Introduction
Chronic subdural hematoma (CSDH) occurs frequently
in middle-aged and aged people (>50 years), and the incidence
rate in individuals aged >70 years is 20 times as high as that
in the general population (1). Due
to the global population aging, a marked increase in the number of
CSDH cases is foreseeable (2).
Burr-hole craniotomy (BHC) and twist-drill craniotomy (TDC) are two
of the most commonly used therapeutic methods for CSDH, and the
comparison of their curative effects has always been a hotspot in
clinical research.
Evidence-based studies by Weigel et al
(3) and Lega et al (4) indicated that BHC is more efficient and
safer than TDC. However, an increasing number of studies have
suggested that TDC should be used as a preferred clinical regimen.
In particular, while the randomized controlled studies of Muzii
et al (5), Gökmen et
al (6) and Singh et al
(7) did not prove that TDC was
superior according to the major clinical indexes ‘recurrence rate’
and ‘mortality rate’. However, the recent evidence-based studies by
Ducruet et al (8) and
Almenawer et al (9) pushed
the question back to its origin, as the authors argued that TDC has
more advantages due to the shorter surgery times and minimally
invasive characteristics.
Previous studies generally use non-unified
definitions of the outcomes, and among them, the major indexes
refer to different clinical implications (3,10–20). For
instance, CSDH ‘recurrence’, the most commonly used index to
describe the major outcomes, is often confused with ‘secondary
operation’ (5,6,18,21). To
this end, the clinical evaluation system for CSDH was redesigned
with the cure rate as the major evaluation index, and the present
prospective clinical randomized controlled trial was designed to
further determine the advantages and disadvantages of the two
therapeutic methods.
Patients and methods
Definition of TDC
Skull drilling evacuation of hematoma with a skull
drilling diameter of >5 mm is defined as minimally invasive
craniotomy or TDC, that with a drilling diameter of >5 and
>30 mm is defined as BHC and that with larger surgical wounds is
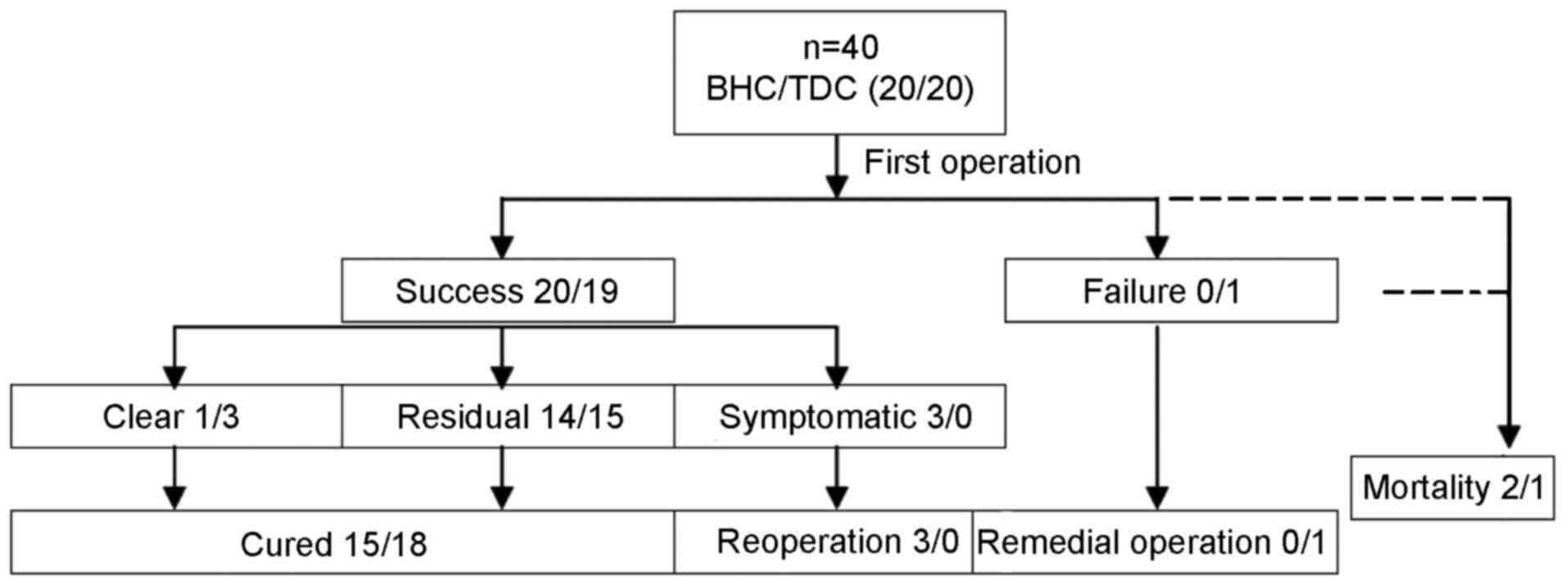
defined as general craniotomy. In the flow chart in Fig. 1, the clinical course of patients with
CSDH is illustrated according to previous studies (22).
