Introduction
Glioblastoma multiforme (GBM) originates from glial
cells and is the most prevalent and critical form of brain cancer
(1). GBM cells are characterized by
their propensity to invade surrounding brain tissue adjacent to the
main tumor mass (2). Although
surgical resection combined with radiotherapy and/or chemotherapy
is currently used as a standard treatment for GBM, the median
survival rate of patients with GBM is <15 months (3,4). Tumor
recurrence, as a primary reason for the high mortality rate of GBM
patients, is principally caused by residual tumor cells that remain
within tissues following various treatments (5). Cellular apoptosis is negatively
correlated with cell growth, and the inhibition of cell apoptosis
is primarily responsible for cancer cell survival. Therefore,
induction of apoptosis is considered to be a potential therapeutic
strategy for the eradication or suppression of cancer cell growth
and tumor progression (6,7). However, the mechanism responsible for
regulating cancer cell survival in GBM remains unknown and warrants
investigation.
Results suggest that insulin-like growth factor 1
(IGF1) and its receptor tyrosine kinase [insulin-like growth factor
1 receptor (IGF1R) are key regulators in the development and
progression of cancer (8). Previous
studies have demonstrated that the IGF1/IGF1R pathway is involved
in regulating tumorigenesis, cell growth and metastasis in many
cancers, and that some physiological functions, including cell
cycle progression, apoptosis and differentiation, are associated
with the IGF1/IGF1R pathway (9,10). In
colon cancer cells, it has also been documented that activation of
IGF1/IGF1R induced by incubation with tumor necrosis factor lead to
cellular resistance to apoptosis (11). Furthermore, inhibition of the
IGF1/IGF1R pathway may reduce tumorigenesis and attenuate tumor
progression through the induction of apoptosis in cancer cells
(12). However, the role of
IGF1/IGF1R signaling in the survival of GBM cells and the
corresponding molecular mechanisms are not well understood.
In the present study, it was observed that exogenous
IGF1 or overexpression of IGF1R protected against apoptosis and
promoted the survival of GBM cells. Mechanistic assays also
indicated that the inhibitory effects of IGF1/IGF1R on GBM
apoptosis were mediated by the phosphoinositide 3-kinase
(PI3K)/protein kinase B (AKT) pathway. These results suggest that
targeting of IGF1/IGF1R signaling may aid to improve the efficacy
of treatments for GBM.
Materials and methods
Cell culture
The glioblastoma cell line U87 was obtained from the
American Type Culture Collection (Manassas, VA, USA). U87 cells
were maintained in an incubator at 37 °C and 5% CO2. The
cells were cultured in Dulbecco's modified Eagle's medium (DMEM)
with 10% fetal bovine serum (FBS) and 105 U/l
penicillin-streptomycin (all from Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) for three days.
Cell viability assay
U87 cells were seeded into 96-well plates in DMEM
with 10% FBS at a density of 5×103 cells/well and
incubated at 37°C for 12 h. Cells were then treated at 37 °C with
200 µM H2O2 (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), 2.5, 5, 10 and 20 ng exogenous IGF (PeproTech,
Rocky Hill, NJ, USA), 10 µM wortmannin (Beyotime Institute of
Biotechnology, Haimen, China) and 2 µg IGF1R plasmid (Addgene,
Inc., Cambridge MA, USA) for 48 h (13). U87 cells cultured in 10% FBS DMEM
treated with PBS were used as control. Following treatment, cell
proliferation was assessed using a Cell Counting Kit 8 (CCK8) Cell
Proliferation Assay kit (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's instructions. Briefly, CCK8
reagent (5 µl) was added to each well and incubated at 37 °C for 2
h, and the number of viable cells was calculated from absorbance
measurements at 450 nm.
Lactate dehydrogenase (LDH) release
assay
The release of LDH from U87 cells cultured in 10%
FBS DMEM was measured to determine cell viability according to the
manufacturer's protocol of a CytoTox-One™ Homogenous Membrane
Integrity Assay kit (Promega Corporation, Madison, WI, USA). Cells
were seeded at a density of 5,000 cells per well. Fluorescence
(excitation at 480 nm; emission maximum at 560–590 nm) was assessed
using a Spectramax Gemini XS Microplate Spectrofluorometer
(Molecular Devices LLC, Sunnyvale, CA, USA). The cytotoxicity of
treated U87 cells was calculated as the relative increase in LDH
compared with that of untreated control cells.
