Introduction
As a type of cerebrovascular disease, lacunar
infarction (LI) seriously threatens the health and life of the
middle aged and elderly people. LI accounts for approximately 30%
of cerebral infarctions, and incidence rate of LI shows an
increasing trend year by year. Adenosine triphosphate-binding
cassette transporter A1 (ABCA1) is an integral membrane protein
with ATP as the energy resource. ABCA1 can promote the release of
free phospholipids and free cholesterol from cells. The released
phospholipids and cholesterol will bind to apolipoprotein A1
(ApoA-I) on the cell surface to form high-density lipoprotein
cholesterol (HDL-C), thus being involved in the reverse transport
of cholesterol in the human body and playing an
anti-atherosclerosis role (1,2). Related
data have shown (3,4) that ABCA1 gene mutation can
induce HDL-C deficiency accompanied by atherosclerosis.
ABCA1 gene single nucleotide polymorphism (SNP) is closely
related to the development of atherosclerosis and plasma lipid
level (5), and ABCA1 R219K is
the common SNP (6–8). At present, the roles of ABCA1
R219K in LI complicated with atherosclerosis still have not been
well studied.
Patients and methods
General materials
A total of 112 LI patients complicated with
arteriosclerosis treated in Ningbo First Hospital from March 2015
to September 2016 were enrolled as observation group. Inclusion
criteria: i) patients who met the diagnostic criteria of LI
confirmed via magnetic resonance imaging (MRI) or computed
tomography (CT); ii) patients who were complicated with
arteriosclerosis iii) patients who signed the informed consent.
Exclusion criteria: i) patients with severe dysfunctions in liver,
kidney or other organs; ii) patients with cancer. At the same time,
342 healthy volunteers were selected from physical examination
center to serve as control group. There were no significant
differences in the general information between the two groups
(P>0.05) (Table I).
 | Table I.General data of the patients and the
controls. |
Table I.
General data of the patients and the
controls.
| Items | Observation group
(n=112) | (n=342) | Control group
t/χ2 value | P-value |
|---|
| Sex
(male/female) | 59/53 | 174/168 | 0.049 | 0.824 |
| Age (years) | 20–59 | 20–60 |
|
|
| Average age
(years) | 68.85±7.89 | 68.37±7.48 | 0.581 | 0.562 |
| Body mass index
(kg/m2) | 23.55±2.37 | 23.42±2.53 | 0.479 | 0.632 |
| Educational
level |
|
|
|
|
| Junior
high school and below | 15 (13.39) | 43
(12.57) | 0.102 | 0.951 |
| Senior
high school and technical secondary school | 59 (52.68) | 178 (52.05) |
|
|
| Junior
college and above | 38 (33.93) | 121 (35.38) |
|
|
Diagnostic criteria for LI: i) Acute lacunar
infarction can be seen as a circle, ellipse or fissure with clear
boundary on diffusion-weighted imaging (DWI). T1MI showed low
intensity signal. TT2WI and FLAIR sequence images showed high
intensity signal. ii) In chronic lacunar infarction, the signal
intensity was low on both T1MI and FLAIR images, and the diameter
was <20 mm. iii) There was no cognitive impairment before onset
of lacunar infarction.
Diagnostic criteria for arteriosclerosis: Carotid
artery medial thickness >1.00 mm.
Biochemical detection
Venous blood (10 ml, fasting for 8 h) was collected
from each participant, followed by centrifugation at 2,500 × g for
5 min to collect serum. Serum samples were stored at −70°C before
use. Levels of serum triglyceride (TG), total cholesterol (TC),
HDL-C, low-density lipoprotein cholesterol (LDL-C), ApoA-I were
detected using a full-automatic biochemical analyzer (model BS-800;
Mindray Medical International Ltd., Shenzhen, China). The Lp(a) and
ApoB was detected by ELISA (enzyme linked immunosorbent assay).
Genomic DNA extraction and polymerase chain reaction
(PCR) amplification. Venous blood (3 ml, fasting for 8 h) was
collected from each participant of two groups, and placed in
ethylene diamine tetraacetic acid (EDTA) anticoagulant tube to
extract the genomic DNA using TRIzol reagent (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the instructions.