Definition of recurrent CSDH
The recurrence of CSDH is defined as the repeated
accumulation of hematoma in the ipsilateral subdural hematoma
cavity confirmed by imaging after the initial treatment. Repeated
treatment by decompression drainage in the case of CSDH recurrence
with symptoms or aggravated symptoms is known as reoperation. The
first operation frequently fails due to certain technical issues or
operative complications, including acute subdural hemorrhage,
ineffective shunt or poor drainage. At this point, an emergency
remedial surgery is required to reconstruct the drainage for
intracranial decompression, which is often performed within a short
time after the first operation. In the present study, remedial
surgery within 48 h was used as a short-term efficacy index for
assessing the success rate of surgery. As a control, the cure rate
was used as a long-term index of assessing the surgical efficacy.
Patients regarded as cured from CSDH were those with improvement of
neurologic impairment after the first operation, including the
complete clearance of hematoma without recurrence, residual
hematoma in the subdural space identified during follow-up imaging,
asymptomatic or no symptom aggravation. Therefore, patients who
underwent a second surgery, namely a remedial operation and
reoperation, and who died during follow-up, were not deemed as
being cured (22).
Inclusion criteria
Patients diagnosed with CSDH via head CT or magnetic
resonance imaging (MRI) at Huai'an First People's Hospital (Huai'an
China) between January 2016 and January 2017 were enrolled in the
present study. Inclusion criteria were a clear correlation of CSDH
with neurologic impairment symptoms and signs confirmed via
neurological examination; the requirement of hematoma drainage and
decompression; and an age >18 years for either sex. Patients
with CSDH caused by systemic diseases were excluded, and surgical
contraindications were excluded through appropriate biochemical
examinations, electrocardiogram and chest CT. Patients who met the
inclusion criteria were enrolled and randomly grouped after they
and their families were informed about the details of the present
study, the surgical risks and the relevant safety measures, and
provided written informed consent. The present study was approved
by the Ethics Committee of Huai'an First People's Hospital.
Random grouping method
A total of 40 random numbers were generated using
Statistical Product and Service Solutions (SPSS) v21.0 software
(IBM Corp., Armonk, NY, USA). After the patients provided their
signature to confirm the experimental scheme, one number was
randomly selected from the 40 numbers and the patients were
accordingly enrolled into the pre-set groups (23). Three clinicians were independently
responsible for the grouping, surgery and follow-up,
respectively.
Surgical procedures for TDC
According to the CT scan positioning, the center on
the thickest layer was selected as a puncture point and the
arteries were avoided. The electric hand drill with a 2-cm
minimally invasive intracranial hematoma puncture needle was used
to penetrate the skull and dura mater along the locating point to
the hematoma center into the subdural hematoma cavity. The drill
was then removed, the drainage hose was connected and the drill
head was pulled out. The appearance of a dark red bloody fluid
overflow was considered to indicate a successful puncture, and the
rapid improvement in symptoms were used for verification. The
highest point of drainage tube was maintained 10–15 cm higher than
the head puncture point, and the symptoms were improved; the
drainage tube was temporarily clipped and then opened after 2 h,
and the total drainage time was 48 h. The minimally invasive
puncture drainage was successful in 19 patients, and the drainage
tube was removed after 48 h without the traditional irrigation
according to previous studies (24–29).
