Introduction
Tendon and ligament injuries are among the most
commonly encountered health problems, with about 16.4 million
occurring every year in the United States (1). Cell-based therapies have been
introduced with encouraging outcomes in preclinical evaluations and
shows attractive future direction for clinical therapy of tendon
injuries (2,3). Bone marrow mesenchymal stem cells
(BMSCs) are promising approaches for ligament and tendon
reconstruction, and mostly used stem cell type to help tendon
repair (4–6). In particular, a recent study found that
the use of transforming growth factor (TGF)-β1 and CTGF could
initiated and maintained highly efficient tenogenesis of BMSCs
(7).
Bone morphogenetic protein (BMP) 14, also known as
cartilage-derived morphogenetic protein-1 (CDMP-1) and growth
differentiation factor (GDF)-5, like other BMPs (BMP12 and BMP13),
is a member of the TGF-β family and has been shown to accelerate
tendon healing in animal models (8,9). The
effect of BMP12 on induces tenogenic differentiation of
adiposederived stromal cells (ASCs) and BMP13 on induces
chondrogenic differentiation of murine mesenchymal progenitor cells
have been well understooded (10,11), but
the function of BMP14 on tenogenic differentiation has not yet been
investigated. Mounting evidences are showing that, BMP14 could
increase tendon tensile strength in an achilles tendon injury rat
model (12,13). In a recent in vitro study, it
was demonstrated that interposition of a multilayered collagen
patch seeded with muscle-derived MSCs and BMP14 into the repair
site enhanced flexor tendon healing compared with a similar patch
using cells alone (14), but its
molecular mechansim needs further confirmation.
Sirtuin 1 (Sirt1) is conserved protein
NAD+-dependent histone deacylases, which is linked to
various physiological process, including cell proliferation,
apoptosis and inflammation (15,16).
Several studies have demonstrated important roles of Sirt1 in
macrophages and chondrocyte differentiation (17,18), and
resveratrol induced Sirt1 activation promote sneuronal
differentiation of human BMSCs (19). Furthermore, Sirt1 signaling pathway
may be involved in rabbit flexor tendon repair and may be targeted
for therapeutic intervention in flexor tendon injury (20), but its function role in tenogenic
differentiation has not been reported. Previuos studies shows that,
in HaCaT cells, UV radiation could induce Sirt1 down-regulation,
ROS-mediated c-Jun N-terminal kinase (JNK) activation is involved
in the process, and JNK inhibitor and antioxidant NAC could recover
Sirt1 lost due to UV radiation (21). Sirt1 has recently been demonstrated
to deacetylate Smad3 and Smad7 (22), these data indicating a direct
function of Sirt1 in the regulating JNK and Smad activity.
Many transcription factors play important roles in
the BMSCs differentiation (23,24).
Including that, farnesoid X receptor (FXR), a nuclear receptors,
operates as a ligand-activated transcription factor (25), its activation stimulates BMSCs
osteoblastic differentiation, whereas its inhibition leads to an
adipocyte-like phenotype (26).
Peroxisome proliferator-activated receptor (PPAR)-γ, another
lignad-activated transcription factor belonging to the nucleus
hoemone receptor superfamily (27),
could inhibit BMSCs differentiation toward myofibroblasts (28).
Based on these observations, we suppose that whether
BMP14 could induce BMSCs tendon differentiation and what molecular
mechanism is involved. So, BMSCs were isolated, and the dose and
time effects of BMP14 on BMSCs differentiation were examined
targeting tendon markers and the molecular mechanism of BMP14
induced tendon differentiation was explored. Collectively, our
research advances the current understandings of BMP14 induced
tenogenic differentiation of BMSCs and provide a basis for future
cellular and molecular approache for tendon tissue engineering and
repair.
Materials and methods
Materials
α-MEM medium, 0.25% trypsin, fetal bovine serum
(FBS) and antibiotic/antimycotic solutions were obtained from
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
anti-CD29 (ab52971), anti-CD44 (ab25340) anti-Sirt1 (ab110304),
anti-PPARγ (ab45036), anti-Collagen type I (ab34710), anti-Collagen
type III (ab7778), anti-tenomodulin (ab203676) and anti-SCX
(ab58655) primary antibodies were obtained from Abcam (Cambridge,
MA, USA). Anti-ERK1/2 (no. 4695), acetylated-lysine (no. 9441),
anti-phospho-ERK1/2 (no. 4370), anti-JNK (no. 9258),
anti-phospho-JNK (no. 4668), anti-Smad2/3 (no. 8685),
anti-phospho-Smad2/3 (no. 11979), anti-Smad1 (no. 6944),
anti-phospho-Smad1 (no. 5753) antibodies were purchased from Cell
Signaling Technology, Inc., (Danvers, MA, USA), β-actin (A1978)
monoclonal antibody was from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany). CD44H monoclonal antibody (12-0444-82), CD73 monoclonal
antibody (11-0739-42), CD90.1 Monoclonal antibody (A16370) and CD45
monoclonal antibody (11-0461-82) were purchased from eBioscience
(Thermo Fisher Scientific, Inc.). BMP14 (SRP4580) was purchased
from Sigma-Aldrich; Merck KGaA. LDN-193189 (S2618) and SP600125
(S1460) were obtained from Selleck (Shanghai, China).
Animals
All animal protocols were approved by the
Institutions Animal Care and Use Committee of Jingchu Institute of
Technology and complied with the guidelines of the Jingchu
Institute of Technology's Regulations of Animal Experiments.
Wistar-Kyoto (WKY) rats were obtained from Model Animal Research
Center of Nanjing University. The rats were housed in a
pathogen-free barrier facility with a 12 h light/dark cycle and
were given free access to food and water. Rats were sacrificed
under CO2 asphyxia (20% volume of container per min) at
8–12 weeks of age and hind limbs were removed in preparation for
bone marrow isolation.
BMSC isolation and identification
Bone marrow from the hind limbs of the WKY rat was
isolated as previously described (29) and the expressions of cell surface
markers on isolated BMSCs were measured using flow cytometry
(30). Briefly, cells were collected
and fixed in 4% paraformaldehyde, then stained with FITC-conjugated
mouse anti-rat CD44, CD73, CD90, and CD45, and affinity purified
mouse IgG was used as control. Fluorescence activated cell sorting
was performed with FACS Calibur (BD Biosciences, Franklin Lakes,
NJ, USA) and data were analyzed with FlowJo software (Tree Star,
Ashland, OR, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
After treatment, BMSCs were lysed with TRIzol
(Thermo Fisher Scientific, Inc.) and total RNA was extracted
according to the manufacturer's instructions. RNA concentration was
determined by UV spectrophotometry (NanoDrop 2000; Thermo Fisher
Scientific, Inc.). 1 µg RNA was reversely transcribed into cDNA in
a 20 µl reaction using the M-MMLV Reverse Transcriptase with RNasin
Ribonuclease Inhibitors (Promega Biotech Co., Ltd., Beijing,
China). 1 µl of the cDNA was used for subsequent RT-qPCR reactions
with SYBR Green qPCR Master Mix (Biotool, Shanghai, China)
according to the manufacturer's instructions using StepOnePlus
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The PCR program was set as the follows: 95°C for 10 min,
followed by 95°C for 15 sec, 60°C for 30 sec, and 72°C for 30 sec
for 40 cycles. The relative levels of target gene expression was
analyzed using the comparative Cq (2−∆∆Cq) method
(31). GAPDH was used as endogenous
reference gene. The primer sequences used were: collagen I
(forward: 5′-AAGGCCCACGGGGACCTGTT-3′, reverse:
5′-GGGCCAGGCACGGAAACTCC-3′); collagen III (forward:
5′-AGCTGGACCAAAAGGTTGATG-3′, reverse: 5′-GACCTCGTGCTCCAGTTAGC-3′);
Scx (forward: 5′-AGAGACGGCGGCGAGAAC-3′, reverse:
5′-AATCGCCGTCTTTCTGTCACG-3′); GAPDH (forward:
5′-CCTGGCCAAGGTCATCCAT-3′, reverse:
5′-GAGTTGAGCAGCGTCTGGAT-3′).