Caspase-3 activity assay
A total of 5×106 U87 cells were lysed in
caspase buffer [10 mM Tris-HCl, 10 mM
NaH2PO4, 130 mM NaCl, 1% (v/v) Triton X-100],
and extracts were clarified by centrifugation at 16,099 × g for 10
min at 4 °C. Protein concentration was assessed using the Bradford
Protein Assay kit (Beyotime Institute of Biotechnology), and
caspase activity was assayed using a fluorometric method with a
luminescence spectrometer (Sinergy HT; BioTek Instruments, Inc.,
Winooski, VT, USA) at 460 nm as previously described (14). After measuring the concentrations,
Acetyl tetrapeptide coupled to 4-methycoumaryl-7-amide (Ac-DEVD-AMC
substrate; PeptaNova GmdH, Sandhausen, Germany), used as a
fluorogenic substrate for caspase-3, was mixed with the extracts
for ~4 h at 37 °C. Caspase activity was calculated as absorbance/µg
protein.
Western blot analysis
A total of 5×106 U87 cells were lysed in
extraction buffer (Tris 50 mM, pH 7.4, NaCl 150 mM, Triton X-100
1%, EDTA 1 mM, and PMSF 2 mM). Extracts were clarified by
centrifugation at 16,099 × g for 10 min at 4 °C, and protein
concentrations were determined using a Bradford Protein Assay kit
(Beyotime Institute of Biotechnology). A total of 50 µg cell
protein was loaded per lane of a 12% SDS PAGE gel for separation by
electrophoresis. Separated proteins were then transblotted onto
nitrocellulose membranes (Whatman, Inc., Florham Park, NJ, USA) and
immunoprobed with primary antibodies against IGF1R (ab39675; Abcam,
Cambridge, MA, USA; 1:1,000), Fas ligand (sc-6237), B-cell lymphoma
2 (Bcl-2; sc-492), Akt (sc-1618), phosphorylated (p)-Akt (sc-33437)
and beta-actin (sc-1616; Santa Cruz Biotechnology, Inc., Dallas,
CA, USA; 1:8,000 for beta-actin and 1:500 for the remaining
antibodies) at 4°C overnight. The blots were incubated with
secondary antibodies of horseradish peroxidase conjugated (HRP)
mouse anti-goat IgG (sc-2354) or mouse anti-rabbit IgG-HRP
(sc-2357; both Santa Cruz Biotechnology, Inc.; 1:5,000) at room
temperature for 1 h, then reacted with an enhanced
chemiluminescence substrate (Pierce; Thermo Fisher Scientific,
Inc.). Resulting band intensities were recorded on an X-ray film.
Quantity One software v4.62 (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) was utilized to assess the protein bands, and all
experiments were repeated three times.
Statistical analysis
Statistical analysis was performed using SPSS
version 13.0 software (SPSS Inc., Chicago, IL, USA). Data were
expressed as the mean ± standard error of the mean of three
independent experiments, and a Student's t-test was used to
determine the statistical significance of results. Differences were
considered to be significant when P<0.05.
Results
IGF1 promotes survival and suppresses
apoptosis in GBM cells
To detect the role of IGF1 in the survival of GBM
cells, 200 µM H2O2 was used to establish a
cell apoptotic model. Different concentrations of IGF1 (2.5, 5, 10
and 20 ng) were initially administered to determine the optimal
concentration of IGF1 for subsequent experiments in U87 cells.