Through the routine post-operative review of the head CT, severe
complications were excluded and the drainage tube was removed after
48 h.
Surgical procedures for BHC
A scalp incision with a length of ~4 cm was made
with a skull drill of 12 mm in diameter and the drill was slightly
expanded by using the rongeur. The dura mater was cut in a cross
shape and washed with warm saline, and one drainage tube was placed
into it.
Post-operative management
To prevent recurrence, all patients were routinely
administered statins every night. After the operation, the tube was
clipped for 2 h after it was opened for drainage for 48 h and
hematoma was significantly reduced on CT; the symptoms were
improved and the silicone drainage tube was eventually pulled out.
After the operation, in a comfortable supine position, the elderly
patients were required to lie in bed and rest, but they were not
strictly confined to the bed (11).
The incision was disinfected and the dressing was replaced once per
day. All 40 patients were followed up by independent clinicians. A
new head imaging was reviewed and neurological scoring according to
the mRS was performed at 3 months after the operation.
Statistical analysis
All data from the data set were input into SPSS
v21.0 for windows software (IBM Corp.). Measurement data were
presented as the mean ± standard deviation. The independent-samples
t-test was used for intergroup comparison and the χ2
test was used for enumeration data. The difference values of mRS
scores prior to and after the operation and the ratio vs. the
pre-operative value were compared between the two groups using the
Mann-Whitney U test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Demographic data of the patients
A total of 176 cases of CSDH were treated using
different surgical methods at Huai'an First People's Hospital from
January 2016 to January 2017. This population comprised 132 males
and 44 females aged 19–89 years with an average age of 63.2±10.11
years. Definite diagnoses were made by brain CT or head MRI
examination. On CT, the low-density, equal-density, slightly
high-density or mixed-density crescent abnormal fluid areas in the
subdural space frequently had a space-occupying effect. The brain
parenchyma was pressed, the brain fissure disappeared, and the
lateral ventricle and midline structure deviated to the opposite
side to varying degrees. The symptoms/complaints of the patients
included intracranial hypertension, psychiatric symptoms,
hemiplegia and disturbance of consciousness. Among the 40 patients
enrolled, 33 were males and 7 were females, and the age ranged from
19–86 years, with 34 cases (85%) aged >60 years and 26 cases
(65%) with a history of trauma (BHC group, 14 and TDC group, 12).
The duration from injury to admission ranged from 3 weeks to 4
months. A total of 14 cases (35%) denying a history of trauma were
aged >60 years, and there was a significant age difference
between the patients without and with history of trauma (72.36±6.37
vs. 62.73±15.36 years; F=3.81; t=2.23; P=0.03). It was indicated
that a history of trauma may be associated with the formation of
CSDH in patients <60 years, but not necessarily in patients
>60 years. A total of 38 (95%) out of 40 patients had unilateral
hematoma, while 2 cases (5%) had bilateral hematoma; no skull
fracture and brain parenchymal injury were identified during the
imaging examination, and the bleeding volume was 30–210 ml.
Clinical manifestations
A total of 17 cases (42.5%) had chronic progressive
medical characteristics of intracranial hypertension, including
headache, nausea and optic disc edema. A total of 29 cases (72.5%)
presented with focal neurologic impairment due to the hematoma
compression, including hemiparesis, epilepsy, alalia and eating
difficulty, including 6 cases (15%), in which the impairment was
severe with manifestations including disturbance of consciousness,
psychiatric symptoms, behavioral abnormalities and mental
retardation, and 5 cases (12.5%) with urinary incontinence prior to
admission, but whose consciousness as well as respiratory pulse and
other vital signs were stable. The medical history of the cases was
as follows: 20 cases (50%) were complicated with varying degrees of
hypertension and diabetes mellitus, and 2 cases (5%) had a history
of underlying cerebrovascular disease with unilateral hemiplegia.
