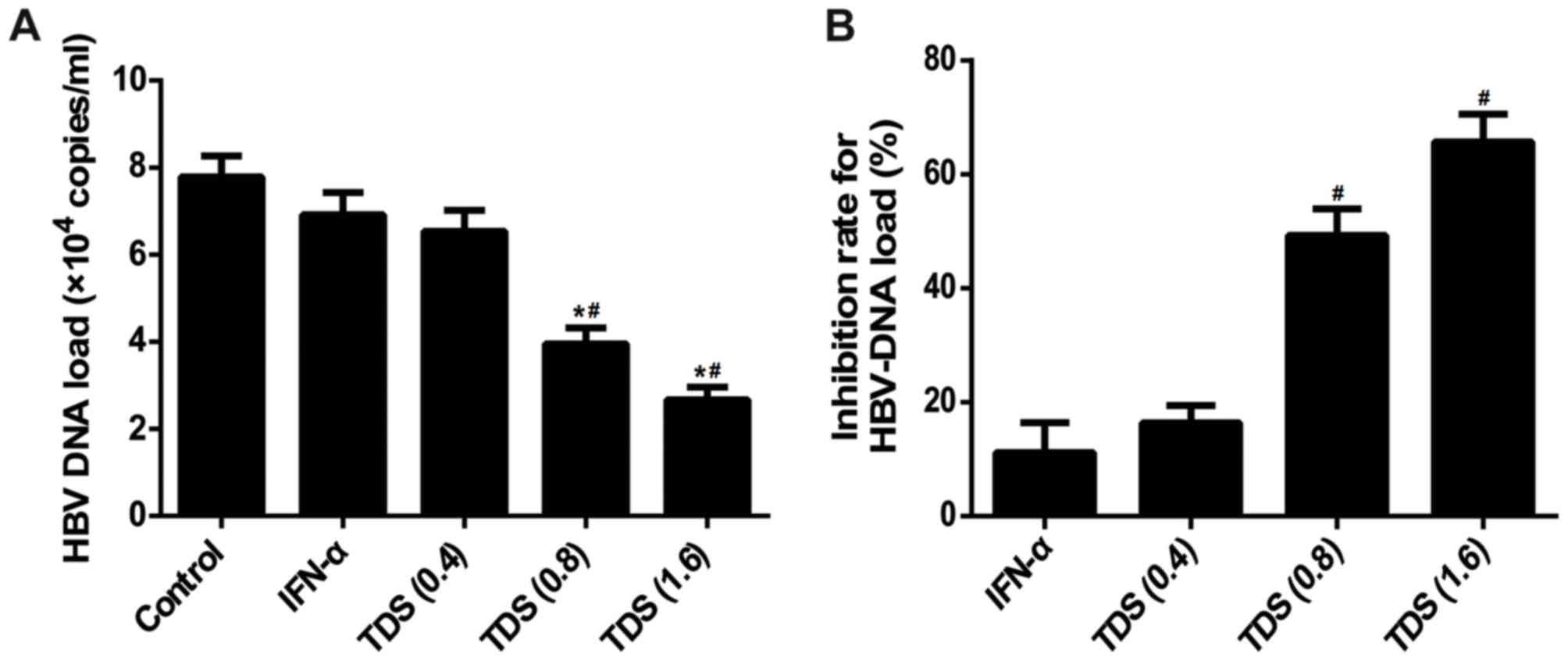
Effect of TDS treatment on HBV-DNA
load in HepG2.2.15 cell culture medium
To further confirm the anti-HBV activity of TDS, the
levels of HBV-DNA in the cell supernatant were assessed. The
results indicated that, compared with control group, treatment of
TDS (0.8 and 1.6 mg/ml) significantly decreased HBV-DNA levels in
the culture medium, with inhibition ratios of 49.3 and 65.7%,
respectively (Fig. 2A and B).
However, no significant difference was observed in the IFN-α group
when compared with the control group. The results revealed that TDS
treatment suppresses HBV-DNA replication in HepG2.2.15 cells.