The amplification was performed with a 25 µl reaction system using
the PCR amplification instrument (Shanghai Huanxi Medical Co.,
Ltd., Shanghai, China). Primers were designed and synthesized by
Shenzhen Huada Gene Co., Ltd. (Table
II). PCR amplification reaction conditions: 94°C for 5 min,
followed by 35 cycles of 94°C for 30 sec, 60°C for 30 sec and 72°C
for 1 min, and 72°C for 5 min. Primer sequences used in PCR
reactions are shown in Table II PCR
product was purified using Qiaquick column (Qiagen, Hilden,
Germany). PCR products were digested with exonuclease I (New
England Biolabs, Inc., Ipswich, MA, USA) and sequenced at a
concentration of 50 ng/µl. Sequencing results were analyzed by 3730
gene analyzer (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The process was in accordance with the instructions of incision
enzyme (New England Biolabs, Inc.) for digestion of the PCR
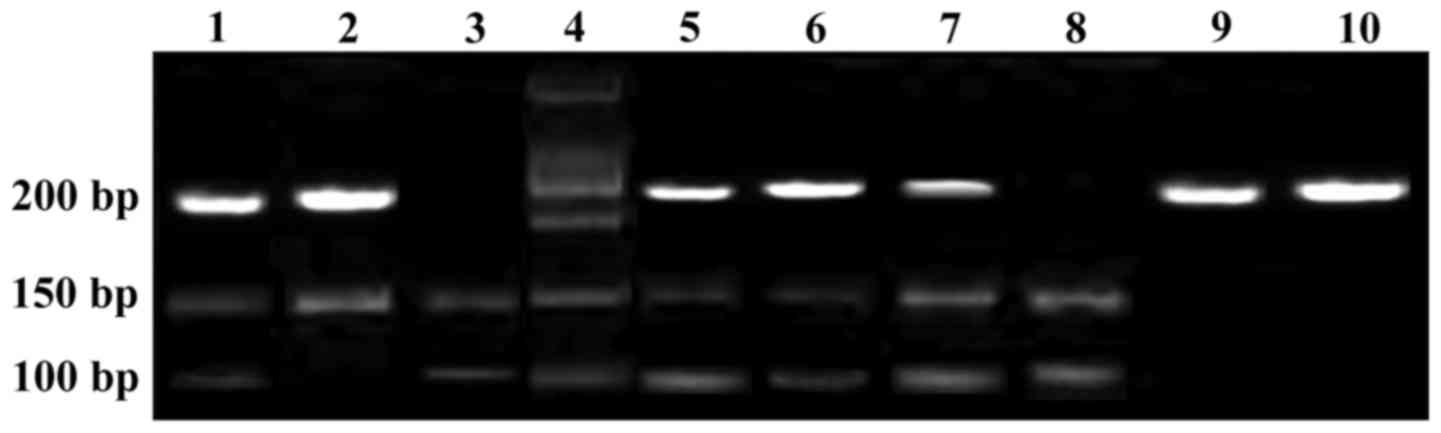
products. RR fragment was 177 bp; RK fragment was 177 bp, which was
cut into 107 bp and 70 bp; KK type fragment was 177 bp, and then
cut into 107 bp and 70 bp. Electrophoresis in 2% agarose gel at 50
V voltage for 3 h. The product was dyed with acetethidine bromide.