Fluorescent immunocytochemistry
BMSCs at passage 2 were initiated in 24-well chamber
slides at a density of 2,500 cells per well in basal medium. At the
end of culture, cells were fixed in 4% paraformaldehyde overnight,
rinsed in phosphate-buffered saline (PBS) and blocked using 10%
sheep serum in PBS with 0.1% Triton X-100. CD29, CD44 and Scx
primary antibodies were diluted at 1:200 and 1:100 respectively and
incubated overnight at 4°C, then Donkey Anti-Rabbit IgG H&L
(Alexa Fluor® 647) (ab150075) and Donkey Anti-Rabbit IgG
H&L (Alexa Fluor® 488) (ab150073) were used at a
1:1,000 dilution for 1 h in a solution containing 1% bovine serum
albumin (BSA). After extensive washes in PBS, slides were mounted
by using SlowFade® Gold Anti fade Mountant with DAPI
(S36942; Thermo Fisher Scientific, Inc.), and observed by
fluorescence microscopy (Olympus IX81; Olympus Corporation, Tokyo,
Japan).
Induction of BMSC differentiation
To examine the effect of BMP14 on tenogenic
differentiation, BMSCs were treated with BMP14 for different dose
and different time. The dose of BMP14 was chosen based on the
results from this study, which resulted in the strongest tenogenic
effects on BMSCs among all the doses and time examined. For western
blot assay, BMSCs were cultured in 6-well plates at a density of
2×105 cells per well, then cells were treated with
either BMP14 (50 ng/ml) for the indicated periods in duplicates. In
cases when the inhibitor LDN-193189 (1 µM) or SP600125 (1 µM) was
applied, the cells were pretreated with either of the drugs for 15
min before the addition of BMP14.
Western blot assay
BMSCs cells were collected, lysed, and subjected to
protein extraction with RIPA buffer. Twenty-five micrograms of
total lysate were separated on sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and then
transferred onto nitrocellulose membranes. Specific monoclonal
anti-Sirt1 (1:1,000 dilution), anti-Collagen Type I (1:1,000
dilution), anti-Collagen Type III (1:1,000 dilution)
anti-tenomodulin (1:1,000 dilution) and Anti-SCX antibody (1:1,000
dilution) primary antibodies (Abcam) and anti-ERK1/2 (1:1,000
dilution), anti-phospho-ERK1/2 (1:1,000 dilution), anti-JNK
(1:1,000 dilution), anti-Phospho-JNK (1:1,000 dilution),
anti-Smad2/3 (1:1,000 dilution), anti-phospho-Smad2/3 (1:1,000
dilution), anti-Smad1 (1:1,000 dilution), anti-phospho-Smad1
(1:1,000 dilution) were used, and anti-rabbit HRP conjugated
secondary antibody (no. 7074; Cell Signaling Technology, Inc.,
Danvers, MA, USA) was used. West Pico Chemiluminescent (Pierce;
Thermo Fisher Scientific, Inc.) was used as the substrate to
visualize protein bands, which were quantified using densitometry
image analysis software (Image Master VDS; Pharmacia Biotech,
Uppsala, Sweden). Normalization was made against β-actin (1:3,000
dilution) expression.
IP-western blot analysis
Cell lysate was incubated overnight at 4°C with
PPARγ antibody and then 2 h with protein A/G beads (B23012;
Biotool). After washing with PBS, bound proteins were separated in
10% SDS-PAGE, transferred to polyvinylidene fluoride (PVDF)
membrane, blocked with 5% milk in TBST, and incubated with
acetylated-lysine, Sirt1 or PPARγ antibodies overnight at 4°C.
After washing with TBST, the membrane was incubated at room
temperature for 1 h with anti-mouse antibody conjugated with
horseradish peroxidase (HRP). Signals were revealed by enhanced
chemiluminescence (ECL) followed by exposure.
Lentivirus infection
BMSC cells were infection with lentivirus vectors
expressing Sirt1 or shSirt1 which were obtained from GenePharm Co.,
Ltd. (Shanghai, China). ShRNA sequences were as follows: Shsirt-1:
GAAGTGCCTCAGATATTAA, Shscramble: GCGCGCTTTGTAGGATTCG.
Statistics
Data were presented as means ± standard error mean
of three independent experiments. Data between groups were analyzed
by Student's t-test or one-way analysis of variance followed by
Bonferroni-Dunn multiple comparison. All statistical analyses were
conducted using SPSS version 16.0 software (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Characterization of BMSCs
There are two kinds of stem cells in the bone
marrow: Mesenchymal and hematopoietic stem cells (32). To initiate the characterization of
BMSCs, immunofluorescent staining was used to identify the BMSCs
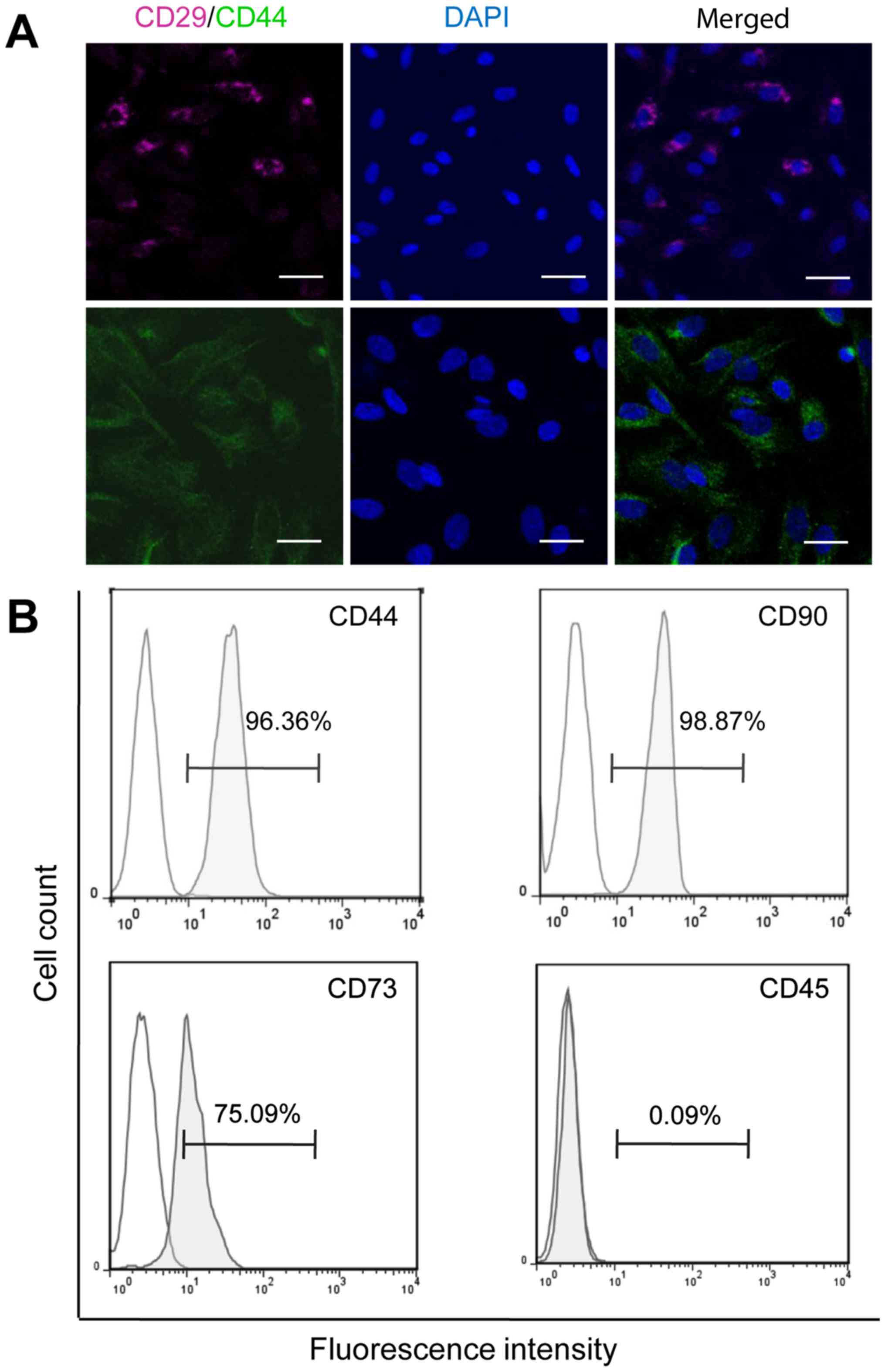
with surface markers CD29 and CD44 (33). As shown in Fig. 1A, CD29 and CD44 were both positively
detected on BMSCs at passage 2.