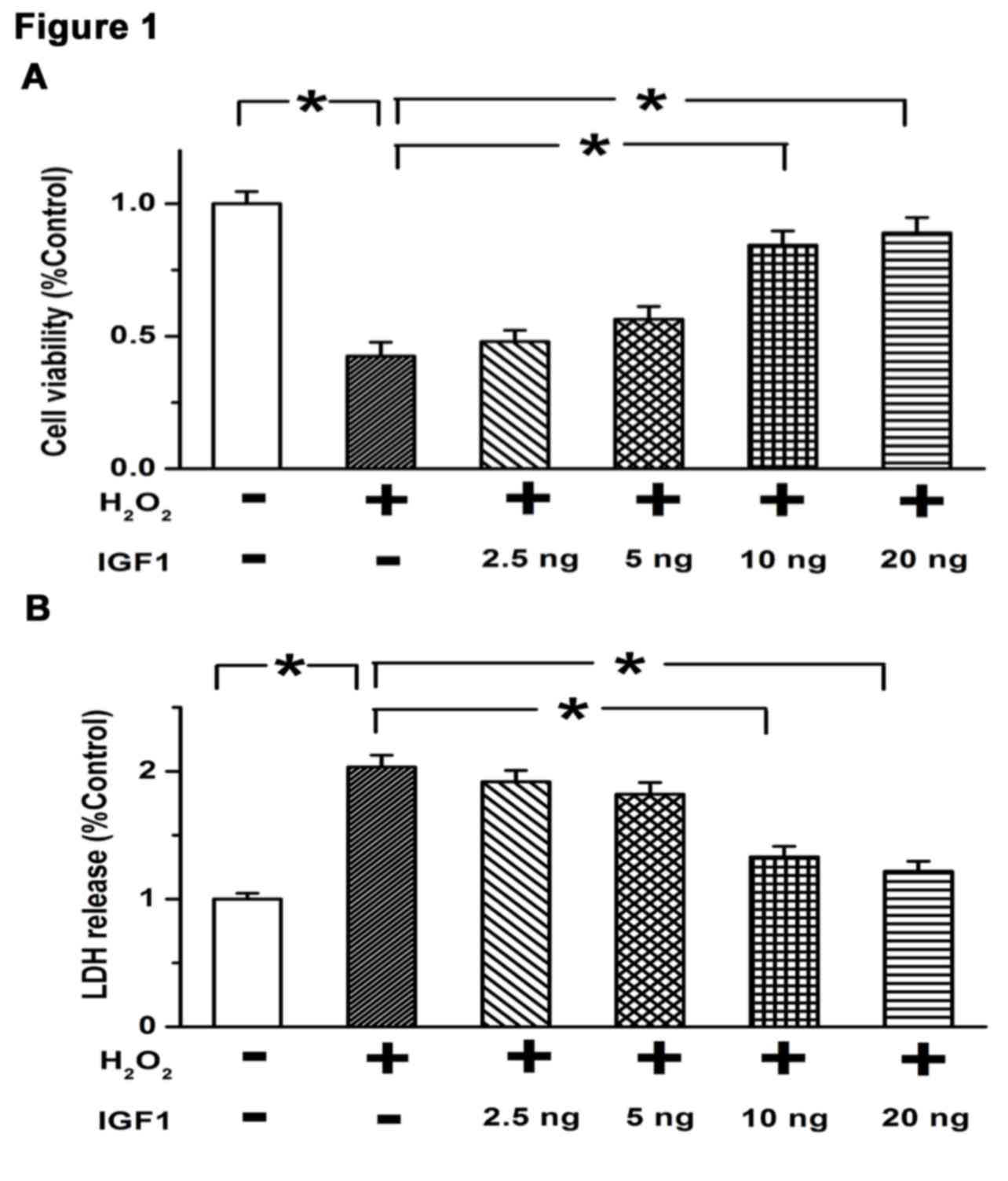
Results of a CCK8 assay indicated that treatment with
H2O2 significantly decreased the viability of
U87 cells compared with controls (P<0.05; Fig. 1A), while IGF1 concentrations of 10
and 20 ng significantly restored cell viability (both P<0.05;
Fig. 1A). Similarly, a cell death
assay demonstrated that LDH release was markedly enhanced by 200 µM
H2O2, while 10 and 20 ng IGF1 significantly
attenuated the release of LDH induced by H2O2
(both P<0.05; Fig. 1B). These
results suggest that IGF1 promotes cell growth and inhibits cell
death at doses of ≥10 ng, indicating that the optimal dose of IGF1
in regulating the survival of GBM cells is 10 ng.