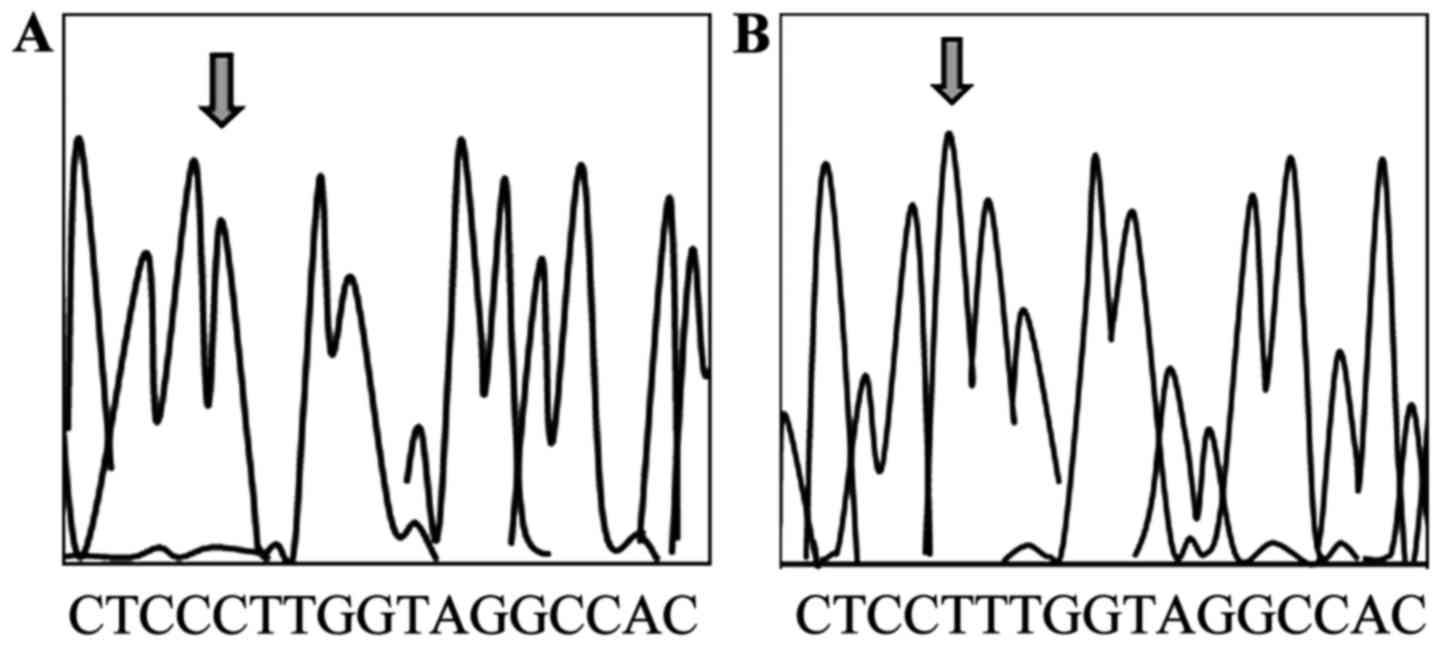
DNA Analyzer (Applied Biosystems; Thermo Fisher Scientific, Inc.)
was used for PCR product sequencing analysis to confirm that the
product was correct (Figs. 1 and
2).
 | Table II.Primers used in PCR reactions. |
Table II.
Primers used in PCR reactions.
| Genes | Sequences |
|---|
| ABCA1 | F:
5′-ACCGAAGTAAGGAGTTGCTCATA-3′ |
|
| R:
5′-GTGATATGGCATCGTTGCATTT-3′ |
| β-actin | F:
5′-ACTGGCATTGTGATGGACTC-3′ |
|
| R:
5′-AGGAAGGAAGGCTGGAAGAG-3′ |
Statistical processing
Statistical analysis was performed using the
Statistical Product and Service Solutions (SPSS) 20.0 (IBM Corp.,
Armonk, NY, USA) software package. Most of the measurement data met
the approximately normal distribution, while lipoprotein (a)
[Lp(a)] met the skewed distribution, and it was tested after
logarithmic transformation. The t-test was used for the intergroup
comparisons of measurement data, and F-test was used for the
comparisons among three groups; Chi-square test was used for
categorical data.
Results
Comparison of general data between two
groups
There were no significant differences in general
data between two groups (P>0.05) (Table I).
Comparison of blood lipid level
between the two groups
There were no significant differences in serum
levels of TC, LDL-C and Lp(a) between the two groups (P>0.05).
Levels of ApoB and TG in observation group were significantly
higher than those in control group, but the levels of HDL-C and
ApoA-I were significantly lower than those in control group
(P<0.05) (Table III).
 | Table III.Comparison of blood lipid level
between the two groups. |
Table III.