Furthermore, 2 cases (5%) had a history of tumor, 1 case (2.5%) had
a history of renal insufficiency and 1 case (2.5%) used to take
antiplatelet drugs, e.g., aspirin, prior to admission. In all
patients enrolled in the present study, statin drugs were used to
prevent hematoma recurrence. No significant differences in the
general clinical characteristics were identified between the two
groups (Table I).
 | Table I.Comparison of general clinical
characteristics and outcomes between the BHC and TDC groups. |
Table I.
Comparison of general clinical
characteristics and outcomes between the BHC and TDC groups.
| Parameter | BHC | TDC | P-value |
|---|
| n | 20 | 20 | |
| Age (years) | 66.00±16.74 | 66.20±10.11 | 0.96 |
| Male sex | 17 (85) | 16 (80) | 0.68 |
| Medical
characteristics |
|
Trauma | 14 (70) | 12 (60) | 0.51 |
|
Hypertension | 5 (25) | 10 (50) | 0.10 |
|
Diabetes | 1 (5) | 0 | 0.31 |
|
Aspirin | 0 | 1 (5) | 0.31 |
| mRS score |
|
Pre-operation | 2.55±1.47 | 2.74±1.49 | 0.70 |
| 48 h
post-operation | 1.35±1.53 | 1.42±1.35 | 0.88 |
| 3
months post-operation | 1.40±1.98 | 0.74±1.67 | 0.27 |
| Outcomes |
|
Remedial surgery | 0 | 1 (5) | 0.31 |
|
Hematoma clear | 1 (5) | 3 (15) | 0.29 |
|
Hematoma residual | 14 (70) | 15 (75) | 0.67 |
|
Secondary operation | 3 (15) | 0 | 0.07 |
|
Death | 2 (10) | 1 (5) | 0.55 |
| LOS
(days) | 14.75±5.95 | 9.00±2.91 | 0.00 |
Major outcomes
One case in the TDC group received the remedial
operation due to no obvious improvement of symptoms and poor
hematoma drainage on head CT review at 48 h after the operation
(TDC vs. BHC; P=0.31; Table I).
However, it cannot be reasoned that the failure rate of TDC was
significantly higher than that of BHC. In the BHC group, 3 patients
had a pre-operative mRS score of 5 points, among which the hematoma
of 2 cases was significantly improved on head CT review at 48 h
after the operation, but the mRS score was still 5 points.
Furthermore, 2 patients died at 32 days after discharge (36 days
post-operation) and 45 days after discharge (49 days
post-operation). In the TDC group, 4 cases had a pre-operative mRS
score of 5 points, among which 1 case was discharged 5 days after
admission, but his mRS score was 4 points, and he died of epilepsy
and pulmonary infection after 1 month. The hemiplegia symptoms of
one 80-year-old case were not alleviated, the patient could not
take care of themselves, and the head CT at the 3-month follow-up
indicated no increase in the hematoma. No difference in the
mortality rate was identified between the two groups (P=0.55). In
addition, the head CT at the 3-month follow-up indicated that the
intracranial hematoma of a total of 4 patients in the two groups
was completely removed, including 3 cases in the TDC group and 1
male case aged 19 years in the BHC group (TDC vs. BHC; P=0.29;
Table I). In the remaining patients,
residual hematoma was present after 3 months (Fig. 2). The BHC group contained 3 cases of
intracranial hypertension, 2 of which were diagnosed with a
recurrence of CSDH, and received the secondary operation at 30 and
47 days after the first operation. One other case was diagnosed
with a secondary intracranial infection and received the incision
and drainage again due to fever, scalp incision swelling and other
central nervous system infection symptoms, as well as a
mixed-density shadow in the subdural space on head CT. In the TDC
group, those patients with improved symptoms within 48 h of surgery
had stable symptoms until the 3-month follow-up, and none of the
patients was required to undergo a secondary operation (TDC vs.