IGF1 inhibits the extrinsic and
intrinsic pathways of apoptosis
To identify the role of IGF1 in apoptosis, it was
determined whether the intrinsic and/or extrinsic pathways of
apoptosis were affected by IGF1. Key molecules in each pathway were
detected in response to IGF1 treatment. Caspse-3, a final executor
of cell apoptosis, is activated by various stimuli in the process
of cell apoptosis (15). Expression
of Fas ligand is specifically increased during the extrinsic
pathway of apoptosis, and Bcl-2, as an anti-apoptotic protein
localized to the outer mitochondrial membrane, is involved in the
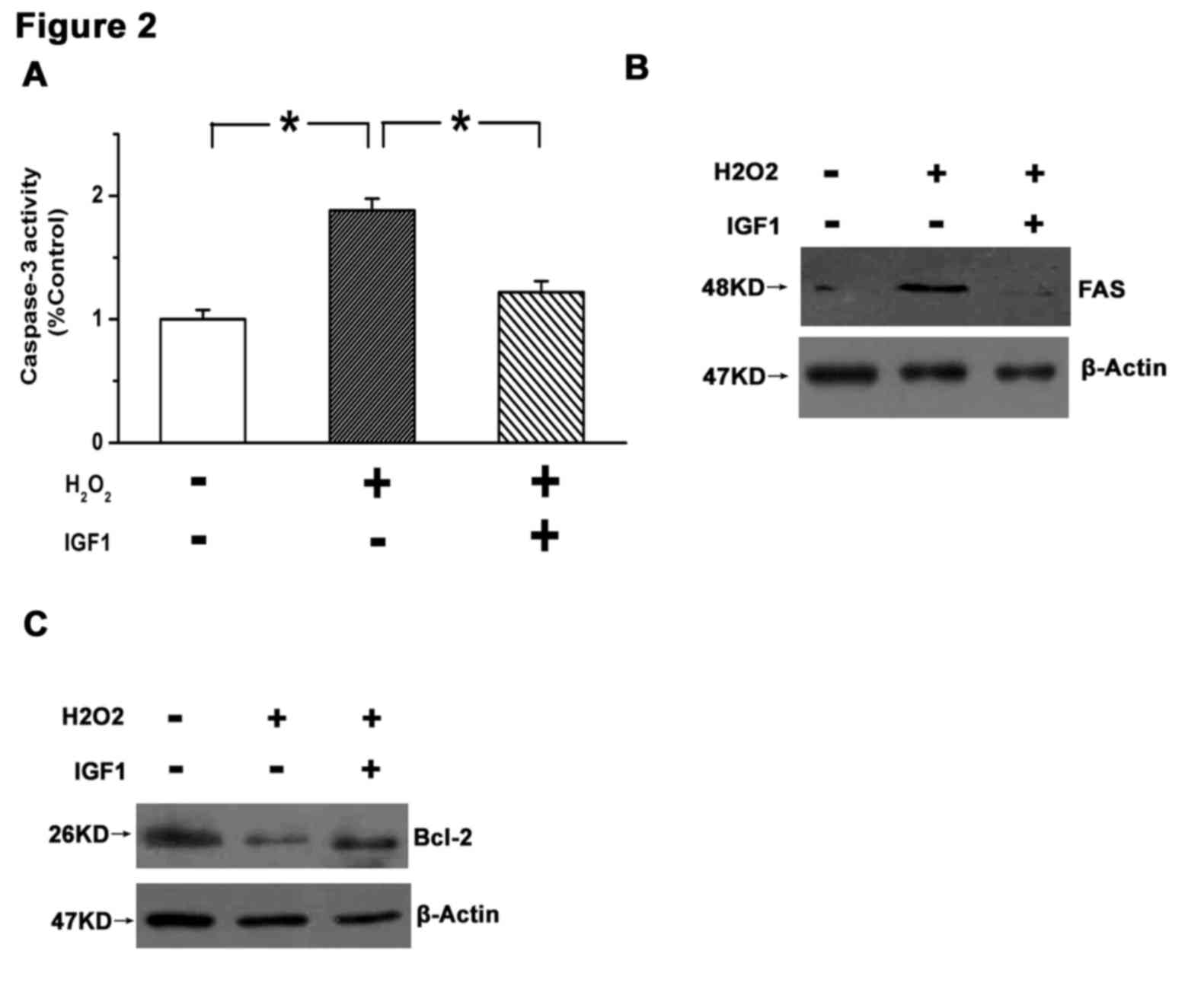
intrinsic pathway of cell apoptosis (15). The present results demonstrated that
caspase-3 was significantly activated in U87 cells following
H2O2 treatment (P<0.05 vs. control), and
that this effect was significantly attenuated by 10 ng IGF1
(P<0.05; Fig. 2A). Furthermore,
H2O2 increased the expression of Fas and
decreased the expression of Bcl-2, while treatment with IGF1
reversed these changes (Fig. 2B and
C). These results indicate that IGF1 may promote the survival
of GBM cells.
