Introduction
Neonatal purulent meningitis (PM) is a kind of
meningitis in fetuses caused by bacterial infection within 4 weeks
after birth. It has high incidence rate and mortality. Survival
infants may have neural sequelae of varied degrees, which seriously
endangers the life and health of perinatal infants. Early and
effective treatment is of great significance in reducing the
mortality and sequelae of pediatric patients. However, clinical
manifestations of neonatal PM in the early stage are nonspecific.
Therefore, early diagnosis has become a thorny issue. In recent
years, cytokines have become an active issue of study to
neonatologist researchers. Understanding the high risk and
predisposing factors of neonatal PM, and the inflammatory factors
and pathogenic bacteria in cerebrospinal fluid, and confirming
diagnoses early, are helpful to take effective measures to prevent
its occurrence, and have a profound significance for the reduction
of pediatric patient mortality and the improvement of the
short-term and long-term quality of life of neonates. By studying
the pathogenic bacteria and inflammatory factors of neonatal PM,
this study aimed to provide the basis for rational clinical
treatment.
Patients and methods
Clinical data
Seventy-four PM neonates, who had complete clinical
data and were treated in Daqing Longnan Hospital from January 2012
to December 2015, were collected as observation group. All these
neonates met the diagnostic criteria of PM. In the observation
group, there were 52 males and 22 females aged 1–28 days (with a
mean of 19.13±8.74 days), with a gestational age of 28–42 weeks (of
which 32 neonates were <32 weeks, 25 neonates were 32–37 weeks,
and 17 neonates were ≥37 weeks), and a birth weight of 1,285–4,570
g (with an average weight of 3,592±658 g, including <2,500 g in
20 neonates and ≥2,500 g in 54 neonates); 28 neonates had premature
rupture of membranes, 40 neonates had intrapartum asphyxia, and 6
neonates had amniotic fluid turbidity. There were 13 early-onset
neonates (within 3 days after birth) and 61 were late-onset (during
4–28 days after birth). Control group included 74 neonates
hospitalized with non-PM during the same period, including 20
neonates with infectious pneumonia, 14 neonates with septicemia, 10
neonates with asphyxia, 10 neonates with jaundice, 6 neonates with
respiratory distress syndrome, 5 neonates with hypoxic ischemic
encephalopathy, 4 neonates with umbilical infection, 3 neonates
with amniotic fluid aspiration, and 2 neonates with meconium
aspiration. Among them, 51 neonates were male and 23 neonates were
female; the age in days was 1–28 days, with an average of
19.17±9.02 days; the gestational age was 28–42 weeks, of whom 17
neonates were <32 weeks, 40 neonates were 32–37 weeks, and 17
neonates were ≥37 weeks; 10 neonates were <2,500 g in birth
weight, and 64 neonates were ≥2,500 g in birth weight; 4 neonates
had premature rupture of membranes, 14 neonates had intrapartum
asphyxia, and 1 neonate had amniotic fluid turbidity. The study was
approved by the Ethics Committee of Daqing Longnan Hospital
(Daqing, China) and written informed consents were signed by the
patient's guardians.
Inclusion and exclusion criteria
Inclusion criteria: i) neonates with the clinical
manifestations of neonatal PM (symptoms: abnormal body temperature,
milk refusal, convulsion, and abnormal reaction; signs:
intracranial hypertension performance); ii) neonates whose results
in cerebrospinal fluid routine examination and biochemical test
were in line with PM changes; iii) neonates who had positive
pathogens in cerebrospinal fluid or blood culture. Exclusion
criteria: i) neonates who had positive fungi in cerebrospinal fluid
or blood culture; ii) neonates who were positive in cerebrospinal
fluid or blood culture but the samples might have been
contaminated.
Methods
Records of general data
Records of general data includes abortion history
and age of the mother of the pediatric patient, and sex, age, birth
weight and gestational age of the pediatric patient, as well as the
occurrence of premature rupture of membranes, intrapartum asphyxia,
umbilical or pulmonary infection.
Detection of inflammation indexes in
cerebrospinal fluid
A total of 2 ml of cerebrospinal fluid was collected
from all pediatric patients via strict aseptic operation, and sent
to clinical laboratory of the hospital for bacterial culture and
identification which were performed by a specialist. Operations
were conducted in accordance with related quality indexes.