BHC; P=0.07). It was also identified that patients with a high
pre-operative score retained these increased scores at 48 h after
the operation (P<0.001), indicating that the symptoms may be
obviously improved by the two types of surgery within 48 h. The
average LOS after TDC was 9.00±2.91 days, which was significantly
shorter than that after BHC (14.75±5.95 days; P<0.01). To
further compare the curative effects of the two types of operation,
the differences in mRS scores prior to the operation vs. 48 h
post-operation (Vpre-48 h), prior to the operation vs. 3
months post-operation (Vpre-3 m) and 48 h vs. 3 months
after the operation (V48 h-3 m) were determined, and the
ratio of these three variables vs. the pre-operative mRS score was
also calculated [V(pre-48 h)/pre, V(pre-3
m)/pre and V(48 h-3 m)/pre, respectively]. The
Mann-Whitney U test was performed and the results indicated that
the variation values Vpre-3 m and V48 h-3 mof
the mRS sore at 3 months after the operation and the ratios
V(pre-3 m)/pre and V(48 h-3 m)/pre in the TDC
group were obviously different compared with those in the BHC group
(P<0.05; Table II), suggesting
that the improvement of neurological function in the TDC group
after the operation was more obvious than that in the BHC group.
According to the traditional definition, the remedial operation (1
case in the TDC group) and secondary operation (3 cases in the BHC
group) were considered to indicate a recurrence of CSDH in the
present study, and the recurrence rate was not significantly
different between the two groups (P=0.29; Table I). Although 18 patients (90%) in the
TDC group were cured (the operation was successful and the survival
time was >3 months without recurrence), there was no significant
difference in the cure rate compared with that in the BHC group
(P=0.21).
 | Table II.Comparison of VmRS between
the two surgery groups. |
Table II.
Comparison of VmRS between
the two surgery groups.
|
| BHCM | TDC |
|
|---|
|
|
|
|
|
|---|
|
VmRS | n | Mean | Sum | n | Mean | Sum | P-value |
|---|
| Vpre-48
h | 20 | 19.93 | 398.00 | 20 | 21.08 | 421.00 | 0.72 |
| Vpre-3
m | 20 | 16.40 | 328.00 | 19 | 23.79 | 452.00 | 0.04 |
| V48 h-3
m | 20 | 16.23 | 32450 | 19 | 23.97 | 455.50 | 0.03 |
| V(pre-48
h)/pre | 20 | 22.03 | 440.50 | 20 | 18.98 | 379.50 | 0.40 |
| V(pre-3
m)/pre | 20 | 16.68 | 333.50 | 19 | 23.50 | 446.50 | 0.03 |
| V(48 h-3
m)/pre | 20 | 15.70 | 314.00 | 19 | 24.53 | 466.00 | 0.01 |
Discussion
CSDH is a common neurological disease and frequently
occurs in the elderly. With the global population aging, its
incidence rate is high. BHC and TDC are the two most commonly used
drainage techniques for subdural hematoma. Due to non-unified
definitions and inconsistent evaluation indexes, previous studies
as a whole have not convincingly demonstrated which one of the two
techniques provides more benefits (22). Three randomized controlled studies
had the following shortcomings: The definition of recurrence in the
study by Muzii et al (5) was
based on the imaging results, which is different from the
traditional evaluation based on symptom recurrence; however, the
other indexes in their study were evaluated in a similar manner. In
the study by Gökmen et al (6), the recurrence of CSDH was considered as
requirement for a secondary operation. The study by Singh et
al (7) had similar clinical
implications to Gökmen et al (6), but the detailed description of
definitions was lacking. Oh et al (14) identified in a review that as an
inconsistency, the definition of ‘recurrence’ mainly included
remedial operation, remnants and secondary operation. Although the
recurrence rate has been a major index for evaluating the efficacy
in the past, it has also been the most inconsistently defined
parameter.
The definition of recurrence based on residual
hematoma may be the major cause for the variety of results. In the
present study, the proportion of patients with clear intracranial
residual hematoma on head CT at the 3-month follow-up after the
operation was 72.5%, which was consistent with the results of
Weigel et al (3,20). In previous studies, the presence of
post-operative residual hematoma is the most common clinical
outcome for patients receiving treatment for CSDH. At present, it
remains to be clarified whether the residual hematoma is the
unabsorbed hematoma after the first onset and treatment,
compensatory product formed due to the difficult re-expansion and
failed filling of residual cavity in patients with brain atrophy or
new subdural hematoma. Since the recurrence of CSDH was determined
based on residual hematoma in the past (11,24–29) and
monitoring of the changes in residual hematoma varies, the
definition of ‘recurrence’ is not consistent among studies.