Comparison of blood lipid level
between the two groups.
| Groups | n | TG (mmol/l) | TC (mmol/l) | HLD-C (mmol/l) | LDL-C (mmol/l) | ApoA-I (g/l) | ApoB (g/l) | Lp(a) (mg/l) |
|---|
| Control group | 342 | 1.37±0.54 | 4.68±0.45 | 1.47±0.27 | 2.73±0.33 | 1.38±0.31 | 0.94±0.14 | 216.6±76.3 |
| Observation
group | 112 | 1.72±0.65 | 4.66±0.38 | 1.18±0.22 | 2.76±0.28 | 1.27±0.27 | 0.87±0.16 | 222.4±72.5 |
| t value |
| 6.092 | 0.127 | 7.326 | 0.204 | 8.336 | 9.005 | 0.086 |
| P-value |
| <0.05 | >0.05 | <0.05 | >0.05 | <0.05 | <0.05 | >0.05 |
Comparison of ABCA1 R219K genotypes
between the two groups
There were no significant differences in the RR, RK
and KK frequencies and allele frequency of ABCA1 R219K
genotype between the two groups (P>0.05) (Table IV).
 | Table IV.Comparison of ABCA1 R219K genotypes
between the two groups. |
Table IV.
Comparison of ABCA1 R219K genotypes
between the two groups.
|
|
| ABCA1 R219K
genotype [n (%)] | Allele frequency
(%) |
|---|
|
|
|
|
|
|---|
| Groups | n | RR | RK | KK | R | K |
|---|
| Control group | 342 | (33.5) | (51.5) | (15.0) | 56.4 | 43.6 |
| Observation
group | 112 | (28.2) | (54.6) | (17.2) | 55.5 | 44.5 |
| χ2
value |
| 0.156 | 0.163 | 0.285 | 0.204 | 0.216 |
| P-value |
| >0.05 | >0.05 | >0.05 | >0.05 | >0.05 |
Effects of ABCA1 R219K genotypes on
blood lipid level
The levels of HDL-C, TG, TC, LDL-C, ApoA-I, ApoB and
Lp(a) showed no significant differences among different genotypes
of ABCA1 R219K (P>0.05) (Table
V).
 | Table V.Effects of ABCA1 R219K genotypes on
blood lipid level. |
Table V.
Effects of ABCA1 R219K genotypes on
blood lipid level.
| Types | TG (mmol/l) | TC (mmol/l) | HLD-C (mmol/l) | LDL-C (mmol/l) | ApoA-I (g/l) | ApoB (g/l) | Lp(a) (mg/l) |
|---|
| RR type
(n=146) | 1.41±0.87 | 4.72±0.76 | 1.32±0.27 | 2.71±0.42 | 1.36±0.27 | 0.84±0.12 | 213.6±80.5 |
| RK type
(n=234) | 1.53±0.72 | 4.82±0.88 | 1.34±0.34 | 2.76±0.56 | 1.37±0.29 | 0.88±0.21 | 227.6±78.3 |
| KK type (n=74) | 1.46±0.68 | 4.75±0.76 | 1.41±0.65 | 2.82±0.52 | 1.41±0.32 | 0.86±0.25 | 225.7±71.6 |
| F-value | 2.654 | 1.054 | 1.658 | 2.287 | 1.892 | 1.634 | 1.927 |
| P-value | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 |
Discussion
ABCA1 is a type of common and highly conserved
transmembrane protein that plays a key role in mediating the
reverse transport of cholesterol (9–12).
ABCA1 R219K gene polymorphism has been proved to have
effects on the blood lipids, but effects of R219K gene polymorphism
on human blood lipid profile remains unknown. Most studies suggest
that the TG level is decreased and the HDL-C level is increased in
K allele carriers (13–15). However, our data showed that there
was no significant difference in HDL-C level between different
R219K genotypes and control group.
Arteriosclerosis is a pathological basis of
pathogenesis of cardiovascular and cerebrovascular diseases, as
well as the main factor of the incidence of those diseases. Studies
have shown that degree of arteriosclerosis is reduced in
ABCA1 R219K mutation carriers (16). In addition, in ABCA1 R219K
mutation carriers, cerebral infarction lesions can be easily
repaired, and the incidence of coronary heart disease is also
reduced. Some scholars (17,18) studied patients with ischemic stroke
and found that R allele frequency in patients with cerebral
infarction is lower than that in healthy population. However,
further analysis found that ABCA1 R219K gene polymorphism is
not an independent risk factor for ischemic stroke. Some scholars
(19) found that K allele frequency
in ABCA1 R219K has a protective effect on patients with
coronary heart disease and ischemic stroke.
As a common type of ischemic stroke, LI can be
induced by various factors, such as hypertension and cerebral
arteriosclerosis (20–23). Correlation between LI complicated
with arteriosclerosis and ABCA1 gene polymorphism remains
unclear. ABCA1 R219K gene polymorphism can increase the
beneficial blood lipid profile and reduce the severity of
arteriosclerosis. At the same time, atherosclerosis can promote the
occurrence and development of hypertension (24–28).