Additional cerebrospinal fluid was taken for the detection of
inflammation indexes [β 2 microglobulin (β2MG) and C-reactive
protein (CRP)]. All operations were carried out strictly according
to the instructions.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
18.0 software (SPSS Inc., Chicago, IL, USA) was used for data
analysis. χ2 test was adopted for enumeration data
comparison among groups. Normal measurement data were recorded as
mean ± standard deviation, and t-test was employed for comparison
among groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical manifestations of neonatal
PM
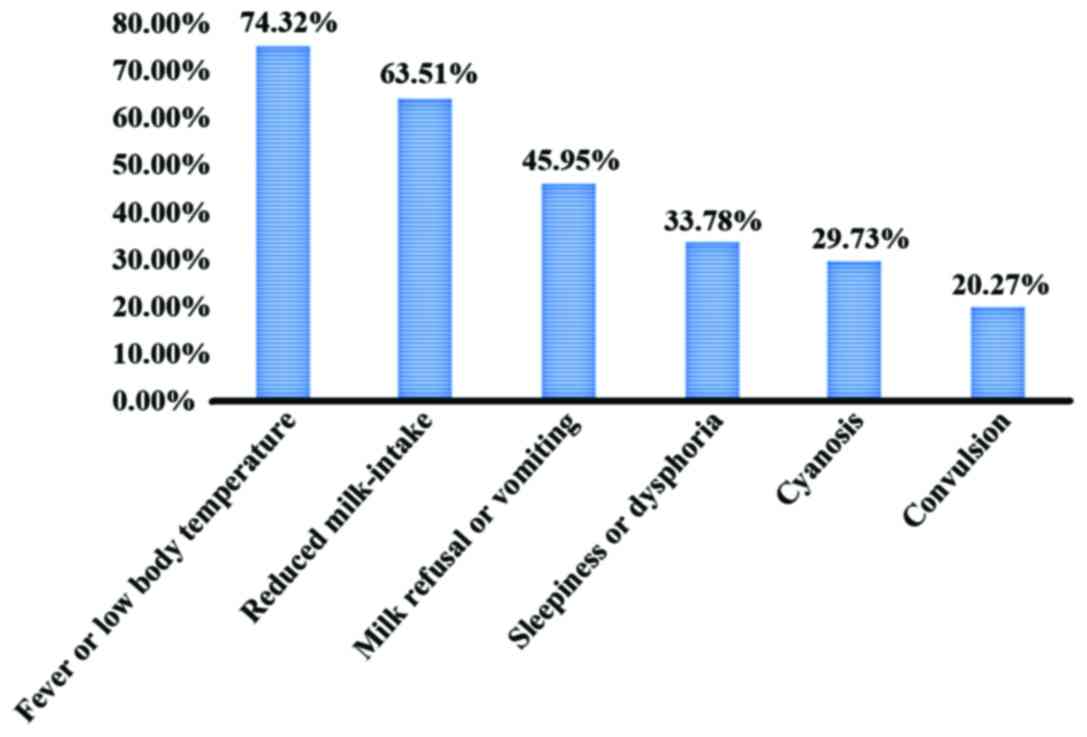
Neonatal PM might have no symptoms in early stage,
and lack specific clinical manifestations. The main symptoms of
neonatal PM were fever, reduced milk-intake, milk refusal or
vomiting and sleepiness or dysphoria, among which, fever or low
body temperature accounted for 74.32% (55/74), reduced milk-intake
accounted for 63.51% (47/74), milk refusal or vomiting accounted
for 45.95% (24/74), sleepiness or dysphoria accounted for 33.78%
(25/74), cyanosis accounted for 29.73% (22/74), and convulsion
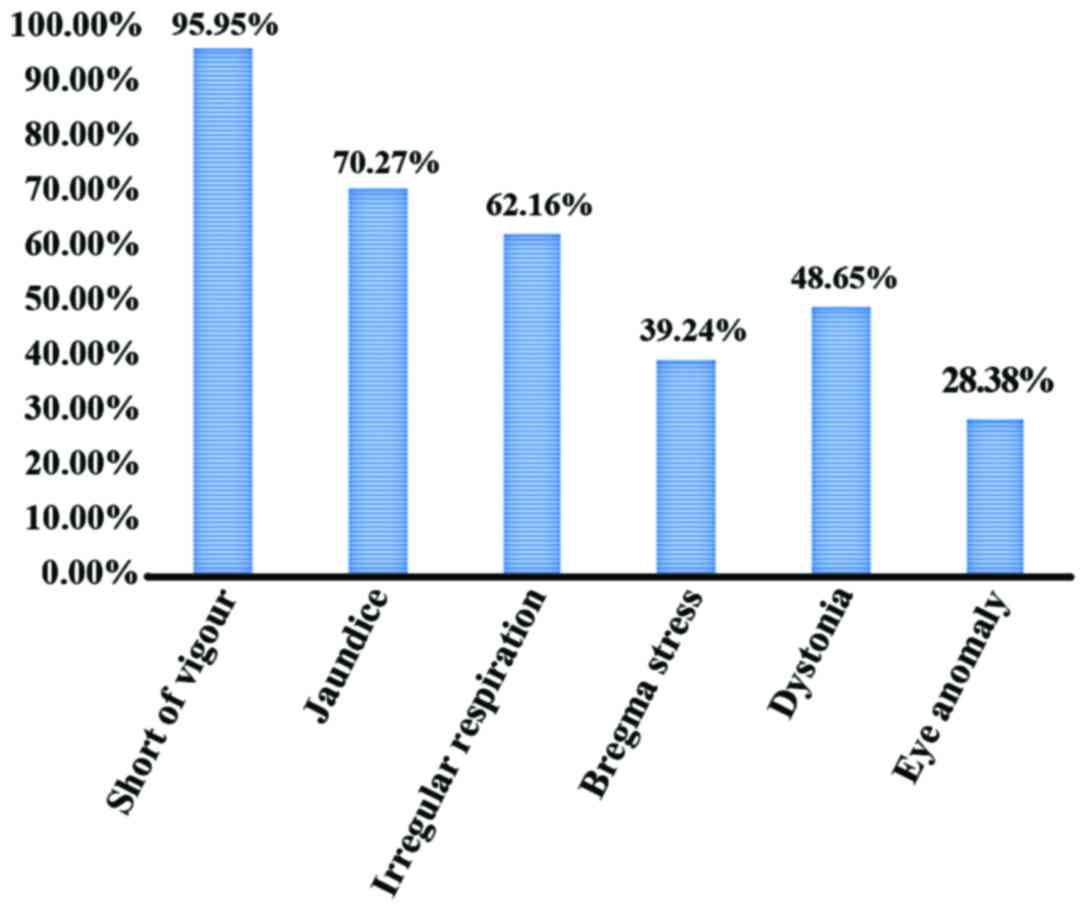
accounted for 20.27 (15/74). The main signs were low spirit,
jaundice, irregular respiration, bregma stress and dystonia. Among
them, short of vigour occupied 95.95% (71/74), jaundice 70.27%
(52/74), irregular respiration 62.16% (46/74), bregma stress 39.24%
(35/74), dystonia 48.65% (36/74), and eye anomaly occupied 28.38%
(21/74) (Figs. 1 and 2).
Analyses of high risk factors
The results showed that the high risk factors of
neonatal PM were not significantly correlated with abortion history
and age of the mother of the pediatric patient, sex of the