Overexpression of IGF1R inhibits
apoptosis and promotes survival in GBM cells
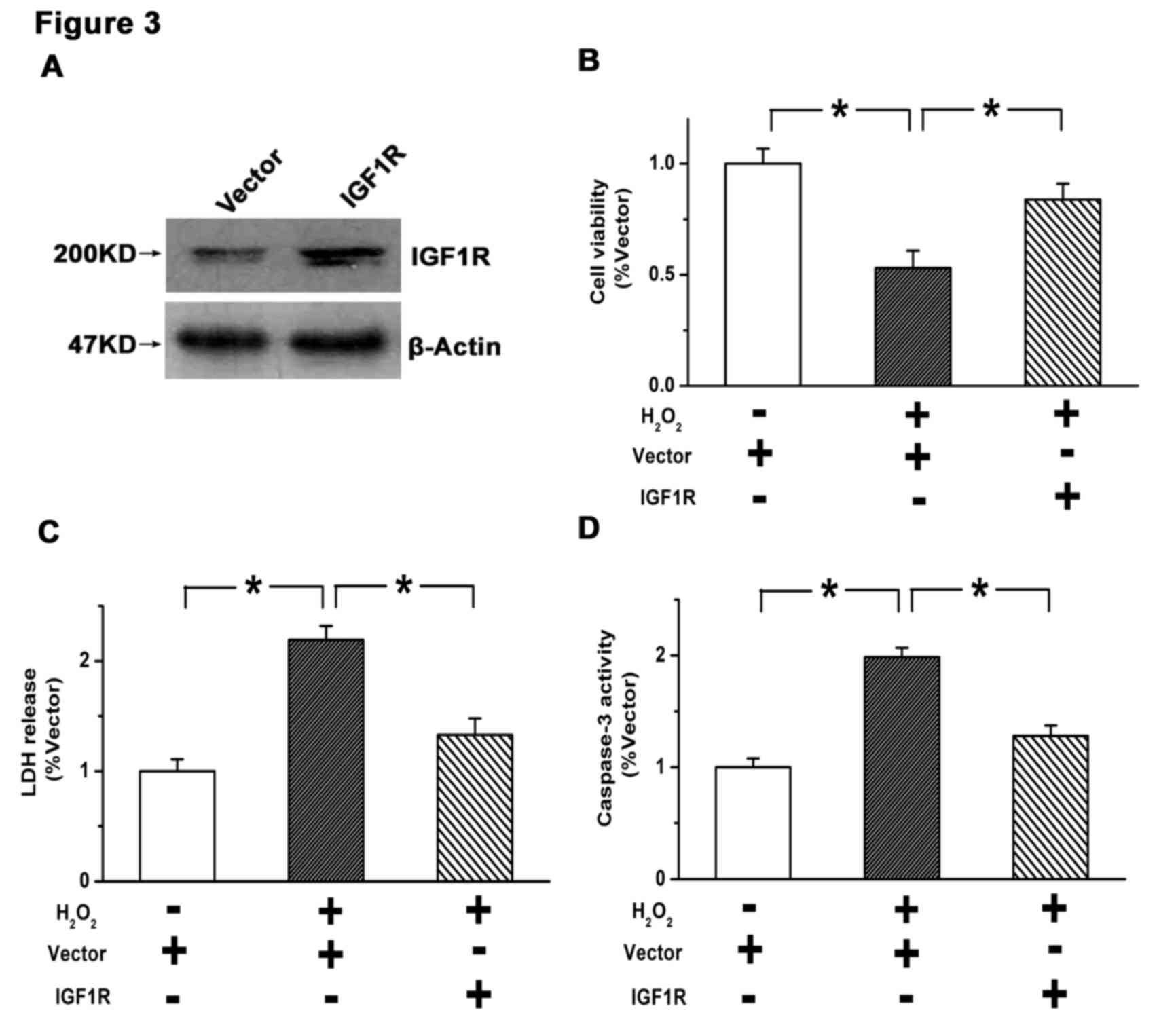
Stable overexpression of IGF1R was established in
U87 cells by lentiviral infection. As depicted in Fig. 3A, the efficiency of IGF1R
overexpression was verified by western blot analysis. A subsequent
CCK8 assay indicated that the viability of U87 cells was
significantly suppressed by H2O2 treatment
(P<0.05 vs. control), which was significantly attenuated by
overexpression of IGF1R (P<0.05; Fig.