However, it was found in this study that ABCA1 R219K
polymorphism had no correlation with LI complicated with
arteriosclerosis. This study is still challenged by the small
sample size, and we only focused on one SNP type only, which may
affect the results. Future studies with larger number of samples
are still needed to confirm the conclusion.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YX and ZL conceived and designed the study,
collected, analyzed and interpreted the patient data. YX drafted
the manuscript. ZL revised the manuscript critically for important
intellectual content. Both authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Ningbo First Hospital (Ningbo, China). Signed informed consents
were obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim JS, Lee HS, Park HY, Kim SS, Kang HG,
Kim NH, Park JS and Kim Y: Endothelial function in lacunar
infarction: A comparison of lacunar infarction, cerebral
atherosclerosis and control group. Cerebrovasc Dis. 28:166–170.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iranmanesh F and Farahmand H: Large vessel
atherosclerotic infarction and lacunar lesions. Acta Neurol Taiwan.
16:203–206. 2007.PubMed/NCBI
|
|
3
|
Lodder J: Size criterion for lacunar
infarction. Cerebrovasc Dis. 24:156–157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hong NR, Seo HS, Lee YH, Kim JH, Seol HY,
Lee NJ and Suh SI: The correlation between carotid siphon
calcification and lacunar infarction. Neuroradiology. 53:643–649.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yatsuya H, Folsom AR, Wong TY, Klein R,
Klein BE and Sharrett AR: ARIC Study Investigators: Retinal
microvascular abnormalities and risk of lacunar stroke:
Atherosclerosis Risk in Communities Study. Stroke. 41:1349–1355.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng C, Hua T, Xu Y, Liu XY and Huang J:
Arterial remodeling of basilar atherosclerosis in isolated pontine
infarction. Neurol Sci. 36:547–551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao L, Jin H, Yang B, Zhang S, Han S, Yin
F and Feng Y: Correlation between ABCA1 gene polymorphism and
aopA-I and HDL-C in abdominal aortic aneurysm. Med Sci Monit.
22:172–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suwanwela NC and Chutinetr A: Risk factors
for atherosclerosis of cervicocerebral arteries: Intracranial
versus extracranial. Neuroepidemiology. 22:37–40. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagai Y, Kitagawa K and Matsumoto M:
Implication of earlier carotid atherosclerosis for stroke and its
subtypes. Prev Cardiol. 6:99–103. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zafar A: Diabetic patients are at a higher
risk of lacunar infarction and dyslipidemia: Results of a
comparative pilot study from King Fahad Hospital of the University,
Saudi Arabia. Neurosciences (Riyadh). 22:20–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Darabi M, Rabbani M, Ani M, Zarean E,
Panjehpour M and Movahedian A: Increased leukocyte ABCA1 gene
expression in post-menopausal women on hormone replacement therapy.
Gynecol Endocrinol. 27:701–705. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Edgel KA, Leboeuf RC and Oram JF: Tumor
necrosis factor-alpha and lymphotoxin-alpha increase macrophage
ABCA1 by gene expression and protein stabilization via different
receptors. Atherosclerosis. 209:387–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ota M, Fujii T, Nemoto K, Tatsumi M,
Moriguchi Y, Hashimoto R, Sato N, Iwata N and Kunugi H: A
polymorphism of the ABCA1 gene confers susceptibility to
schizophrenia and related brain changes. Prog Neuropsychopharmacol
Biol Psychiatry. 35:1877–1883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yao MH, Guo H, He J, Yan YZ, Ma RL, Ding
YS, Zhang JY, Liu JM, Zhang M, Li SG, et al: Interactions of six
SNPs in ABCA1 gene and obesity in low HDL-C disease in Kazakh of
China. Int J Environ Res Public Health. 13:176–181. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shalia K, Saranath D and Shah VK:
Peripheral blood mononuclear cell ABCA1 transcripts and protein
expression in acute myocardial infarction. J Clin Lab Anal.