The expression of the cell markers specific for
mesenchymal and hematopoieticstem cells was analyzed with flow
cytometry. All analyzed cells were positive for mesenchymal cell
markers CD44, CD73 and CD90, but negative for hematopoietic cell
marker CD45 (Fig. 1B), confirming
the identity of isolated BMSCs.
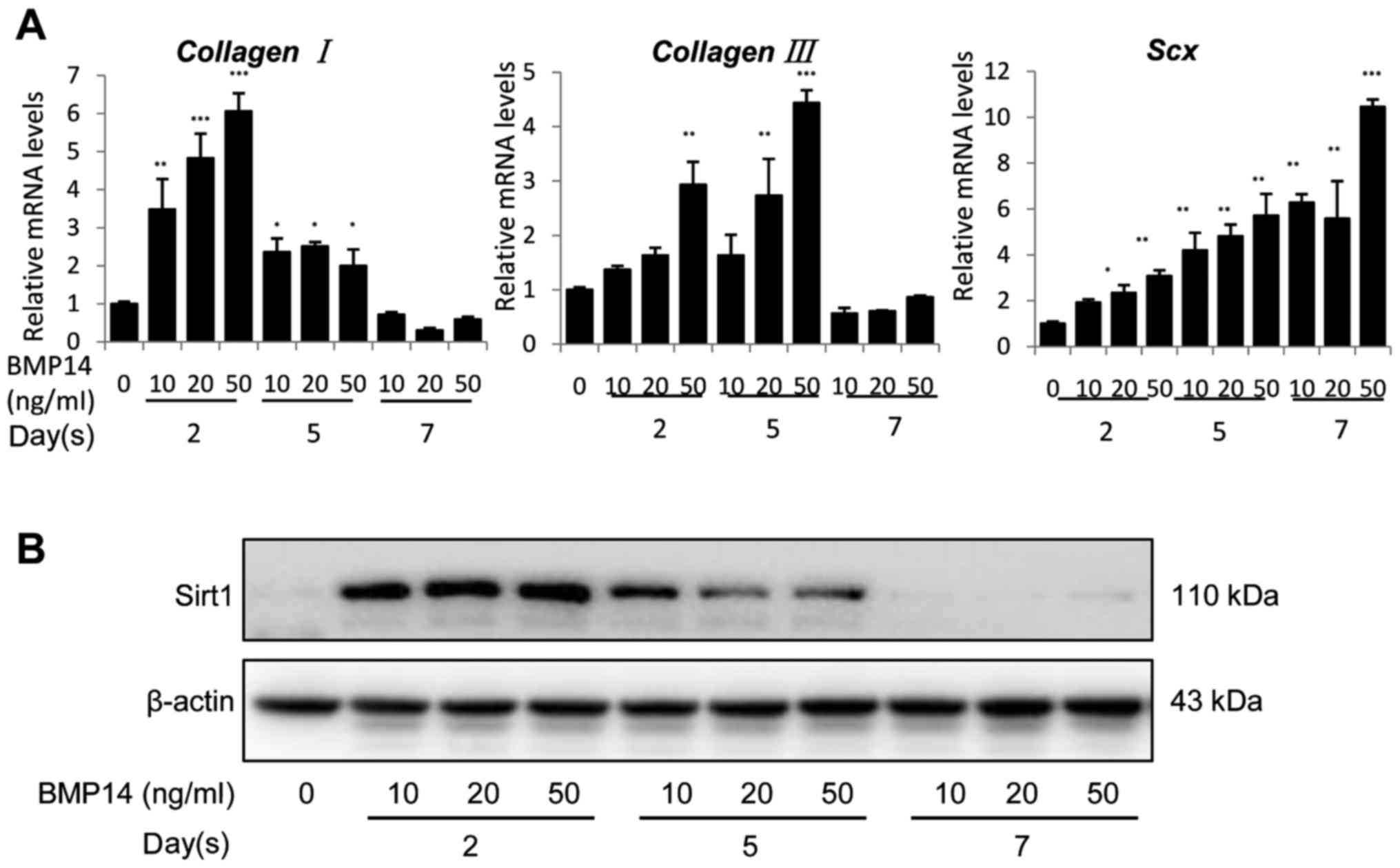
BMP14 induce tenogenic gene expression
in BMSCs
The dose and time effects of BMP14 on BMSC
differentiation were further investigated using RT-qPCR assay
targeting the Scx and the tendon matrix genes collagen
I and collagen III. Scx is a tendons and ligaments
specific marker (34) which
regulates TNMD expression (35), and
TNMD is required for tendon fibroblasts (TFs) proliferation and
tendon maturation (36). Our results
showed that BMP14 dramatically increased collagen I
expression in mRNA levels by up to 3.5–6-fold at day 2, but
decreased to ~2.5-fold at day 5 and ~0.75-fold at day 7 (Fig. 2A left). 50 ng/ml BMP14 also caused a
substantial increase in collagen III expression (Fig. 2A middle) at day 2 and day 5, it also
increased Scx mRNA expression in BMSCs by up to 11-fold in a
dose and time dependent manner (Fig.
2A right). Furthermore, BMP14 promotes the Sirt1 protein
content in BMSCs at day 2 but dramatically decreased at day 5 and
day 7 (Fig. 2B). This may response
to the reduced differentiation potential after long time culture
(37). These results suggest that
BMP14 regulates the differentiation of BMSCs by a mechanism
involving the expression of Sirt1.
Sirt1 gain of function promotes BMP14
induced tenogenic differentiation of BMSCs
Overexpression of Sirt1 was identified using western
blotting and qPCR as shown in Fig.
3A, and Sirt1 could increased BMP14 induced tendon marker gene
collagen I, collagen III and Scx mRNA expression in
BMSCs (Fig. 3B), indicating that
Sirt1 overexpression could promote BMP14 induced tenogenic
differentiation of BMSCs. Western blot assay showed that Sirt1
could also increase the tendon marker collagen I, TNMD and Scx
protein expression in BMSCs caused by BMP14 (Fig. 3C), Sirt1 overexpression shows
increased tendon markers mRNA and protein expression than control
group, sirt1 combine with BMP14 shows more increased tendon marker
levels than BMP14 or Sirt1 group. Immunofluorescence staining using
Scx antibody shows the samilar result as western blot assay, and
the fluorescence intensity was quantified (Fig. 3D). Co-immunoprecipitation (Co-IP)
results showed that BMP14 treatment and Sirt1 overexpression
decreased PPARγ acetylation levels in BMSCs, BMP14 plus Sirt1 could
further decrease the acetylation levels (Fig. 3E).