3B). Similarly, the elevated rate of cell death induced by
H2O2 (P<0.05 vs. control) was
significantly reduced by overexpression of IGF1R (P<0.05), as
determined by an LDH release assay (Fig.
3C). Furthermore, the increased activity of caspase-3 induced
by H2O2 (P<0.05 vs. control) was
significantly reduced by IGF1R overexpression (P<0.05; Fig. 3D). These results suggest that
activation of the IGF1/IGF1R pathway suppresses apoptosis and
promotes survival in GBM cells.
IGF1/IGF1R inhibits cell apoptosis by
activating the PI3K/AKT pathway
The PI3K/AKT pathway is a critical pathway in the
regulation of cell apoptosis. Therefore, it was determined whether
a regulatory relationship existed between IGF1/IGF1R and the
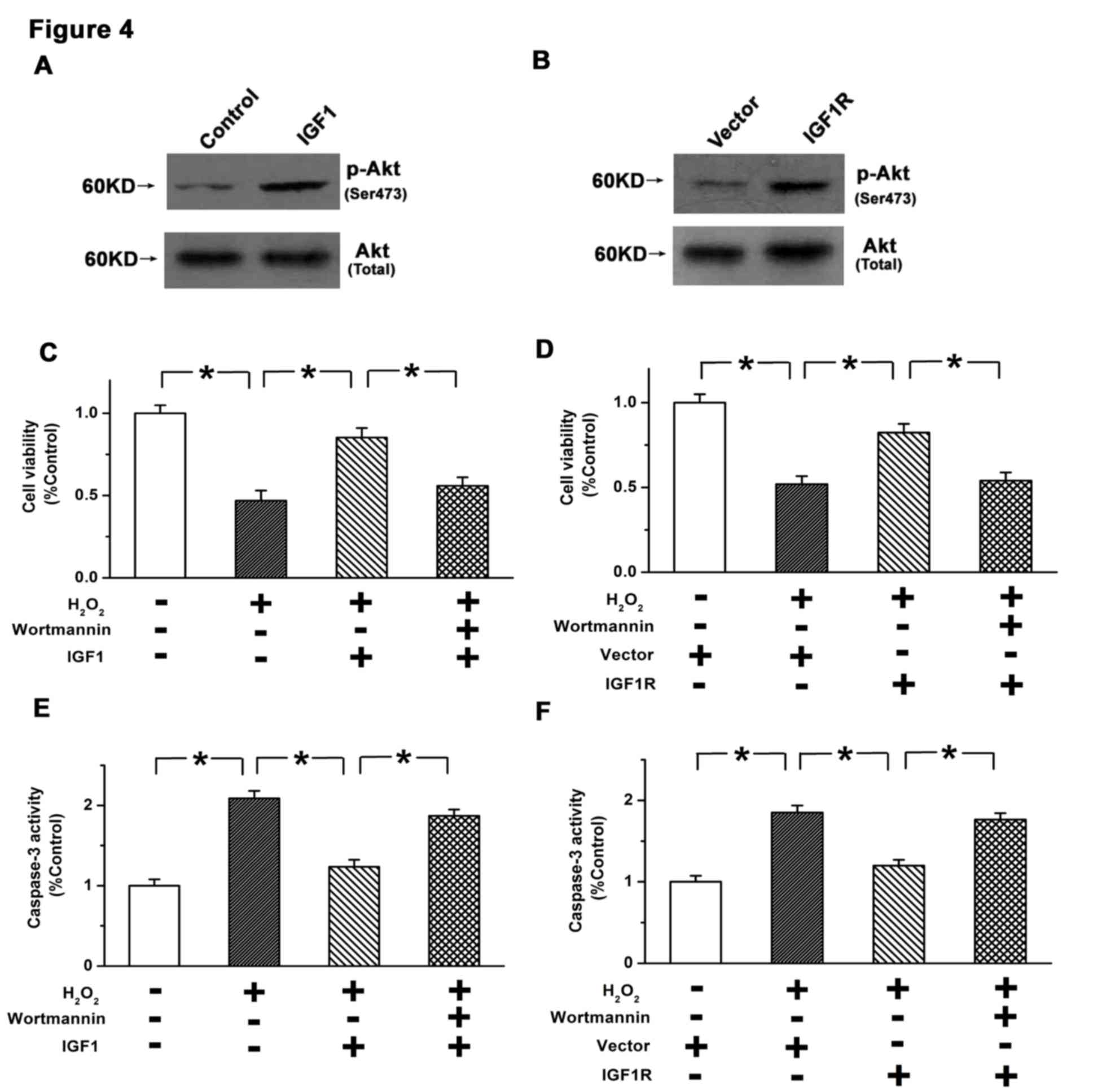
PI3K/AKT pathway in U87 cells. As depicted in Fig. 4A and B, it was observed that both
exogenous IGF1 treatment and overexpression of IGF1R induced the
phosphorylation of AKT. Furthermore, wortmannin, as an inhibitor of
the PI3K/AKT pathway (16), was used
to determine the role of PI3K/AKT in the inhibition of cell
apoptosis by IGF1/IGF1R. It was observed that the increases in cell
viability induced by exogenous IGF1 or IGF1R overexpression were
significantly reversed by 10 µM wortmannin (P<0.05; Fig. 4C and D). Furthermore, inhibition of
caspase-3 activation by IGF1/IGF1R was significantly attenuated by
wortmannin (P<0.05; Fig. 4E and
F). Therefore, the inhibitory effects of IGF1/IGF1R on GBM
apoptosis may be mediated by PI3K/AKT signaling.