pediatric patient, and delivery mode, but obviously related to the
premature rupture of membranes, premature birth, body weight
<2,500 g, neonatal asphyxia, and umbilical or pulmonary disease
of the pediatric patient, respectively, suggesting that neonates
with premature rupture of membranes before birth, younger
gestational age and lower body weight are more susceptible to PM
infection (Table I).
 | Table I.Analyses of predisposing factors
between two groups of neonates. |
Table I.
Analyses of predisposing factors
between two groups of neonates.
| Observation
factor | PM group (n=74) | Control group
(n=74) | t/χ2
value | P-value |
|---|
| Abortion history of
the mother |
|
|
|
|
| No | 49 | 47 | 1.912 | 0.132 |
| Yes | 25 | 27 |
|
|
| Age of the mother
(years) |
|
|
|
|
|
<35 | 46 | 50 | 2.134 | 0.089 |
| ≥35 | 28 | 24 |
|
|
| Sex of the pediatric
patient |
|
|
|
|
| Male | 52 | 51 | 1.429 | 0.178 |
|
Female | 22 | 23 |
|
|
| Delivery mode |
|
|
|
|
|
Spontaneous delivery | 51 | 53 | 1.623 | 0.151 |
| Forceps
or fetal aspiration delivery |
|
|
|
|
| Cesarean
delivery | 18 | 17 |
|
|
| Premature rupture of
membranes |
|
|
|
|
| No | 46 | 70 | 15.413 | <0.001 |
| Yes | 28 | 4 |
|
|
| Gestational age
(weeks) |
|
|
|
|
|
<32 | 32 | 17 | 11.645 | 0.003 |
|
32–37 | 25 | 40 |
|
|
| ≥37 | 17 | 17 |
|
|
| Body weight (g) |
|
|
|
|
|
<2,500 | 20 | 10 | 8.732 | 0.006 |
|
≥2,500 | 54 | 64 |
|
|
| Neonatal
asphyxia |
|
|
|
|
| No | 34 | 60 | 12.208 | 0.001 |
|
Yes | 40 | 14 |
|
|
| Umbilical or
pulmonary infection |
|
|
|
|
| No | 31 | 50 | 9.536 | 0.005 |
|
Yes | 43 | 24 |
|
|
Comparison of inflammatory indicators
between two groups of neonates
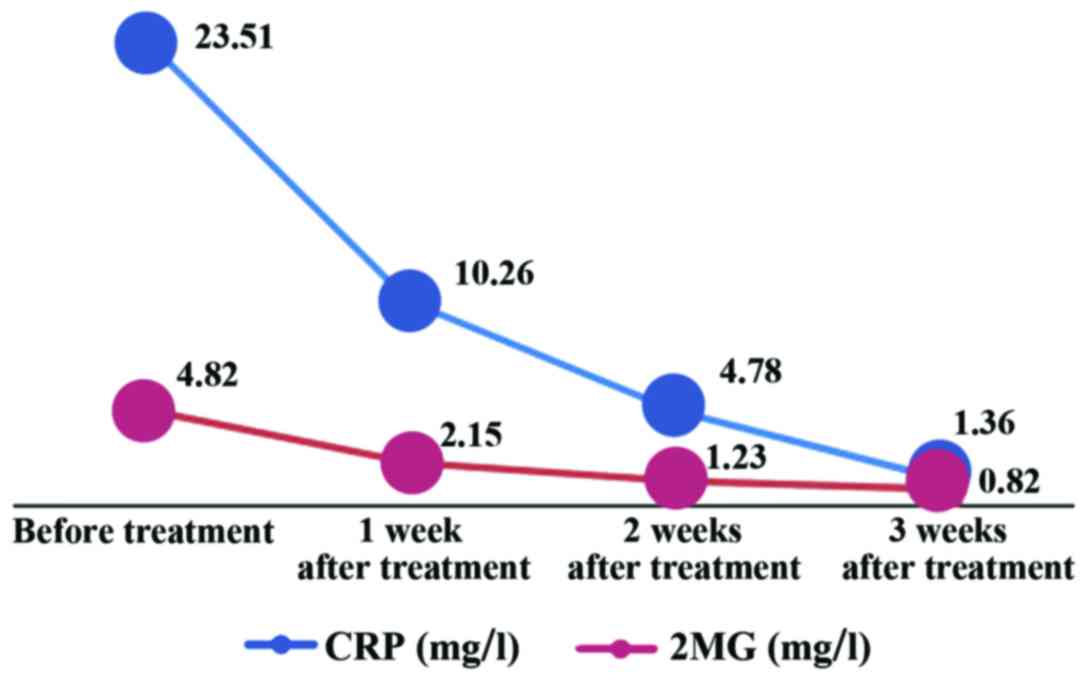