Effect of TDS treatment on duck HBV
(DHBV)-DNA levels, inflammation and HBsAg in duck livers
Subsequently, the present study assessed the
anti-HBV effect of TDS in vivo. As presented in Fig. 3A, duck serum DHBV-DNA levels were
significantly decreased in the TDS group (20 g/kg) following
treatment for 7, 14 and 21 days compared with that of the control
group. Following treatment for 21 days and withdrawal of the drug
for 5 days, the levels of duck serum DHBV-DNA in the TDS groups (10
and 20 g/kg) were significantly reduced when compared with the
control group. Following drug withdrawal for 5 days, the levels of
DHBV-DNA did not relapse in the TDS groups (10 and 20 g/kg).
However, relapse following cessation of TDS was observed in the
IFN-α and TDS (5 g/kg) groups.
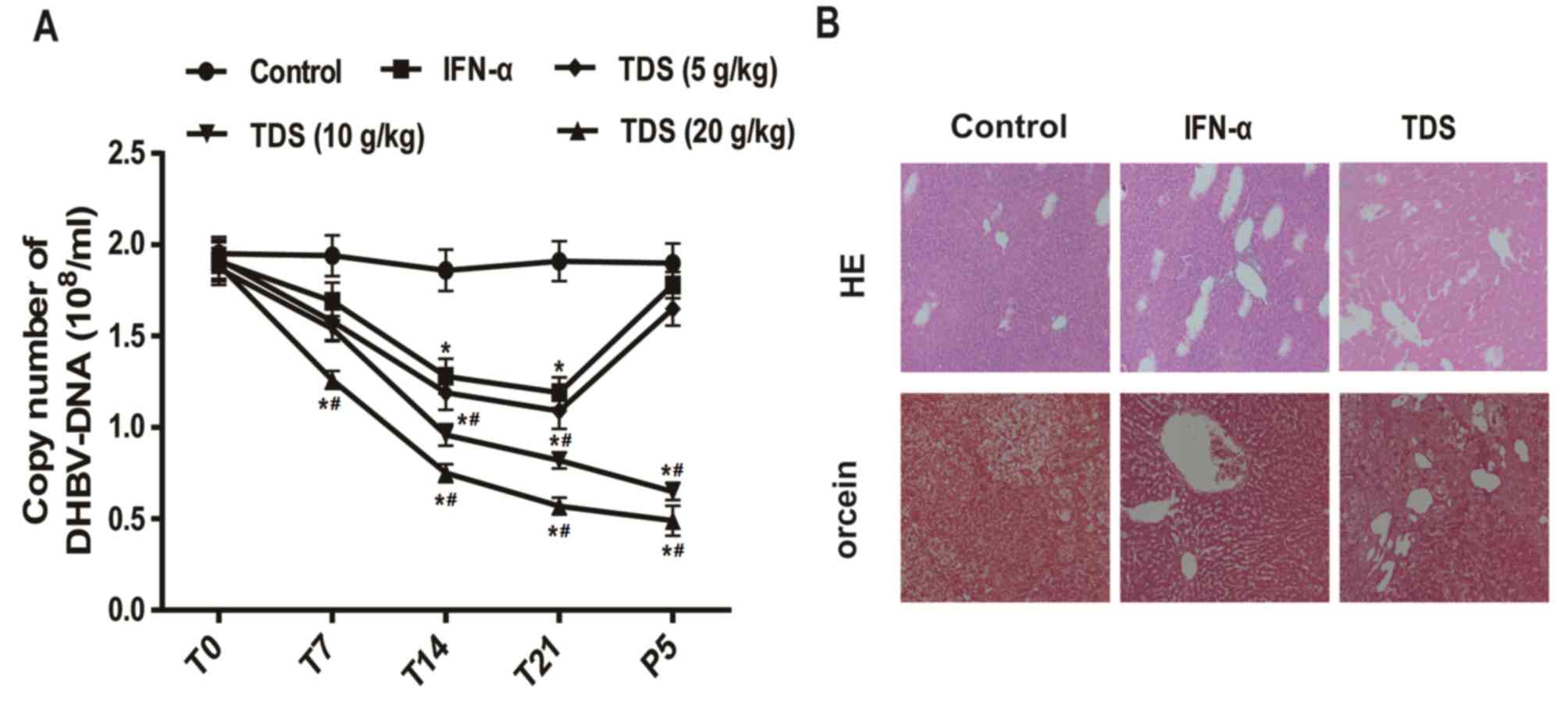 | Figure 3.TDS treatment reduced DHBV-DNA
levels, inflammation and HBsAg signals in livers. (A) DHBV-DNA
levels in the duck serum at different times (T0, T7, T14, T21 and
P5) after IFN-α (4,000 IU/injections/d in 0.5 ml) and different
concentrations of TDS (5, 10 and 20 g/kg/day) treatment. (B)
Representative examples of H&E and orcein staining of the duck
livers (magnification, 200×) in control, IFN-α and TDS (20 g/kg)
treated groups following treatment for 21 days. *P<0.05 vs.