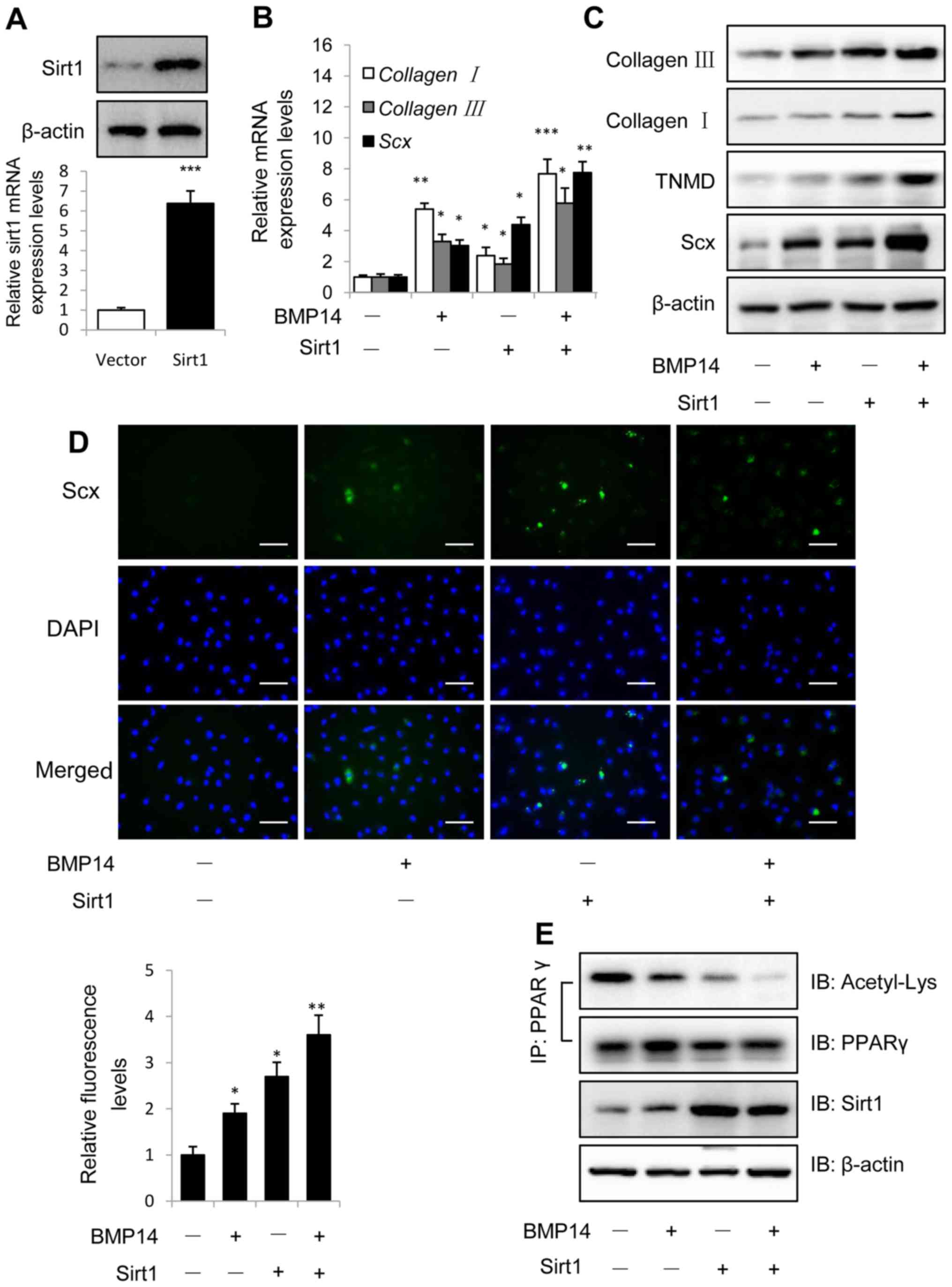 | Figure 3.Overexpression of Sirt1 promotes
BMP14 induced tenogenic differentiation of BMSCs. (A) Sirt1 protein
and mRNA levels were determined by western blot and RT-qPCR at 48 h
post infection with the vector control or Sirt1 lentivirus. β-actin
was used as the loading control. (B) mRNA expression of collagen
I, collagen III and Scx and (C) protein expression of
collagen I, collagen III, TNMD and Scx were detected using RT-qPCR
and western blot analysis with or without 50 ng/ml BMP14 treatment
for 48 h. (D) Fixed BMSCs were stained with Scx antibodies and
Alexa Fluor 488 goat anti-rabbit secondary antibodies (green) and
DAPI (blue). Scale bars, 200 µm. (E) PPARγ acetylation levels in
BMSCs were analyzed using immunoprecipitation with anti-PPARγ
antibodies and immunoblotting with anti-Acetyl-Lys and anti-PPARγ
antibodies. The total cell lysate was immunoblotted with anti-sirt1
and anti-β-actin antibodies. Each bar represents the mean ±
standard error of the mean. The results were repeated in three
independent experiments. *P<0.05, **P<0.01 and ***P<0.001
vs. the control. RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; BMP, bone morphogenetic protein; BMSC,
bone marrow mesenchymal stem cell; Sirt1, sirtuin 1; Scx,
scleraxis; TNMD, tenomodulin; PPARγ, peroxisome
proliferator-activated receptor γ; IP, immunoprecipitation; IB,
immunoblotting. |
Knockdown Sirt1 could inhibit the
BMP14 induced tenogenic differentiation of BMSCs
Knockdown of Sirt1 was performed using shSirt1
lentivirus infection for 48 h and evaluated with western blot and
real-time PCR assay (Fig. 4A).
Knockdown Sirt1 could decreased BMP14 induced tendon marker gene
collagen I, collagen III and Scx mRNA expression in
BMSCs (Fig. 4B and C). Western blot
assay showed that knockdown Sirt1 could also decrease the tendon
marker collagen I, TNMD and Scx protein expression in BMSCs caused
by BMP14 (Fig. 3C), only knockdown
sirt1 shows decreased tendon markers mRNA and protein expression
than control group. Shsirt1 combine with BMP14 shows increased
tendon marker levels than shsirt1, but decreased levels than BMP14.
Immunofluorescence staining using Scx antibody shows the similar
result as western blot assay (Fig.
4D), and the fluorescence intensity was quantified.