The results revealed that the average of CRP in
cerebrospinal fluid in the neonatal PM group was 23.51±12.63 mg/l
and that in the control group was 1.01±0.78 mg/l; the mean of β2MG
in cerebrospinal fluid was 4.82±0.95 mg/l in the neonatal PM group
and 0.80±0.56 mg/l in the control group; neonatal PM group had
overtly increased levels of CRP and β2MG compared with those in the
control group (P<0.05) (Table
II). In the neonatal PM group, the CRP levels in cerebrospinal
fluid after treatment for 1, 2 and 3 weeks were 10.26±7.42,
4.78±3.36 and 1.36±0.85 mg/l, respectively, and the levels of β2MG
were 2.15±0.84, 1.23±0.67, and 0.82±0.51 mg/l, respectively.
Compared with those before treatment, CRP and β2MG levels in
cerebrospinal fluid in the neonatal PM group were obviously reduced
after treatment for 1, 2 and 3 weeks (P<0.05) (Fig. 3).
 | Table II.Comparisons of inflammatory
indicators between two groups of neonates. |
Table II.
Comparisons of inflammatory
indicators between two groups of neonates.
| Indicator
(mg/l) | Observation group
(n=74) | Control group
(n=74) | t/χ2
value | P-value |
|---|
| CRP in
cerebrospinal fluid | 23.51±12.63 | 1.01±0.78 | 15.792 | 0.001 |
| β2MG in
cerebrospinal fluid | 4.82±0.95 | 0.80±0.56 | 11.834 | 0.005 |
Composition of bacterial strain
detected in the past four years
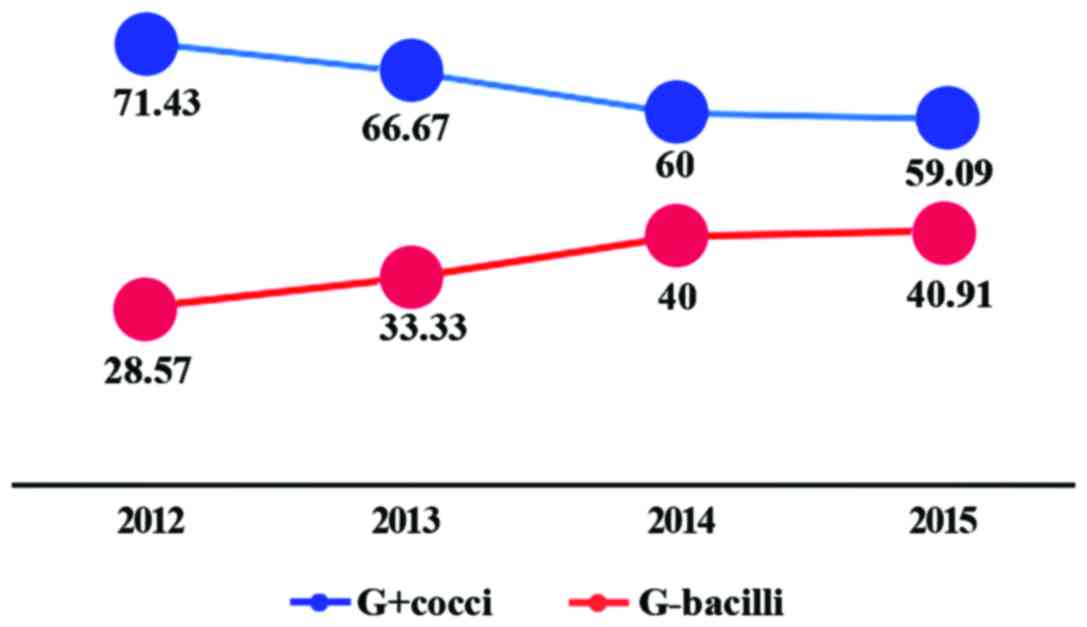
During 2012–2015, there was an upward tendency in
positive rate of Gram-negative bacilli (G-bacilli) year by year,
and the positive rates were 28.75, 33.33, 40.00 and 40.91%,
respectively; however, the positive rate of Gram-positive cocci
(G+cocci) showed a decline trend year by year, and the positive
rates were 71.43, 66.67, 60.00 and 59.09%, respectively (Fig. 4).