control group, #P<0.05 vs. IFN-α group. DHBV, duck
hepatitis B virus; T, treatment; P, drug withdrawal; IFN-α,
interferon-α; TDS, transgenic Dunaliella salina; H&E,
hematoxylin and eosin. |
Histological observation was then performed using
H&E and orcein staining. The degree of swelling in liver cells
was notable and the expression of infiltrating lymphocytes was
positive in the control group. However, these results were
alleviated following IFN-α and TDS treatment. In addition, HBsAg
was observed as brown granules in the control group. However, a
decrease in the signals for HBsAg was observed in IFN-α and TDS
groups compared with the control group (Fig. 3B). These results demonstrated that
TDS and IFN-α treatment alleviated inflammation and HBsAg in duck
livers.
Discussion
IFNs are considered to serve a key role in the
control of viral infections (28). A
previous study has indicated that interferons suppress HBV
replication in transgenic mice that produce a high level of HBV
(29). IFN-α has previously been
used to treat HBV infections; however, IFN-α treatment generates
sustained virological response in a small quantity of patients
(30). The incorporation of TA1 into
the fusion gene of IFN-α/IFN-γ may be a promising strategy for the
development of anti-HBV drugs (31).
The present study demonstrated that IFN-TA1 in a TDS model
efficiently reduced HBsAg and HBeAg secretion and HBV-DNA
replication in HepG2.2.15 cells in vitro. Furthermore, TDS
treatment suppressed DHBV-DNA levels, inflammation and HBsAg
signals in duck livers in vivo.
The HBV marker is an essential tool for the
assessment of HBV infection (32).
Following infection, viral replication occurs inside hepatocytes
and consequently HBV DNA, and viral proteins, including HBeAg and
HBsAg can be easily detected in serum. The levels of these clinical
markers are commonly used to evaluate the disease stage of patients
(33,34). Therefore, the inhibition of HBV
replication may inevitably reduce the secretion of HBeAg and HBsAg
(35). In the present study, the
HepG2.2.15 cell line, which contains multiple copies of the HBV
genome and is capable of secreting HBV virions into the
supernatant, was used as in vitro model (16). The results demonstrated that TDS
markedly inhibited the levels of HBV DNA and HBsAg and HBeAg in the
culture medium of HepG2.2.15 cells. Additionally, TI values for
HBsAg and HBeAg were higher in the TDS group than those of the
IFN-α group, indicating that TDS treatment enhances the effect of
treatment in vitro.
DHBV is closely associated with human HBV in regard
to its mode of replication, genomic organization and hepatotropism
(36). DHBV in its natural host, the
duck, has been used as an animal model in a preclinical study of
drugs designed for the treatment of HBV (37). The results of the present study
demonstrated that TDS was a potent inhibitor of DHBV replication in
ducks congenitally infected with DHBV. In addition, DHBV-DNA levels
were markedly reduced in the high dosage TDS group (20 g/kg)
following treatment for 7, 14 and 21 days and withdrawal of the
drug for 5 days compared with that in the control group. The levels
of DHBV-DNA did not relapse in the high and medium dosage groups of
TDS (20 and 10 g/kg, respectively) following drug withdrawal for 5
days. However, relapse following cessation of TDS was observed in
the low dosage TDS (5 g/kg) and IFN-α groups. Additionally, the
histological analysis of duck liver confirmed that TDS and IFN-α
treatment alleviated inflammatory and HBsAg signals in duck livers.
Therefore, these results revealed that TDS strengthens the
anti-DHBV effects in vivo.
In conclusion, the present study demonstrated that
TDS, which produces IFN-TA1 recombinant proteins, effectively
inhibited HBsAg and HBeAg secretion and HBV-DNA replication in
vitro and suppressed DHBV replication and inflammation in
vivo. The present results indicate that D. salina may be
used as a bioreactor for the production of IFN-TA1 recombinant
proteins. In addition, the anti-HBV effect of TDS is greater than
that of IFN-α, which may be an effective antiviral medicine in
future treatment of HBV.
Acknowledgements
Not applicable.