In order to avoid confusing results due to the
unclear definition of recurrence, the terminology of the present
study was based on studies by Neal et al (30) and Singla et al (31), and a novel index system was
established. As one of the major indexes for evaluating the
long-term efficacy of surgery, patients with removal of CSDH or
residual CSDH without any new neurologic impairment in the
follow-up imaging after 3 months were deemed as cured. The cured
patients only underwent one operation. No additional surgery was
required based on the examination outcomes, including various
criteria for assessing the recurrence and reasons for determining a
secondary operation. The cure rate was used as the major index for
evaluating the efficacy, which has applicability in the clinic.
Based on the above, the results of the present study
indicated that 75 and 90% of patients in the BHC group and TDC
group, respectively, were cured, and there was no statistically
significant difference between the two groups. However, it is
indicated that BHC and TDC do not have the same effectiveness.
Therefore, the improvement level of the mRS score, expressed as the
VmRS value, was used as another evaluation index with
the aim to clarify the effectiveness of the two surgeries through
further evaluating the overall changes in mRS scores prior to and
after the two types of operation in each group.
The differences in mRS scores between the
time-points prior to the operation, at 48 h and 3 months after
operation were calculated (Vpre-48 h, Vpre-3
m and V48 h-3 m), and the ratio of these three
variables to the pre-operative mRS score was also calculated
[V(pre-48 h)/pre, V(pre-3 m)/pre and
V(48 h-3 m)/pre]. The Mann-Whitney U test was performed
and the results indicated that the variation values Vpre-3
m and V48 h-3 m of mRS score at 3 months after the
operation and the ratios V(pre-3 m)/pre and V(48
h-3 m)/pre in the TDC group exhibited obvious differences
compared with those in the BHC group (P<0.05), suggesting that
the improvement of neurological function in the TDC group at 3
months after the operation was more obvious than that in the BHC
group. Therefore, it may be assumed that with the increase of
sample size, the long-term cure rate in the TDC group may be
superior to that in BHC group. This present result is superior to
that of the recent study by Wang et al (32), which indicated that TDC and BHC have
comparable clinical outcomes in the treatment of patients with
CSDH.
As previous studies, the present study identified no
significant difference in the mortality rate of CSDH patients after
treatment with the two different surgeries (3,8). In the
present study, the overall failure rate of TDC was 5%, which was
within the previously reported range (0–36.4%) (8). The present study also proved that the
success rate of the first BHC was higher (100%), but it did not
suggest that the failure rate of TDC is higher than that of BHC
(P=0.31). The failure rate may be associated with factors including
the surgical conditions, proficiency of the surgeon, technical
factors and the overall condition of the patients.
In the present study, the rate of symptom recurrence
and secondary operation within 3 months after BHC was up to 15%,
which was consistent with the fact that the long-term improvement
in mRS score after the operation in the BHC group was lower than
that in the TDC group, proving the rationality and completeness of
the evaluation system applied. However, it may not be reasonable to
use the secondary operation rate as a major clinical index to
assess the efficacy of the first operation. In addition, as
mentioned above, among previous studies, the definition of
‘secondary operation’ is as inconsistent as that of ‘recurrence’,
and it lacks the practicality for clinical use as an index for
measuring the surgical efficacy.
In the present study, no significant differences
were identified in the cure rate and the mortality rate of patients
with CSDH after the two types of surgical treatment. However, the
mRS score in the TDC group at 3 months after the operation was
significantly more improved compared with that in the BHC group,
and the overall LOS in the TDC group was significantly shorter
compared with that in the BHC group. Therefore, the TDC regimen was
indicated to be superior to the BHC regimen.