Discussion
It has been established that the progression and
development of glioblastoma is primarily due to the overgrowth of
cancer cells, determined by the balance between the growth and
death of cells (17). Previous
results have indicated that a dysfunction in cell apoptosis may be
responsible for the initiation of many human diseases, including
glioblastoma (18). Inhibition of
cell apoptosis is frequently observed in the tissues of
glioblastoma patients, and thus the regulation of cell apoptosis is
considered to be a potential strategy in preventing the progression
of glioblastoma (19). Therefore,
studies into the regulatory mechanisms of apoptosis in GBM cells
are required to improve the efficacy of molecular therapy. In the
present study, it was observed that IGF1/IGF1R protected against
apoptosis and promoted the survival of GBM cells, and that the
regulatory effects of IGF1/IGF1R on apoptosis were potentially
mediated by PI3K/AKT signaling.
Previous studies have demonstrated that IGF1R, as a
key regulator of mitogenesis and tumorigenicity, participates in
regulating cell transformation, cell growth and cell cycle
progression (20,21). IGF1 is a specific ligand of IGF1R,
and upon binding to IGF1R, activates a cascade of intracellular
molecular pathways, which may occur during the development and
progression of a number of diseases, including cancer (22). IGF1/IGF1R and downstream targets are
important in the regulation of cell transformation (23), invasion (24), tumor metastasis (25) and cell survival (9). Although overexpression of IGF1 and
IGF1R has been observed in a subset of cancer tissues from patients
with GBM (26), the consequences of
IGF1/IGF1R overexpression on the survival of GBM cells and the
corresponding molecular mechanisms are not well understood. In the
present study, it was observed that IGF1 promoted cell survival and
suppressed cell death at doses of ≥10 ng. Furthermore, both
exogenous IGF1 and IGF1R overexpression inhibited the intrinsic and
extrinsic pathways of apoptosis in GBM cells. These results
indicated that activation of IGF1/IGF1R promoted the survival of
GBM cells by inhibiting apoptosis.
The PI3K/AKT pathway, as a critical pro-survival
signaling pathway, modulates cell growth, proliferation, apoptosis
and survival in a number of cell types (27). It has been documented that increased
phosphorylation of AKT at Ser/Thr sites was associated with the
inhibition of cell apoptosis (28).
Moreover, aberrant activation of the PI3K/AKT pathway has been
observed in a number of tumor tissues, including GBM, and
inhibitors of the PI3K/AKT pathway have been investigated as a
potential anticancer therapy (29).
However, the regulatory mechanisms underlying the PI3K/AKT pathway
in the progression of GBM remain unknown. In the present study, it
was demonstrated that IGF1/IGF1R promoted the activation of
PI3K/AKT signaling in GBM cells. Specifically, the results
indicated that both exogenous IGF1 and IGF1R overexpression led to
increased phosphorylation of AKT. Furthermore, the inhibitory
effects of IGF1/IGF1R on the apoptosis of GBM cells were attenuated
by inhibition of the PI3K/AKT pathway. These results suggested that
IGF1/IGF1R promotes the growth and survival of GBM cells, at least
in part through the PI3K/AKT pathway.