Composition of pathogenic
bacteria
Single bacterial strain was found in all
cerebrospinal fluid samples of the 74 neonates who were diagnosed
with PM and were bacteriologically positive. All of them were
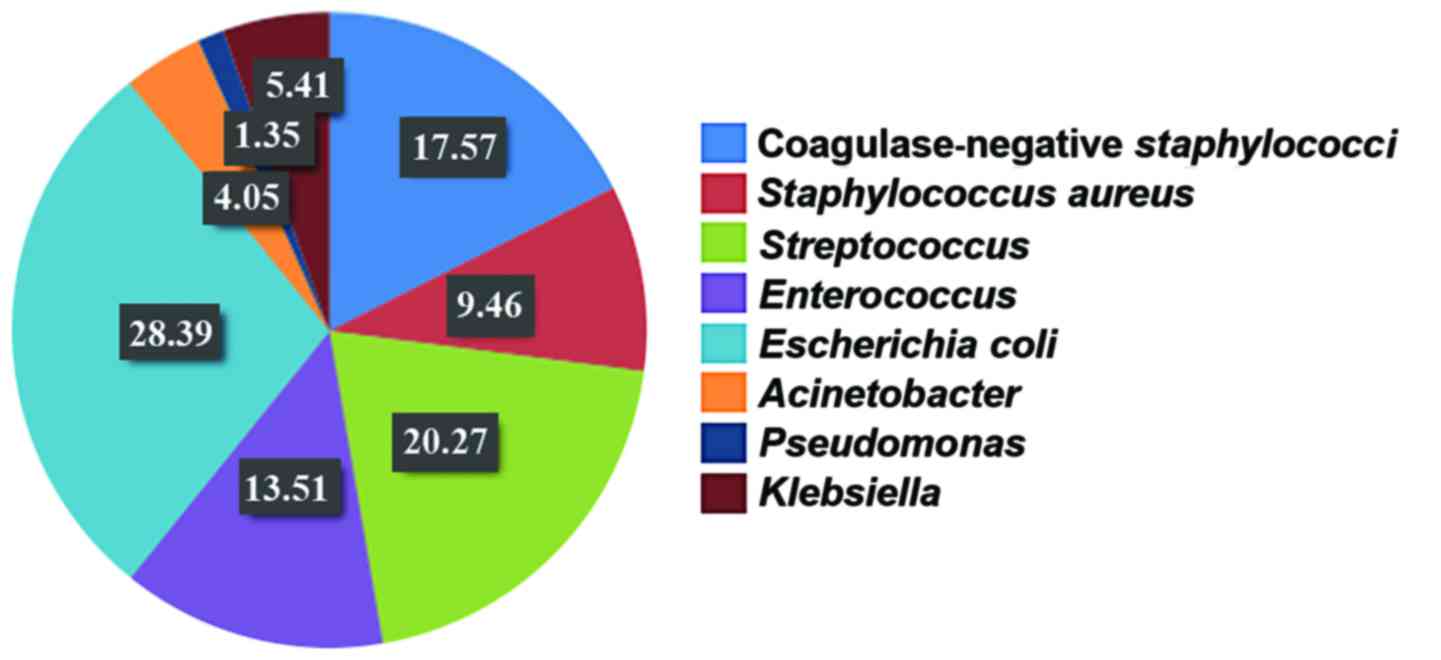
aerobic bacteria. Among them, 45 strains were G+cocci, which
accounted for 60.81% and included 13 strains of coagulase-negative
staphylococci (CNS) (17.57%), 7 strains of Staphylococcus
aureus (9.46%), 15 strains of Streptococcus [20.27%,
including 12 strains of group B Streptococcus (GBS, 4
strains were detected in 2012–2013, and 8 strains were detected in
2014–2015)], and 10 strains of Enterococcus (13.51%); 29
strains were G-negative bacilli, accounting for 39.19% and
including 21 strains of Escherichia coli (28.39%), 3 strains
of Acinetobacter (4.05%), 1 strain of Pseudomonas
(1.35%) and 4 strains of Klebsiella (5.41%). The top three
were successively Escherichia coli, CNS and
Streptococcus (Fig. 5).