In conclusion, the effectiveness of TDC in the
treatment of CSDH is not lower than that of BHC, and it is
characterized by a simpler operation and smaller damage; at the
same time, the LOS of patients receiving TDC is also obviously
shorter than that of patients after BHC, and the improvement of
neurological score of patients who received TDC was better in the
long-term. Therefore, TDC is superior to BHC for the treatment of
CSDH.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key Project
of Science and Technology Development Fund of Nanjing Medical
University (grant no. 2015NJMUZD074).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CX, BC, LXu, LXi and XY were involved in the study
design; MW, XH, QC, JZ, ML and ZL were involved in data collection;
XT, GC and FX were involved in data analysis; CX and ML prepared
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Huai'an First People's Hospital. Written informed consent was
obtained from the patients and/or their guardians.
Patient consent for publication
Patients or their guardians provided written
informed consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Abecassis IJ and Kim LJ: Craniotomy for
treatment of chronic subdural hematoma. Neurosurg Clin N Am.
28:229–237. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Santarius T and Hutchinson PJ: Chronic
subdural haematoma: Time to rationalize treatment? Br J Neurosurg.
18:328–332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weigel R, Schmiedek P and Krauss JK:
Outcome of contemporary surgery for chronic subdural haematoma:
Evidence based review. J Neurol Neurosurg Psychiatry. 74:937–943.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lega BC, Danish SF, Malhotra NR, Sonnad SS
and Stein SC: Choosing the best operation for chronic subdural
hematoma: A decision analysis. J Neurosurg. 113:615–621. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Muzii VF, Bistazzoni S, Zalaffi A,
Carangelo B, Mariottini A and Palma L: Chronic subdural hematoma:
Comparison of two surgical techniques. Preliminary results of a
prospective randomized study. J Neurosurg Sci. 49:41–47.
2005.PubMed/NCBI
|
|
6
|
Gökmen M, Sucu HK, Ergin A, Gokmen A and
Lu Bezircio H: Randomized comparative study of burr-hole
craniostomy versus twist drill craniostomy; surgical management of
unilateral hemispheric chronic subdural hematomas. Zentralbl
Neurochir. 69:129–133. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh SK, Sinha M, Singh VK, Parihar A,
Srivastava C, Ojha BK and Chandra A: A randomized study of twist
drill versus burr hole craniostomy for treatment of chronic
subdural hematomas in 100 patients. Indian J Neurotrauma. 8:83–88.
2011. View Article : Google Scholar
|
|
8
|
Ducruet AF, Grobelny BT, Zacharia BE,
Hickman ZL, DeRosa PL, Andersen KN, Sussman E, Carpenter A and
Connolly EJ Jr: The surgical management of chronic subdural
hematoma. Neurosurg Rev. 35:155–169. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Almenawer SA, Farrokhyar F, Hong C,
Alhazzani W, Manoranjan B, Yarascavitch B, Arjmand P, Baronia B,
Reddy K, Murty N and Singh S: Chronic subdural hematoma management:
A systematic review and meta-analysis of 34,829 patients. Ann Surg.
259:449–457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu CS: Inconsistent data resources weaken
the quality of research results. Ann Surg. 262:e121–e122. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abouzari M, Rashidi A, Rezaii J,
Esfandiari K, Asadollahi M, Aleali H and Abdollahzadeh M: The role
of postoperative patient posture in the recurrence of traumatic
chronic subdural hematoma after burr-hole surgery. Neurosurgery.
61:794–797. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin X: Comparing twist-drill drainage with
burr hole drainage for chronic subdural hematoma. Chin J Traumatol.
14:170–173. 2011.PubMed/NCBI
|
|
13
|
Nakajima H, Yasui T, Nishikawa M, Kishi H
and Kan M: The role of postoperative patient posture in the
recurrence of chronic subdural hematoma: A prospective randomized
trial. Surg Neurol. 58(385): 387. 2002.
|
|
14
|
Oh HJ, Lee KS, Shim JJ, Yoon SM, Yun IG
and Bae HG: Postoperative course and recurrence of chronic subdural
hematoma. J Korean Neurosurg Soc. 48:518–523. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oishi M, Toyama M, Tamatani S, Kitazawa T
and Saito M: Clinical factors of recurrent chronic subdural
hematoma. Neurol Med Chir (Tokyo). 41:382–386. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ramachandran R and Hegde T: Chronic
subdural hematomas-causes of morbidity and mortality. Surg Neurol.