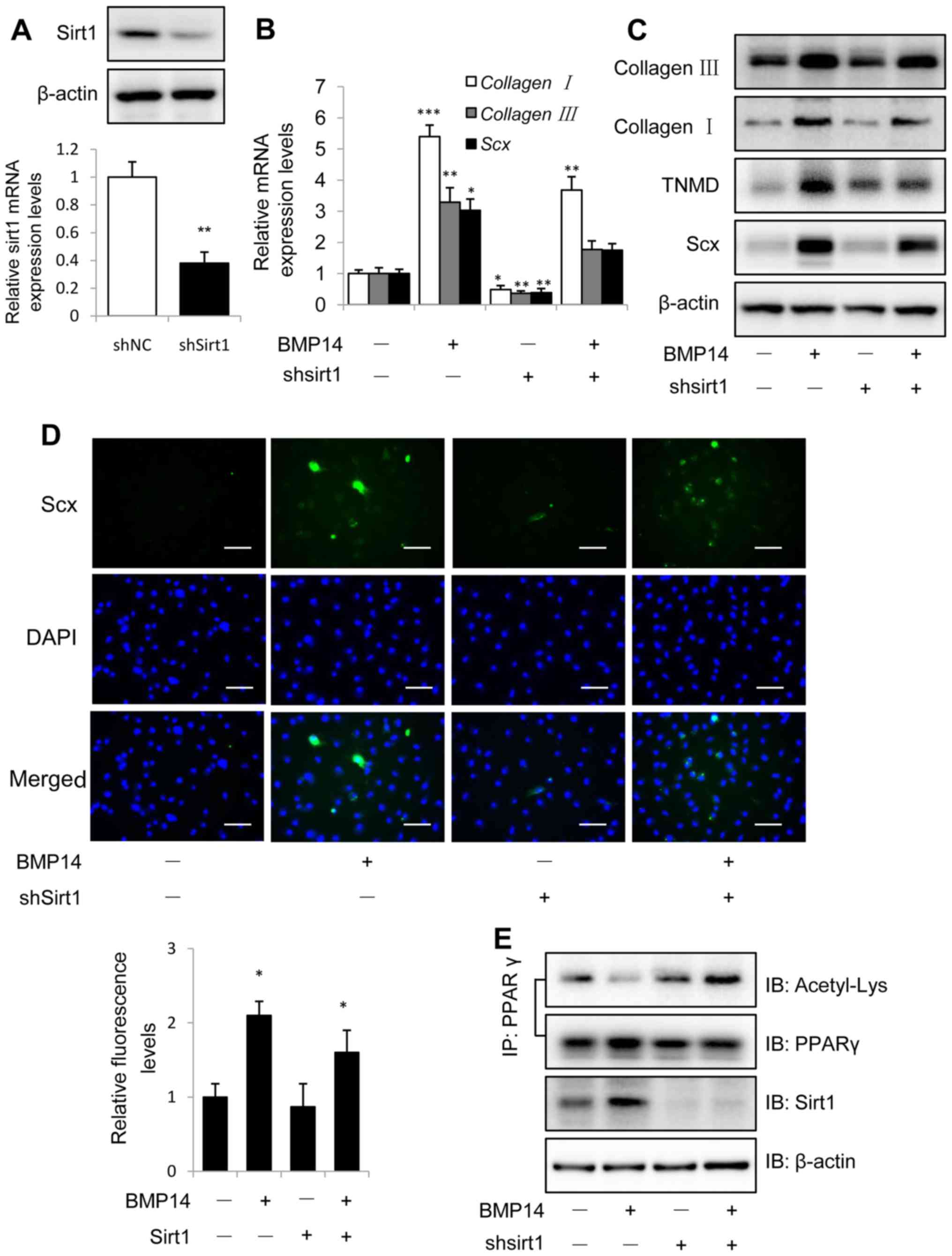 | Figure 4.Knockdown Sirt1 inhibits BMP14
induced tenogenic differentiation of BMSCs. (A) Sirt1 protein and
mRNA levels were determined by western blot analysis and RT-qPCR at
48 h post infection with shNC or shSirt1 lentivirus. β-actin was
used as the loading control. (B) The mRNA expression collagen I,
collagen III and Scx and (C) the protein expression of
collagen I, TNMD and Scx were detected in BMSCs infected with shNC
or shSirt1 using RT-qPCR and western blot analysis, respectively
with or without 50 ng/ml BMP14 treatment for 48 h. (D) Fixed BMSCs
were stained with Scx antibodies and Alexa Fluor 488 goat
anti-rabbit secondary antibodies (green) and DAPI (blue). Scale
bar, 200 µm. (E) PPARγ acetylation in BMSCs was analyzed using
immunoprecipitation with anti-PPARγ antibodies and immunoblotting
with anti-Acetyl-Lys and anti-PPARγ antibodies. The total cell
lysate was immunoblotted with anti-sirt1 and anti-β-actin
antibodies. Each bar represents the mean ± standard error of the
mean. The results were repeated in three independent experiments.
*P<0.05, **P<0.01 and ***P<0.001 vs. the control. BMSC,
bone marrow mesenchymal stem cell; Sirt1, sirtuin 1; NC, negative
control; sh, short hairpin; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; BMP, bone
morphogenetic protein; Sirt1, Sirtuin1; Scx, scleraxis; TNMD,
tenomodulin; PPARγ; peroxisome proliferator-activated receptor γ;
IP, immunoprecipitation; IB, immunoblotting. |
Co-IP results showed that BMP14 treatment decreased
PPARγ acetylation levels in BMSCs, but knockdown Sirt1 could
increase the acetylation levels (Fig.
4E). Since Sirt1 is a protein deacetylase implicated in the
regulation of metabolic activity, so the results of Sirt1 regulate
the acetylation levels of PPARγ is consistence with the previous
findings (38).
BMP14 induced tenogenic
differentiation of BMSCs via the JNK and Smad1 pathway in
Sirt1-dependent manner
BMPs are known to activate both Smad pathways and
non-Smad pathways such as mitogen-activated protein kinase (MAPK)
pathway (39,40). As shown in Fig. 5A, BMP14 induced increased
phosphorylation of Smad1 and JNK, but has no effect on the
phosphorylation of p38 and Smad2/3. We next asked whether Sirt1
could affect the activation of Smad and MAPK pathway in BMSCs
induced by BMP14. To answer this question, lentivirus overexpress
Sirt1 and shSirt1 were used. Interestingly, overexpression of Sirt1
in BMSCs could increase the phosphorylation of Smad1 and JNK, and
knockdown Sirt1 could significantly decrease the phosphorylation
levels (Fig. 5A). Furthermore,
TGF-β/Smad pathway inhibitor LDN-193189 and JNK inhibitor SP600125
were used to detect whether can block the BMP14 decresed the
acetylation of PPARγ effect. As we expected, 1 µM LDN-193189 and
SP600125 both can increase the acetylation of PPARγ (Fig. 5C).
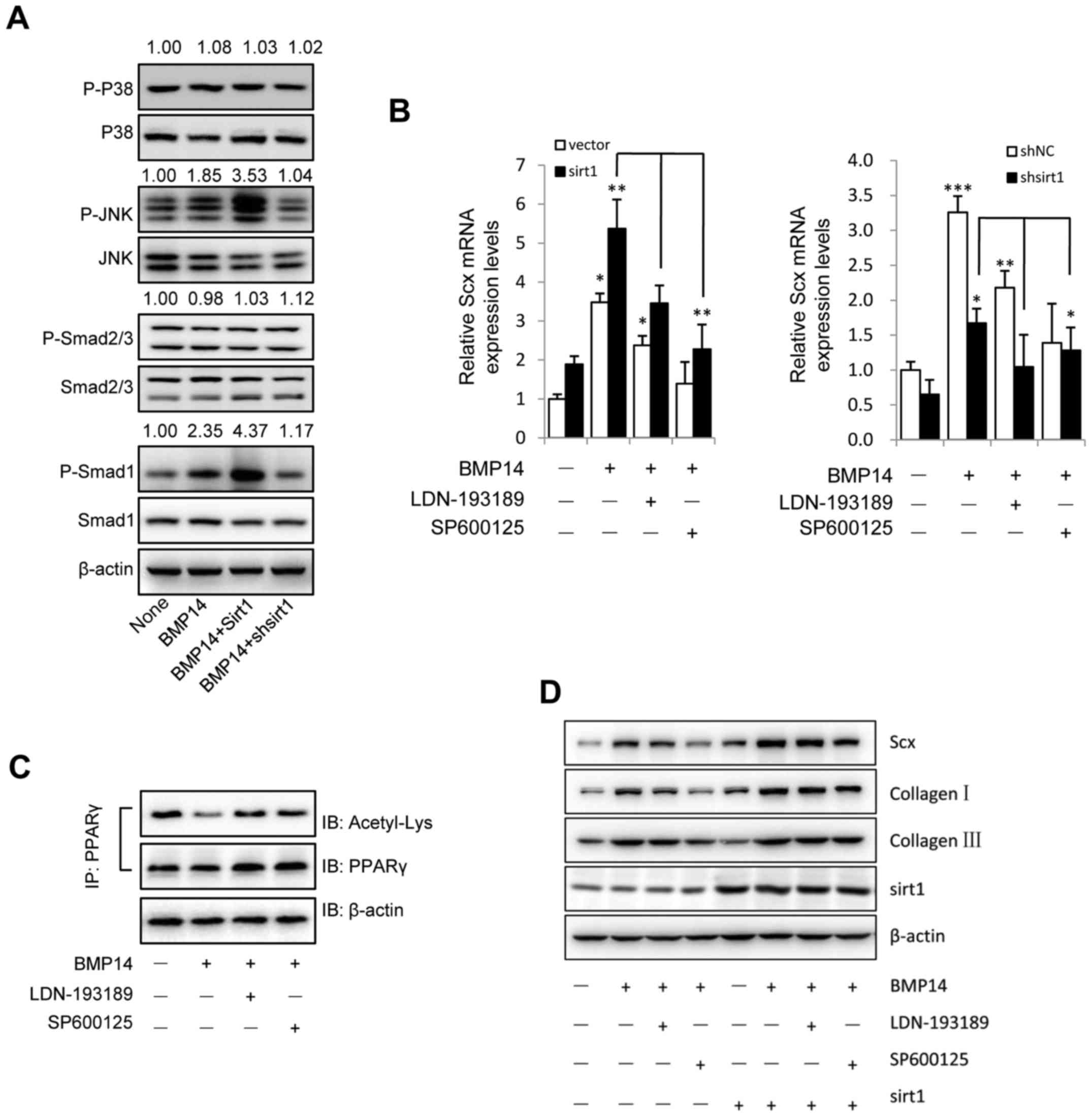 | Figure 5.BMP14 induces tenogenic signaling in
BMSCs via the JNK and Smad1 pathway in a Sirt1-dependent manner.