Funding
The authors are thankful for the financial support
from the Key Project of Science and Technology Department in Henan
Province (grant no. 152102310045).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ wrote the paper and performed the research; PH
and YZ performed the research; XX analyzed the data; SF and CS
designed the research. All authors have read and approved this
manuscript.
Ethics approval and consent to
participate
Animal handling protocols were approved by the
Animal Ethics Committees of The First Affiliated Hospital of
Zhengzhou University (Zhengzhou, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Seeger C and Mason WS: Hepatitis B virus
biology. Microbiol Mol Biol Rev. 64:51–68. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choi JG, Chung YH, Kim JA, Jin YJ, Park
WH, Lee D, Shim JH, Lee YS, Seo DD, Jang MK, et al: High HBV-DNA
titer in surrounding liver rather than in hepatocellular carcinoma
tissue predisposes to recurrence after curative surgical resection.
J Clin Gastroenterol. 46:413–419. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang G, Han M, Chen F, Xu Y, Chen E, Wang
X, Liu Y, Sun J, Hou J, Ning Q and Wang Z: Hepatitis B virus
genotype B and mutations in basal core promoter and pre-core/core
genes associated with acute-on-chronic liver failure: A multicenter
cross-sectional study in China. Hepatol Int. 8:508–516. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mangano C, Squadrito G, Cacciola I,
Carpentieri M, Foti G and Raimondo G: Effectiveness of add-on
pegylated interferon alfa-2a therapy in a lamivudine-treated
patient with chronic hepatitis B. Ann Hepatol. 10:84–87.
2011.PubMed/NCBI
|
|
5
|
Cho H and Kelsall BL: The role of type I
interferons in intestinal infection, homeostasis, and inflammation.
Immunol Rev. 260:145–167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bandurska K, Król I and Myga-Nowak M:
Interferons: Between structure and function. Postepy Hig Med Dosw
(Online). 68:428–440. 2014.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pestka S, Krause CD and Walter MR:
Interferons, interferon-like cytokines, and their receptors.
Immunol Rev. 202:8–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Horras CJ, Lamb CL and Mitchell KA:
Regulation of hepatocyte fate by interferon-γ. Cytokine Growth
Factor Rev. 22:35–43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao B, Wang H, Lafdil F and Feng D: STAT
proteins-key regulators of anti-viral responses, inflammation, and
tumorigenesis in the liver. J Hepatol. 57:430–441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koumbi L: Current and future antiviral
drug therapies of hepatitis B chronic infection. World J Hepatol.
7:1030–1040. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Geng D, Wang Y, Wang P, Li W and Sun Y:
Stable expression of hepatitis B surface antigen gene in Dunaliella
salina (Chlorophyta). J Appl Phycol. 15:451–456. 2003. View Article : Google Scholar
|
|
12
|
Sun Y, Yang Z, Gao X, Li Q, Zhang Q and Xu
Z: Expression of foreign genes in Dunaliella by electroporation.
Mol Biotechnol. 30:185–192. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng S, Li X, Xu Z and Qi J: Dunaliella
salina as a novel host for the production of recombinant proteins.
Appl Microbiol Biotechnol. 98:4293–4300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang HH, Yin WB and Hu ZM: Advances in
chloroplast engineering. J Genet Genomics. 36:387–398. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barzegari A, Hejazi MA, Hosseinzadeh N,
Eslami S, Mehdizadeh Aghdam E and Hejazi MS: Dunaliella as an
attractive candidate for molecular farming. Mol Biol Rep.
37:3427–3430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sells MA, Chen ML and Acs G: Production of
hepatitis B virus particles in Hep G2 cells transfected with cloned
hepatitis B virus DNA. Proc Natl Acad Sci USA. 84:1005–1009. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu X, Xie C, Li YM, Huang ZL, Zhao QY, Hu
ZX, Wang PP, Gu YR, Gao ZL and Peng L: TMEM2 inhibits hepatitis B
virus infection in HepG2 and HepG2.2.15 cells by activating the
JAK-STAT signaling pathway. Cell Death Dis. 7:e22392016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li L, Lei QS, Zhang SJ, Kong LN and Qin B:
Suppression of USP18 potentiates the anti-HBV activity of
interferon alpha in HepG2.2.15 cells via JAK/STAT signaling. PLoS
One. 11:e01564962016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Romani L, Bistoni F, Montagnoli C, Gaziano
R, Bozza S, Bonifazi P, Zelante T, Moretti S, Rasi G, Garaci E and
Puccetti P: Thymosin alpha1: An endogenous regulator of
inflammation, immunity, and tolerance. Ann N Y Acad Sci.