Comparison of pathogenic bacteria
composition between early-onset and late-onset neonatal PM
Streptococcic (mainly GBS) accounted for the largest
proportion in pathogenic bacteria of early-onset neonatal PM, which
was ~30.77% (4/13) and higher than that in pathogenic bacteria of
late-onset neonatal PM [14.75% (9/61), χ2=5.278;
P<0.05]. CNS was frequently found in pathogenic bacteria of
late-onset neonatal PM was ~9.67% (12/61), which was higher than
that in pathogenic bacteria of early-onset neonatal PM [7.69%
(1/13), χ2=4.631; P<0.05]. Although Escherichia
coli accounted for the largest proportion in pathogenic
bacteria of early-onset neonatal PM, at ~29.51% (18/61), it was not
statistically significant from that in pathogenic bacteria of
early-onset neonatal PM [23.08% (3/13), χ2=2.512;
P>0.05] (Table III).
 | Table III.Comparison of pathogenic bacteria
composition between early-onset and late-onset neonatal PM (cases,
%). |
Table III.
Comparison of pathogenic bacteria
composition between early-onset and late-onset neonatal PM (cases,
%).
| Bacterial
strain | Cases | Early-onset | Late-onset |
|---|
| Total | 74 | 13 | 61 |
| CNS | 13 | 1 (7.69) | 12
(19.67)a |
| Staphylococcus
aureus | 7 | 2 (15.38) | 5 (8.20) |
|
Streptococcus | 15 | 4 (30.77) | 11
(18.03)b |
|
Enterococcus | 10 | 1 (7.69) | 9 (14.75) |
| Escherichia
coli | 21 | 3 (23.08) | 18 (29.51) |
|
Acinetobacter | 3 | 1 (7.69) | 2 (3.28) |
|
Pseudomonas | 1 | 0 (0.00) | 1 (1.64) |
|
Klebsiella | 4 | 1 (7.69) | 3 (4.92) |
Discussion
Clinical manifestations of neonatal PM. In
comparison with other age groups of children, neonates have open
fontanel and cranial suture, insufficient muscular strength in the
neck, low immunity and poor blood brain barrier function, so they
are more susceptible to PM (1). Most
pediatric patients just have the symptoms of bloodstream
infections, such as low to moderate fever, milk refusal,
hypokinesia, and subenergetic crying; neonatal PM has unobvious
intracranial hypertension signs and lacks typical meningeal
irritation signs, which easily leads to delaying diagnosis and
improper treatment, resulting in a high fatality rate (2). According to the results in this study,
74 neonates with PM showed no specific clinical manifestations,
with fever in 55 neonates (74.32%) and reduced milk-intake in 47
neonates (63.51%), which is in line with reports of Chang et
al and Lin et al (3,4). As to
signs, there were low spirit (71 cases, 95.95%), jaundice (52
cases, 70.27%), irregular respiration, bregma stress and abnormal
muscle tension. Therefore, once the pediatric patients have
clinical symptoms including fever, milk refusal, low spirit and
jaundice, strongly vigilant observation and treatment are needed
against the complication of PM, and lumbar puncture should be
considered, so as to make clear diagnoses, avoiding missed
diagnoses and mis-diagnoses, and reduce mortality and sequelae.
High risk factors of neonatal PM. Studies have found
that neonatal PM infection is mainly related to three ways
(5). The first way is antepartum
infection, which is mainly caused by maternal factors; if mothers
are infected with bacteremia before delivery, the neonates may be
infected through placental circulation. The second way is
intrapartum infection, which often occur in patients with premature
rupture of membranes; with a long time of delivery, plus relaxed
disinfection in the midwifery, the neonates are infected by
swallowing or inhaling the infected amniotic fluid. The third way
is postnatal infection, which is caused by the invasion of
pathogenic bacteria into blood circulation and then meninx via
natural orifice, umbilicus, damaged skin and mucosa. A study
indicated that (6) neonates are
susceptible to infections due to poor overall immune function
(cellular immunity and humoral immunity); because of imperfect
blood-brain barrier, the infections are not easy to be limited, and
the bacteria can easily penetrate the blood-brain barrier to cause
intracranial infections. Therefore, PM is often a part of
septicemia or is secondary to septicemia. Scholars have considered
that (7) neonates who are suspected
of septicemia in clinical practice should receive cerebrospinal
fluid examination, no matter whether the high-risk factors, neural
symptoms and signs of PM are found or not. The results showed that
the high-risk factors of PM were gestational age, weight <2,500
g, neonatal asphyxia, premature rupture of membranes, and umbilical
or pulmonary infection. Kavuncuoğlu et al (8) reported that the incidence rate of
neonatal PM gradually was increased along with the decreases of
gestational age and birth weight curve. Severe asphyxia not only
reduces the immune function of the body, but also damages the
blood-brain barrier. If the delivery time is too long, and the
disinfection is not strict in the process of midwifery, pediatric
patients with premature rupture of membranes may be infected by
swallowing or inhaling contaminated amniotic fluid.