(A) BMSC cells were infected with Sirt1 overexpressing or shSirt1
lentivirus. Following treatment with 50 ng/ml BMP14, the cell
extracts were blotted with anti-P-p38, p38, P-JNK, JNK, P-smad2/3,
smad2/3, P-smad1 and smad1 antibodies. Quantitative analysis of the
blots was performed using ImageJ software and the relative ratios
are indicated above the blots. (B) mRNA levels of Scx were detected
using RT-qPCR. (C) BMSC cells were treated with 50 ng/ml BMP14 plus
1 µM LDN-193189 and SP600125. Following 48 h the cells were
harvested and cell extracts were immunoprecipitated with PPARγ
antibodies; the blots were immunoblotted with acetyl-lys and PPARγ
antibodies. The total cell lysate was immunoblotted with
anti-β-actin antibody. β-actin was used as the control. BMSC cells
were treated with 50 ng/ml BMP14 with or without 1 µM LDN-193189 or
SP600125. (D) The protein expression of Scx, collagen I, collagen
III and Sirt1 in BMSC cells were detected by western blot analysis.
β-actin was used as the loading control. The data are presented as
the mean ± standard error of the mean. *P<0.05, **P<0.01 and
***P<0.001. RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; BMP, bone morphogenetic protein; Sirt1,
sirtuin 1; BMSC, bone marrow mesenchymal stem cell; sh, short
hairpin; p-, phosphorylated; JNK, c-Jun N-terminal kinase; PPARγ,
peroxisome proliferator-activated receptor γ; Scx, scleraxis; IP,
immunoprecipitation; IB, immunoblotting. |
RT-qPCR and western blot assay demonstrated that
Sirt1 promote Smad1 and JNK mediated Scx mRNA and protein
expression and collagen I, collagen III protein levels, however,
BMP14 treatment had similar proportional increasement between
vector and Sirt1 forced expression, but LDN-193189 or SP600125
decreased the Scx mRNA and protein levels and collagen I, collagen
III protein expression (Fig. 5B),
the lentivirus knockdown Sirt1 showed the same effect. These data
suggest that BMP14 promote effect on tenogenic differentiation
markers through Smad1 and JNK pathway.
Discussion
The current study investigated the effects and
molecular mechanism of BMP14 on BMSC tenogenic differentiation,
thus further demonstrating its role as a tenogenic cue. Besides, we
found that BMP14 promotes BMSCs differentiation in Sirt1-dependent
manner via JNK and Samd1 pathway. Promoting BMP14 induced BMSCs
tenogenic differentiation with a longterm view in therapeutic
strategies for improving tendon to bone healing (41).
BMP14 has been shown to play a role in a variety of
musculoskeletal processes, including joint formation (42), endochondral ossification (43), tendon and ligament maintenance and
repair (44,45) and even brown adipogenesis in systemic
energy expenditure (46). Previous
studies showed that both BMP12 and BMP14 were capable of inducing
tendon marker gene expression in ASCs, but BMP12 was marginally
more potent and selective than BMP14 (10). But the effect of BMP14 on BMSCs was
poorly understood. So, in the present study, we propose a mechanism
by which BMP14 promotes Sirt1-mediated PPARγ deacetylation might
contribute to this process.
Firstly, we observed that 50 ng/ml BMP14 had a
tenogenic differentiation effect on BMSCs in a dose- and
time-dependent manner when measured at day 2. BMP14 upregulates
Sirt1 both at mRNA and protein levels compared to untreated BMSCs.
Several studies have demonstrated important roles of Sirt1 in
macrophages and chondrocyte differentiation (17,18), but
its function role in tenogenic differentiation role has not been
reported.
To comprehensively understand the effect of Sirt1 in
tenogenic differentiation, we performed in vitro western
blot and real-time PCR assays to identify the tenogenic markers
expression of BMSCs cells with over-expression or knockdown of
Sirt1. Our results show that Sirt1 promotes cell tenogenic
differentiation. Consistently, Sirt1 knockdown decrease that
process of BMSCs cells, implying that Sirt1 functions as mediator
in the tenogenic differentiation. As Sirt1 is a NAD-dependent
deacetylase that removes acety group from proteins and modulating
protein activity, Co-IP assay was further used to reveal that the
PPARγ deacetylation may be the reason for the
differentiation-promoting effect of Sirt1.
PPARγ is mainly known to regulate metabolism, immune
responses and cellular proliferation. Recently, its functional role
in adipocyte differentiation via Sirt1 has been investigated
(38). Here, it is confirmed to have
a similar effect in tenogenic differentiation. The deacetylation
levels of PPARγ by BMP14 treatment was even reduced by
overexpression of Sirt1, indicating that Sirt1 could regulate the
deacetylation of PPARγ in BMSCs differentiation.
BMPs comprise the largest subgroup of the TGF
superfamily and also known to activate both Smad pathways and MAPK
pathways (47,48). But in our study only the
phosphorylation of JNK and Smad1 were observed, indicating that ERK
and Smad2/3 are not involved in this process. As previously
reported, Smad and MAPK pathways were downstream of Sirt1, the
activation of Sirt1 could increase the phosphorylation of Smad and
MAPK pathway (49,50). Taken together with the observation
that BMP14 can activate Sirt1 and deacetylate PPARγ, we hypothesis
that BMP14 decreased the acetylation of PPARγ through Smad and JNK
pathway. Interestingly, the treatment with Smad inhibitor
LDN-193189 or JNK inhibitor SP600125 could increase the PPARγ
acetylation, and affect the Sirt1 regulated tenogenic
differentiation markers expression. We hypothesize that, in BMSCs,
BMP14 induced the deacetylation of PPARγ through activation of
Sirt1-Smad1/JNK pathway, deacetylated PPARγ further promote the
tendon differentiation of BMSCs.
In 2004, Wang et al (51) noted that the in vitro
applications of BMP-14 induced cellular activation/proliferation in
primary intervertebral discs (IVD) cells, promoting collagen
synthesis. But higher doses produced inflammatory reactions,
indicating the positive effect of GDF-5 may depend on dosing, and
that multiple treatments can cause side effect like catabolic and
inflammation (51). It has been
shown that growth factors such as TGF-β, insulin-like growth
factor-1 (IGF-1) and BMP2 can induce differentiation of BMSCs into
chondrocytes under appropriate conditions, and has been
successfully applied to tissue engineering and transgenic
technology to help damage the repair of cartilage (52,53).