67:367–373. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rohde V, Graf G and Hassler W:
Complications of burr-hole craniostomy and closed-system drainage
for chronic subdural hematomas: A retrospective analysis of 376
patients. Neurosurg Rev. 25:89–94. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Santarius T, Kirkpatrick PJ, Ganesan D,
Chia HL, Jalloh I, Smielewski P, Richards HK, Marcus H, Parker RA,
Price SJ, et al: Use of drains versus no drains after burr-hole
evacuation of chronic subdural haematoma: A randomised controlled
trial. Lancet. 374:1067–1073. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Torihashi K, Sadamasa N, Yoshida K, Narumi
O, Chin M and Yamagata S: Independent predictors for recurrence of
chronic subdural hematoma: A review of 343 consecutive surgical
cases. Neurosurgery. 63:1125–1129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weigel R, Krauss JK and Schmiedek P:
Concepts of neurosurgical management of chronic subdural haematoma:
Historical perspectives. Br J Neurosurg. 18:8–18. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rughani AI, Lin C, Dumont TM, Penar PL,
Horgan MA and Tranmer BI: A case-comparison study of the subdural
evacuating port system in treating chronic subdural hematomas. J
Neurosurg. 113:609–614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu CS, Lu M, Liu LY, Yao MY, Cheng GL,
Tian XY, Xiao F, Wan Q and Chen F: Chronic subdural hematoma
management: Clarifying the definitions of outcome measures to
better understand treatment efficacy-a systematic review and
meta-analysis. Eur Rev Med Pharmacol Sci. 21:809–818.
2017.PubMed/NCBI
|
|
23
|
Jadad AR and Enkin MW: Randomized
controlled trials: Questions, answers, and musings (Second
Edition). 2008.
|
|
24
|
Erol FS, Topsakal C, Faik OM, Kaplan M and
Tiftikci MT: Irrigation vs. closed drainage in the treatment of
chronic subdural hematoma. J Clin Neurosci. 12:261–263. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuwabara M, Sadatomo T, Yuki K, Migita K,
Imada Y, Shimizu K, Hara T, Oba H and Kurisu K: The effect of
irrigation solutions on recurrence of chronic subdural hematoma: A
consecutive cohort study of 234 patients. Neurol Med Chir (Tokyo).
57:210–216. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iftikhar M, Siddiqui UT, Rauf MY, Malik AO
and Javed G: Comparison of Irrigation versus No Irrigation during
burr hole evacuation of chronic subdural hematoma. J Neurol Surg A
Cent Eur Neurosurg. 77:416–421. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim DH, Kim HS, Choi HJ, Han IH, Cho WH
and Nam KH: Recurrence of the chronic subdural hematoma after
burr-hole drainage with or without intraoperative saline
irrigation. Korean J Neurotrauma. 10:101–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takeda N, Sasaki K, Oikawa A, Aoki N and
Hori T: A new simple therapeutic method for chronic subdural
hematoma without irrigation and drainage. Acta Neurochir (Wien).
148:541–546. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suzuki K, Sugita K, Akai T, Takahata T,
Sonobe M and Takahashi S: Treatment of chronic subdural hematoma by
closed-system drainage without irrigation. Surg Neurol. 50:231–234.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Neal MT, Hsu W, Urban JE, Angelo NM,
Sweasey TA and Branch CJ Jr: The subdural evacuation port system:
Outcomes from a single institution experience and predictors of
success. Clin Neurol Neurosurg. 115:658–664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Singla A, Jacobsen WP, Yusupov IR and
Carter DA: Subdural evacuating port system (SEPS)-minimally
invasive approach to the management of chronic/subacute subdural
hematomas. Clin Neurol Neurosurg. 115:425–431. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang K, Chen D, Cao X and Gao L: A
prospective comparative study of twist drill craniostomy versus
burr hole craniostomy in patients with chronic subdural hematoma.
Turk Neurosurg. 27:60–65. 2017.PubMed/NCBI
|