1112:326–338. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Billich A: Thymosin alpha1. SciClone
Pharmaceuticals. Curr Opin Invest Drugs. 3:698–707. 2002.
|
|
21
|
Feng SY, Xue LX, Liu HT and Lu PJ:
Improvement of efficiency of genetic transformation for Dunaliella
salina by glass beads method. Mol Biol Rep. 36:1433–1439. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meng XP, Yang JY, Qiao XL, Liang LX and
Shou-Min XI: Optimization of construction of fusion gene-BTRCP-CypA
by using SOE-PCR technique. Biotechnology. 23:51–54. 2013.
|
|
23
|
Pant K, Gupta P, Damania P, Yadav AK,
Gupta A, Ashraf A and Venugopal SK: Mineral pitch induces apoptosis
and inhibits proliferation via modulating reactive oxygen species
in hepatic cancer cells. BMC Complement Altern Med. 16:1482016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mi S: A research for screening
anti-hepatitis B virus drugs with the 2.2.15 cell line. Zhonghua Yi
Xue Za Zhi. 72:612–615, 640. 1992.(In Chinese). PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sari M: Effects of production system and
gender on liveweight and body measurements in pekin ducks. Atatürk
Üniversitesi Vet Bil Derg. 8:112–121. 2013.
|
|
27
|
Low HC: Molecular analysis of acute and
chronic duck hepatitis B virus (DHBV) infections in ducks.
(unpublished PhD thesis). University of Adelaide, School of
Molecular and Biomedical Science; Adelaide, South Australia;
2012
|
|
28
|
Tian Y, Chen WL and Ou JH: Effects of
interferon-α/β on HBV replication determined by viral load. PLoS
Pathog. 7:e10021592011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guidotti LG, Morris A, Mendez H, Koch R,
Silverman RH, Williams BR and Chisari FV: Interferon-regulated
pathways that control hepatitis B virus replication in transgenic
mice. J Virol. 76:2617–2621. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hansen BE, Buster EH, Steyerberg EW,
Lesaffre E and Janssen HL: Prediction of the response to
peg-interferon-alfa in patients with HBeAg positive chronic
hepatitis B using decline of HBV DNA during treatment. J Med Virol.
82:1135–1142. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu NF, Huang AL, Zheng RQ, Zhu YB, Xia ZF,
Tang N, Yan G, Gao XL and Wu Y: Anti-HBV effect of fusion protein
(TA1-IFN) in vitro. Zhonghua Gan Zang Bing Za Zhi. 13:252–254.
2005.(In Chinese). PubMed/NCBI
|
|
32
|
Cho HJ, Kim JK, Nam JS, Wang HJ, Lee JH,
Kim BW, Kim SS, Noh CK, Shin SJ, Lee KM, et al: High circulating
microRNA-122 expression is a poor prognostic marker in patients
with hepatitis B virus-related hepatocellular carcinoma who undergo
radiofrequency ablation. Clin Biochem. 48:1073–1078. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ganem D and Prince AM: Hepatitis B virus
infection-natural history and clinical consequences. N Engl J Med.
350:1118–1129. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rehermann B and Nascimbeni M: Immunology
of hepatitis B virus and hepatitis C virus infection. Nat Rev
Immunol. 5:215–229. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu WS, Zhao KK, Miao XH, Ni W, Cai X,
Zhang RQ and Wang JX: Effect of oxymatrine on the replication cycle
of hepatitis B virus in vitro. World J Gastroenterol. 16:2028–2037.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mason WS, Seal G and Summers J: Virus of
Pekin ducks with structural and biological relatedness to human
hepatitis B virus. J Virol. 36:829–836. 1980.PubMed/NCBI
|
|
37
|
Liu Q, Jia R, Wang M, Huang J, Zhu D, Chen
S, Yin Z, Wang Y, Chen X and Cheng A: Cloning, expression and
purification of duck hepatitis B virus (DHBV) core protein and its
use in the development of an indirect ELISA for serologic detection
of DHBV infection. Arch Virol. 159:897–904. 2014. View Article : Google Scholar : PubMed/NCBI
|