BMP14 is one of the important regulators of cartilage formation and
skeletal development in the body. It can promote the proliferation,
adhesion, aggregation and differentiation of articular chondrocytes
(ACS) and mesenchymal cartilage cells (MSCs) in the early stage of
cartilage formation (54). The high
cell induced by BMP14 form micromass, which mimics prechondrogenic
cellular condensation which occurs during embryonic development,
and is widely used as a favorable environment for chondrogenesis
(55).
Taken together, our study demonstrated BMP14 as a
potent tenogenic differentiation cue, shows the potential to induce
BMSCs to form TF-like cells, and further elucidated the signaling
pathway leading to tenogenesis of BMSCs. These findings provide a
cellular and molecular basis for developing novel therapeutic
strategies for tendon healing, and throw light on the usage of
BMP14 in the clinical application, despite the side effect and risk
need further investigation.
Acknowledgements
The authors would like to thank Dr. Qi Zhang (China
Pharmaceutical University, Nanjing, China) for their help with the
isolation and identification of BMSCs from rats, and also Dr.
Mingming Chen (Jingchu Center Hospital Affiliated to the Institute
of Technology, Jingmen, China) for proof-reading and editing the
manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DW and AL performed the immunoblotting and
luciferase analysis, analyzed the results and wrote part of the
manuscript. MT performed RT-qPCR and wrote part of the manuscript.
WH supervised the study, analyzed the luciferase assays and wrote
part of the manuscript. PH and XJ designed and directed the
experiments and wrote the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal protocols were approved by the
Institutions Animal Care and Use Committee of Jingchu Institute of
Technology (Hubei, China) and complied with the guidelines of the
Jingchu Institute of Technology's Regulations for Animal
Experiments.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Furumatsu T, Shimamura Y and Nishida K:
Analysis of musculoskeletal systems and their diseases. Pathology
and treatment for injuries of the tendon and ligament. Clin
Calcium. 25:1205–1211. 2015.PubMed/NCBI
|
|
2
|
Omae H, Sun YL, An KN, Amadio PC and Zhao
C: Engineered tendon with decellularized xenotendon slices and bone
marrow stromal cells: An in vivo animal study. J Tissue Eng Regen
Med. 6:238–244. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gaspar D, Spanoudes K, Holladay C, Pandit
A and Zeugolis D: Progress in cell-based therapies for tendon
repair. Adv Drug Deliv Rev. 84:240–256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hankemeier S, Keus M, Zeichen J,
Jagodzinski M, Barkhausen T, Bosch U, Krettek C and Van Griensven
M: Modulation of proliferation and differentiation of human bone
marrow stromal cells by fibroblast growth factor 2: Potential
implications for tissue engineering of tendons and ligaments.
Tissue Eng. 11:41–49. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen HS, Chen YL, Harn HJ, Lin JS and Lin
SZ: Stem cell therapy for tendon injury. Cell Transplant.
22:677–684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao C, Chieh HF, Bakri K, Ikeda J, Sun
YL, Moran SL, An KN and Amadio PC: The effects of bone marrow
stromal cell transplants on tendon healing in vitro. Med Eng Phys.
31:1271–1275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yin Z, Guo J, Wu TY, Chen X, Xu LL, Lin
SE, Sun YX, Chan KM, Ouyang H and Li G: Stepwise differentiation of
mesenchymal stem cells augments tendon-like tissue formation and
defect repair in vivo. Stem Cells Transl Med. 5:1106–1116. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hotten GC, Matsumoto T, Kimura M, Bechtold
RF, Kron R, Ohara T, Tanaka H, Satoh Y, Okazaki M, Shirai T, et al:
Recombinant human growth/differentiation factor 5 stimulates
mesenchyme aggregation and chondrogenesis responsible for the
skeletal development of limbs. Growth Factors. 13:65–74. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rickert M, Wang H, Wieloch P, Lorenz H,
Steck E, Sabo D and Richter W: Adenovirus-mediated gene transfer of
growth and differentiation factor-5 into tenocytes and the healing
rat Achilles tendon. Connect Tissue Res. 46:175–183. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shen H, Gelberman RH, Silva MJ,
Sakiyama-Elbert SE and Thomopoulos S: BMP12 induces tenogenic
differentiation of adipose-derived stromal cells. PLoS One.
8:e776132013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nochi H, Sung JH, Lou J, Adkisson HD,
Maloney WJ and Hruska KA: Adenovirus mediated BMP-13 gene transfer
induces chondrogenic differentiation of murine mesenchymal
progenitor cells. J Bone Miner Res. 19:111–122. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rickert M: BMP-14 gene therapy increases
tendon tensile strength in a rat model of achilles tendon injury. J
Bone Joint Surg Am. 90:445–446. 2008.PubMed/NCBI
|
|
13
|
Ozasa Y, Gingery A, Thoreson AR, An KN,
Zhao C and Amadio PC: A comparative study of the effects of growth
and differentiation factor 5 on muscle-derived stem cells and bone
marrow stromal cells in an in vitro tendon healing model. J Hand
Surg Am. 39:1706–1713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayashi M, Zhao C, An KN and Amadio PC:
The effects of growth and differentiation factor 5 on bone marrow
stromal cell transplants in an in vitro tendon healing model. J
Hand Surg Eur Vol. 36:271–279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qu Y, Zhang J, Wu S, Li B, Liu S and Cheng
J: SIRT1 promotes proliferation and inhibits apoptosis of human
malignant glioma cell lines. Neurosci Lett. 525:168–172. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takeda-Watanabe A, Kitada M, Kanasaki K
and Koya D: SIRT1 inactivation induces inflammation through the
dysregulation of autophagy in human THP-1 cells. Biochem Biophys
Res Commun. 427:191–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park SY, Lee SW, Kim HY, Lee SY, Lee WS,
Hong KW and Kim CD: SIRT1 inhibits differentiation of monocytes to
macrophages: Amelioration of synovial inflammation in rheumatoid
arthritis. J Mol Med (Berl). 94:921–931. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Buhrmann C, Busch F, Shayan P and
Shakibaei M: Sirtuin-1 (SIRT1) is required for promoting
chondrogenic differentiation of mesenchymal stem cells. J Biol
Chem. 289:22048–22062. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Joe IS, Jeong SG and Cho GW:
Resveratrol-induced SIRT1 activation promotes neuronal
differentiation of human bone marrow mesenchymal stem cells.
Neurosci Lett. 584:97–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Q, Lu H and Yang H: Chitosan prevents
adhesion during rabbit flexor tendon repair via the sirtuin 1
signaling pathway. Mol Med Rep. 12:4598–4603. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao C, Lu S, Kivlin R, Wallin B, Card E,
Bagdasarian A, Tamakloe T, Wang WJ, Song X, Chu WM, et al: SIRT1
confers protection against UVB- and H2O2-induced cell death via
modulation of p53 and JNK in cultured skin keratinocytes. J Cell
Mol Med. 13:3632–3643. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zerr P, Palumbo-Zerr K, Huang J, Tomcik M,
Sumova B, Distler O, Schett G and Distler JH: Sirt1 regulates
canonical TGF-β signalling to control fibroblast activation and
tissue fibrosis. Ann Rheum Dis. 75:226–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen W, Zhang L, Shao SX, Wang HP, Cui SJ,
Zhang YN, Kong XZ, Yin Q and Zhang JP: Transcription factors GATA4
and TBX5 promote cardiomyogenic differentiation of rat bone marrow
mesenchymal stromal cells. Histol Histopathol. 30:1487–1498.