29:242–249. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ogata M, Tsujita M, Hossain MA, Akita N,
Gonzalez FJ, Staels B, Suzuki S, Fukutomi T, Kimura G and Yokoyama
S: On the mechanism for PPAR agonists to enhance ABCA1 gene
expression. Atherosclerosis. 205:413–419. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cerda A, Issa MH, Genvigir FD, Rohde CB,
Cavalli SA, Bertolami MC, Faludi AA, Hirata MH and Hirata RD:
Atorvastatin and hormone therapy influence expression of ABCA1,
APOA1 and SCARB1 in mononuclear cells from hypercholesterolemic
postmenopausal women. J Steroid Biochem Mol Biol. 138:403–409.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Uehara Y, Miura S, von Eckardstein A, Abe
S, Fujii A, Matsuo Y, Rust S, Lorkowski S, Assmann G, Yamada T, et
al: Unsaturated fatty acids suppress the expression of the
ATP-binding cassette transporter G1 (ABCG1) and ABCA1 genes via an
LXR/RXR responsive element. Atherosclerosis. 191:11–21. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsunemi A, Ueno T, Fukuda N, Watanabe T,
Tahira K, Haketa A, Hatanaka Y, Tanaka S, Matsumoto T, Matsumoto Y,
et al: A novel gene regulator, pyrrole-imidazole polyamide
targeting ABCA1 gene increases cholesterol efflux from macrophages
and plasma HDL concentration. J Mol Med (Berl). 92:509–521. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iwamoto N and Yokoyama S: Protein kinase D
regulates the adiponectin gene expression through phosphorylation
of AP-2: A common pathway to the ABCA1 gene regulation.
Atherosclerosis. 216:90–96. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meurs I, Out R, Van Berkel TJ and van Eck
M: Role of the ABC transporters ABCA1 and ABCG1 in foam cell
formation and atherosclerosis. Future Lipidol. 3:675–687.
2017.https://www.tandfonline.com/doi/abs/10.2217/17460875.3.6.675
View Article : Google Scholar
|
|
22
|
Jiang M and Li X: Activation of PPARγ does
not contribute to macrophage ABCA1 expression and ABCA1-mediated
cholesterol efflux to apoAI. Biochem Biophys Res Commun.
482:849–856. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fitz NF, Carter AY, Tapias V, Castranio
EL, Kodali R, Lefterov I and Koldamova R: ABCA1 deficiency affects
basal cognitive deficits and dendritic density in mice. J
Alzheimers Dis. 56:1075–1085. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Villalobos-Comparán M, Antuna-Puente B,
Villarreal-Molina MT, Canizales-Quinteros S, Velázquez-Cruz R,
León-Mimila P, Villamil-Ramírez H, González-Barrios JA,
Merino-García JL, Thompson-Bonilla MR, et al: Interaction between
FTO rs9939609 and the native American-origin ABCA1 rs9282541
affects BMI in the admixed Mexican population. BMC Med Genet.
18:46–52. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Coban N, Gulec C, Ozsait-Selcuk B and
Erginel-Unaltuna N: CYP19A1, MIF and ABCA1 genes are targets of the
RORα in monocyte and endothelial cells. Cell Biol Int. 41:163–176.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Babashamsi MM, Halalkhor S, Firouzjah
Moradi H, Parsian H, Jalali SF and Babashamsi M: Association of
ATP-binding cassette transporter A1 (ABCA1)-565 C/T gene
polymorphism with hypoalpha lipoproteinemia and serum lipids, IL-6
and CRP levels. Avicenna J Med Biotechnol. 9:38–43. 2017.PubMed/NCBI
|
|
27
|
Nelson JK, Koenis DS, Scheij S, Cook EC,
Moeton M, Santos A, Lobaccaro JA, Baron S and Zelcer N: EEPD1 is a
novel LXR target gene in macrophages which regulates ABCA1
abundance and cholesterol efflux. Arterioscler Thromb Vasc Biol.
37:423–432. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vega-Badillo J, Gutiérrez-Vidal R,
Hernández-Pérez HA, Villamil-Ramírez H, León-Mimila P,
Sánchez-Muñoz F, Morán-Ramos S, Larrieta-Carrasco E,
Fernández-Silva I, Méndez-Sánchez N, et al: Hepatic miR-33a/miR-144
and their target gene ABCA1 are associated with steatohepatitis in
morbidly obese subjects. Liver Int. 36:1383–1391. 2016. View Article : Google Scholar : PubMed/NCBI
|