Inflammatory factors of neonatal PM. The major
pathogenesis of patients with PM is the infiltration or aggregation
of a large number of neutrophil in peripheral blood and
cerebrospinal fluid, which plays an important role in the removal
of bacterial infections, but may induce local (meningeal)
inflammatory injuries. CRP is a nonspecific reaction product in
acute phase of inflammatory diseases, and is not significantly
affected by various factors including anti-inflammatory or
immunosuppressive agents, fever, erythrocyte sedimentation rate,
and leukocyte increase. Clinically, CRP detection is an index of
important value during the treatment of inflammatory diseases
(9), and it is an item that can be
used to assess whether the body is infected and whether the disease
is in the active stage (10). The
results in this study revealed that CRP level in neonatal PM group
was significantly higher than that in control group (P<0.05),
and had a certain value for auxiliary diagnosis. However, Enguix
et al (11) reported that CRP
has low specificity and sensitivity to the diagnosis of severe
infectious diseases. Therefore, CRP concentration cannot be used as
a separate indicator for the evaluation of intracranial infection.
Bacterial infections in other parts of the body should be firstly
excluded, and other tests of cerebrospinal fluid should be
combined. β2MG is synthesized by lymphocytes, which is located on
the surface of all nucleated cells and may be elevated in
inflammation. The results in this study showed that the level of
β2MG in neonatal PM group was overtly higher than that in the
control group (P<0.05), and the levels of CRP and β2MG in
cerebrospinal fluid after treatment were evidently lower
(P<0.05), which is consistent with a literature report (12). The increase of β2MG in cerebrospinal
fluid in neonatal PM group may come from: i) central nervous
system, once the immune system in nervous system is activated,
intrathecal β2MG syntheses and cells in cerebrospinal fluid are
increased, and metabolic cycles are accelerated, thereby a large
number of β2MG fall off from the cell surface and enter into the
cerebrospinal fluid; ii) periphery, β2MG in blood may enter into
the cerebrospinal fluid through the damaged blood-brain barrier
(13). Therefore, a comprehensive
analysis on increases of CRP and β2MG in cerebrospinal fluid can
support the diagnosis of neonatal PM.
Changes in the pathogenic bacteria of neonatal PM.
Due to different regions, years and age grades, plus the abuse of
antibiotics in great quantities in clinical practice along with
constant launches of new antibacterial drugs, the strain of
pathogenic bacteria is changing continuously, bringing difficulties
to the diagnosis and treatment, which is one of the important
causes of neonatal death (14). In
developed countries, GBS is the primary pathogenic bacterium of
neonatal PM, followed by G-negative bacilli. In developing
countries, although G-negative bacilli and Staphylococcus
aureus remain dominant, the incidence rate of GBS meningitis is
also increasing gradually (15–17).
Studies have shown that streptococci, enterococci, Escherichia
coli and other G-negative bacilli can cause a mortality of up
to 10%, and no significant differences were found among these
strains, which is similar to the existing report (18). This study indicated that the top 3
pathogens that form the neonatal PM in order were: Escherichia
coli, CNS and Streptococcus. In China, it was considered
that neonatal GBS infection is rare, but in recent years, the
prevalence rate of GBS infection in our department has been
enhanced year by year. In this study, there were 15 cases of
Streptococcus, in which 12 cases were GBS, with only 4
strains in 2012–2013 and 8 strains in 2014–2015, needing to arouse
attention in clinical work. Some studies have reported that
prognosis of neonatal GBS PM and different GBS serums are related,
among which type III serum is closely related to meningitis and its
severity (19). This is because that
type III GBS has a strong adhesion capacity on vascular endothelial
tissue, chorion and neonatal lungs, which is difficult to be
eliminated. It is deemed that GBS infection is mainly related to
mother GBS colonization, especially early-onset infection (20). European and American countries use
universal screening methods to prevent perinatal mother-to-infant
GBS infection, and achieve remarkable results, namely neonatal
early-onset PM septicemia can be reduced by nearly 90% (21). As the incidence rate of GBS in this
region shows an upward trend, and once being infected, the
consequence is severe, and a heavy burden is placed on families and
society, it is necessary to perform a large-sample epidemiological
survey on GBS colonization of pregnant and delivery women in this
region. In early-onset cases, the top 2 pathogenic bacteria are
Streptococcus and Escherichia coli.