2015.PubMed/NCBI
|
|
24
|
Li ZW, Piao CD, Sun HH, Ren XS and Bai YS:
Asiatic acid inhibits adipogenic differentiation of bone marrow
stromal cells. Cell Biochem Biophys. 68:437–442. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Modica S and Moschetta A: Nuclear bile
acid receptor FXR as pharmacological target: Are we there yet? FEBS
Lett. 580:5492–5499. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boufker Id H, Lagneaux L, Fayyad-Kazan H,
Badran B, Najar M, Wiedig M, Ghanem G, Laurent G, Body JJ and
Journé F: Role of farnesoid X receptor (FXR) in the process of
differentiation of bone marrow stromal cells into osteoblasts.
Bone. 49:1219–1231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Evans RM: The steroid and thyroid hormone
receptor superfamily. Science. 240:889–895. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jia S, Liu X, Li W, Xie J, Yang L and Li
L: Peroxisome proliferator-activated receptor gamma negatively
regulates the differentiation of bone marrow-derived mesenchymal
stem cells toward myofibroblasts in liver fibrogenesis. Cell
Physiol Biochem. 37:2085–2100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Evans JF, Rodriguez S and Ragolia L: ACTH
promotes chondrogenic nodule formation and induces transient
elevations in intracellular calcium in rat bone marrow cell
cultures via MC2-R signaling. Cell Tissue Res. 352:413–425. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jin M, Chen Y, Zhou Y, Mei Y, Liu W, Pan C
and Hua X: Transplantation of bone marrow-derived mesenchymal stem
cells expressing elastin alleviates pelvic floor dysfunction. Stem
Cell Res Ther. 7:512016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Andersen CL, Jensen JL and Ørntoft TF:
Normalization of real-time quantitative reverse transcription-PCR
data: A model-based variance estimation approach to identify genes
suited for normalization, applied to bladder and colon cancer data
sets. Cancer Res. 64:5245–5250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Méndez-Ferrer S, Michurina TV, Ferraro F,
Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A,
Enikolopov GN and Frenette PS: Mesenchymal and haematopoietic stem
cells form a unique bone marrow niche. Nature. 466:829–834. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo KT, SchAfer R, Paul A, Gerber A,
Ziemer G and Wendel HP: A new technique for the isolation and
surface immobilization of mesenchymal stem cells from whole bone
marrow using high-specific DNA aptamers. Stem Cells. 24:2220–2231.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schweitzer R, Chyung JH, Murtaugh LC,
Brent AE, Rosen V, Olson EN, Lassar A and Tabin CJ: Analysis of the
tendon cell fate using Scleraxis, a specific marker for tendons and
ligaments. Development. 128:3855–3866. 2001.PubMed/NCBI
|
|
35
|
Shukunami C, Takimoto A, Oro M and Hiraki
Y: Scleraxis positively regulates the expression of tenomodulin, a
differentiation marker of tenocytes. Dev Biol. 298:234–247. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Docheva D, Hunziker EB, Fässler R and
Brandau O: Tenomodulin is necessary for tenocyte proliferation and
tendon maturation. Mol Cell Biol. 25:699–705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Muraglia A, Cancedda R and Quarto R:
Clonal mesenchymal progenitors from human bone marrow differentiate
in vitro according to a hierarchical model. J Cell Sci.
113:1161–1166. 2000.PubMed/NCBI
|
|
38
|
Qiang L, Wang L, Kon N, Zhao W, Lee S,
Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR and Accili D: Brown
remodeling of white adipose tissue by SirT1-dependent deacetylation
of Pparγ. Cell. 150:620–632. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoshida Y, Tanaka S, Umemori H, Minowa O,
Usui M, Ikematsu N, Hosoda E, Imamura T, Kuno J, Yamashita T, et
al: Negative regulation of BMP/Smad signaling by Tob in
osteoblasts. Cell. 103:1085–1097. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Celil AB and Campbell PG: BMP-2 and
insulin-like growth factor-I mediate Osterix (Osx) expression in
human mesenchymal stem cells via the MAPK and protein kinase D
signaling pathways. J Biol Chem. 280:31353–31359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rodeo SA, Sugiguchi F, Fortier LA,
Cunningham ME and Maher S: What's new in orthopaedic research. J
Bone Joint Surg Am. 96:2015–2019. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kan A, Ikeda T, Fukai A, Nakagawa T,
Nakamura K, Chung UI, Kawaguchi H and Tabin CJ: SOX11 contributes
to the regulation of GDF5 in joint maintenance. BMC Dev Biol.
13:42013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kadomatsu H, Matsuyama T, Yoshimoto T,
Negishi Y, Sekiya H, Yamamoto M and Izumi Y: Injectable
growth/differentiation factor-5-recombinant human collagen
composite induces endochondral ossification via Sry-related HMG box
9 (Sox9)expression and angiogenesis in murine calvariae. J
Periodontal Res. 43:483–489. 2008.PubMed/NCBI
|
|
44
|
Oshin AO, Caporali E, Byron CR, Stewart AA
and Stewart MC: Phenotypic maintenance of articular chondrocytes in
vitro requires BMP activity. Vet Comp Orthop Traumatol. 20:185–191.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Aspenberg P: Stimulation of tendon repair:
Mechanical loading, GDFs and platelets. A mini-review. Int Orthop.
31:783–789. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hinoi E, Nakamura Y, Takada S, Fujita H,
Iezaki T, Hashizume S, Takahashi S, Odaka Y, Watanabe T and Yoneda
Y: Growth differentiation factor-5 promotes brown adipogenesis in
systemic energy expenditure. Diabetes. 63:162–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guzman A, Zelman-Femiak M, Boergermann JH,
Paschkowsky S, Kreuzaler PA, Fratzl P, Harms GS and Knaus P: SMAD
versus non-SMAD signaling is determined by lateral mobility of bone
morphogenetic protein (BMP) receptors. J Biol Chem.
287:39492–39504. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sieber C, Kopf J, Hiepen KC and Knaus P:
Recent advances in BMP receptor signaling. Cytokine & Growth
Factor Reviews. 20:343–355. 2009. View Article : Google Scholar
|
|
49
|
Becatti M, Fiorillo C, Barygina V, Cecchi
C, Lotti T, Prignano F, Silvestro A, Nassi P and Taddei N: SIRT1
regulates MAPK pathways in vitiligo skin: insight into the
molecular pathways of cell survival. J Cell Mol Med. 18:514–529.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gao P, Xu TT, Lu J, Li L, Xu J, Hao DL,
Chen HZ and Liu DP: Overexpression of SIRT1 in vascular smooth
muscle cells attenuates angiotensin II-induced vascular remodeling
and hypertension in mice. J Mol Med (Berl). 92:347–357. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang H, Kroeber M, Hanke M, Ries R, Schmid
C, Poller W and Richter W: Release of active and depot GDF-5 after
adenovirus-mediated overexpression stimulates rabbit and human
intervertebral disc cells. J Mol Med (Berl). 82:126–134. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cucchiarini M and Madry H: Overexpression
of human IGF-I via direct rAAV-mediated gene transfer improves the
early repair of articular cartilage defects in vivo. Gene Ther.
21:811–819. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tekari A, Luginbuehl R, Hofstetter W and
Egli RJ: Transforming growth factor beta signaling is essential for
the autonomous formation of cartilage-like tissue by expanded
chondrocytes. PLoS One. 10:e01208572015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Murphy MK, Huey DJ, Hu JC and Athanasiou
KA: TGF-β1, GDF-5, and BMP-2 stimulation induces chondrogenesis in
expanded human articular chondrocytes and marrow-derived stromal
cells. Stem Cells. 33:762–773. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Solursh M: Cell-cell interactions and
chondrogenesisCartilage: Development, Differentiation, and Growth.
Hall BK: 2. Academic Press; New York, NY: pp. 121–141. 1983
|