Streptococcus is the first cause, mainly correlated with
vertical transmission after mother vaginal colonization. In
late-onset cases, Escherichia coli is often found, followed
by CNS. The high incidence of CNS in late-onset cases is deemed to
be related with the large number of Staphylococcus in the
external environment exposed to pediatric patients. The incidence
of streptococci has an increasing trend, frequently found in
early-onset cases, and has poor prognosis, which should arouse high
attention of clinical practice.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
BS, QH and LS conceived and designed the study. HS,
BH and XD were responsible for the collection and analysis of the
patient data. BS and QH interpreted the data and drafted the
manuscript. LS revised the manuscript critically for important
intellectual content. All authors read and approved the final
study.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Daqing Longnan Hospital (Daqing, China). Signed informed consents
were obtained from the guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Klingenberg C, Olomi R, Oneko M, Sam N and
Langeland N: Neonatal morbidity and mortality in a Tanzanian
tertiary care referral hospital. Ann Trop Paediatr. 23:293–299.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El Bashir H, Laundy M and Booy R:
Diagnosis and treatment of bacterial meningitis. Arch Dis Child.
88:615–620. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chang CJ, Chang WN, Huang LT, Huang SC,
Chang YC, Hung PL, Tasi CY, Lu CH, Cheng BC, Lee PY, et al:
Neonatal bacterial meningitis in southern Taiwan. Pediatr Neurol.
29:288–294. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin PC, Chiu NC, Li WC, Chi H, Hsu CH,
Hung HY, Kao HA and Huang FY: Characteristics of nosocomial
bacterial meningitis in children. J Microbiol Immunol Infect.
37:35–38. 2004.PubMed/NCBI
|
|
5
|
Ruan L, Wu D, Li X, Huang Q, Lin L, Lin J,
Chen L, Xu P, Jin J, Yang N, et al: Analysis of microbial community
composition and diversity in postoperative intracranial infection
using high throughput sequencing. Mol Med Rep. 16:3938–3946. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rahman S: Lumbar puncture in neonates
under and over 72 hours of age. J Coll Physicians Surg Pak.
17:646–647. 2007.PubMed/NCBI
|
|
7
|
Hoque MM, Ahmed ASMNU, Chowdhury MAKA,
Darmstadt GL and Saha SK: Septicemic neonates without lumbar
puncture: What are we missing? J Trop Pediatr. 52:63–65. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kavuncuoğlu S, Gürsoy S, Türel Ö, Aldemir
EY and Hoşaf E: Neonatal bacterial meningitis in Turkey:
Epidemiology, risk factors, and prognosis. J Infect Dev Ctries.
7:73–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gambino R: C-reactive protein -
undervalued, underutilized. Clin Chem. 43:2017–2018.
1997.PubMed/NCBI
|
|
10
|
Smith RP and Lipworth BJ: C-reactive
protein in simple community-acquired pneumonia. Chest.
107:1028–1031. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Enguix A, Rey C, Concha A, Medina A, Coto
D and Diéguez MA: Comparison of procalcitonin with C-reactive
protein and serum amyloid for the early diagnosis of bacterial
sepsis in critically ill neonates and children. Intensive Care Med.
27:211–215. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takahashi S, Oki J, Miyamoto A, Moriyama
T, Asano A, Inyaku F and Okuno A: Beta-2-microglobulin and ferritin
in cerebrospinal fluid for evaluation of patients with meningitis
of different etiologies. Brain Dev. 21:192–199. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Murawska E, Szychowska Z and Jarno A: Beta
2 microglobulin in children with neuroinfections. Przegl Epidemiol.
51:457–463. 1997.(In Polish). PubMed/NCBI
|
|
14
|
Sáez-Llorens X and McCracken GH Jr:
Bacterial meningitis in children. Lancet. 361:2139–2148. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin MC, Chi H, Chiu NC, Huang FY and Ho
CS: Factors for poor prognosis of neonatal bacterial meningitis in
a medical center in Northern Taiwan. J Microbiol Immunol Infect.
45:442–447. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cho HK, Lee H, Kang JH, Kim KN, Kim DS,
Kim YK, Kim JS, Kim JH, Kim CH, Kim HM, et al: The causative
organisms of bacterial meningitis in Korean children in 1996–2005.
J Korean Med Sci. 25:895–899. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Furyk JS, Swann O and Molyneux E:
Systematic review: Neonatal meningitis in the developing world.
Trop Med Int Health. 16:672–679. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gaschignard J, Levy C, Romain O, Cohen R,
Bingen E, Aujard Y and Boileau P: Neonatal bacterial meningitis:
444 cases in 7 years. Pediatr Infect Dis J. 30:212–217. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Levent F, Baker CJ, Rench MA and Edwards
MS: Early outcomes of group B streptococcal meningitis in the 21st
century. Pediatr Infect Dis J. 29:1009–1012. 2010.PubMed/NCBI
|
|
20
|
Barichello T, Fagundes GD, Generoso JS,
Elias SG, Simões LR and Teixeira AL: Pathophysiology of neonatal
acute bacterial meningitis. J Med Microbiol. 62:1781–1789. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Verani JR, McGee L and Schrag SJ: Division
of Bacterial Diseases, National Center for Immunization and
Respiratory Diseases, Centers for Disease Control and Prevention
(CDC): Prevention of perinatal group B streptococcal disease -
revised guidelines from CDC, 2010. MMWR Recomm Rep. 59:1–36.
2010.PubMed/NCBI
|