In conclusion, the present study indicated that
IGF1/IGF1R promoted cell survival and inhibited cell apoptosis
through activation of the PI3K/AKT pathway. These results have
identified a potential regulatory mechanism underlying the growth
of cancer cells in GBM, and targeting of IGF1/IGF1R may aid to
improve the efficacy of treatment for GBM.
References
|
1
|
Gurney JG and Kadan-Lottick N: Brain and
other central nervous system tumors: Rates, trends, and
epidemiology. Curr Opin Oncol. 13:160–166. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Henriksson R, Asklund T and Poulsen HS:
Impact of therapy on quality of life, neurocognitive function and
their correlates in glioblastoma multiforme: A review. J
Neurooncol. 104:639–646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu HK, Wen PY and Olson JJ: Exciting new advances in
neuro-oncology: The avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stupp R, Hegi ME, Gilbert MR and
Chakravarti A: Chemoradiotherapy in malignant glioma: Standard of
care and future directions. J Clin Oncol. 25:4127–4136. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu L, Nam YJ, Kung G, Crow MT and Kitsis
RN: Induction of the apoptosis inhibitor ARC by Ras in human
cancers. J Biol Chem. 285:19235–19245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Chen D, Qian H, Tsai YS, Shao S,
Liu Q, Dominguez D and Wang Z: The splicing factor RBM4 controls
apoptosis, proliferation, and migration to suppress tumor
progression. Cancer Cell. 26:374–389. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y and Sun Y: Insulin-like growth
factor receptor-1 as an anti-cancer target: Blocking transformation
and inducing apoptosis. Curr Cancer Drug Targets. 2:191–207. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Resnicoff M, Abraham D, Yutanawiboonchai
W, Rotman HL, Kajstura J, Rubin R, Zoltick P and Baserga R: The
insulin-like growth factor I receptor protects tumor cells from
apoptosis in vivo. Cancer Res. 55:2463–2469. 1995.PubMed/NCBI
|
|
10
|
Dupont J and LeRoith D: Insulin and
insulin-like growth factor I receptors: Similarities and
differences in signal transduction. Horm Res. 55 Suppl 2:S22–S26.
2001.
|
|
11
|
Remacle-Bonnet MM, Garrouste FL, Heller S,
André F, Marvaldi JL and Pommier GJ: Insulin-like growth factor-I
protects colon cancer cells from death factor-induced apoptosis by
potentiating tumor necrosis factor alpha-induced mitogen-activated
protein kinase and nuclear factor kappaB signaling pathways. Cancer
Res. 60:2007–2017. 2000.PubMed/NCBI
|
|
12
|
Peruzzi F, Prisco M, Dews M, Salomoni P,
Grassilli E, Romano G, Calabretta B and Baserga R: Multiple
signaling pathways of the insulin-like growth factor 1 receptor in
protection from apoptosis. Mol Cell Biol. 19:7203–7215. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Entingh-Pearsall A and Kahn CR:
Differential roles of the insulin and insulin-like growth factor-I
(IGF-I) receptors in response to insulin and IGF-I. J Biol Chem.
279:38016–38024. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yasuda Y, Saito M, Yamamura T, Yaguchi T
and Nishizaki T: Extracellular adenosine induces apoptosis in
Caco-2 human colonic cancer cells by activating caspase-9/-3 via
A(2a) adenosine receptors. J Gastroenterol. 44:56–65. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma J, Liang S, Wang Z, Zhang L, Jiang J,
Zheng J, Yu L, Zheng X, Wang R and Zhu D: ROCK pathway participates
in the processes that 15-hydroxyeicosatetraenoic acid (15-HETE)
mediated the pulmonary vascular remodeling induced by hypoxia in
rat. J Cell Physiol. 222:82–94. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gopinath S, Alapati K, Malla RR, Gondi CS,
Mohanam S, Dinh DH and Rao JS: Mechanism of p27 upregulation
induced by downregulation of cathepsin B and uPAR in glioma. Mol
Oncol. 5:426–437. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gomez GG, Volinia S, Croce CM, Zanca C, Li
M, Emnett R, Gutmann DH, Brennan CW, Furnari FB and Cavenee WK:
Suppression of microRNA-9 by mutant EGFR signaling upregulates
FOXP1 to enhance glioblastoma tumorigenicity. Cancer Res.
74:1429–1439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sawa H, Murakami H, Kumagai M, Nakasato M,
Yamauchi S, Matsuyama N, Tamura Y, Satone A, Ide W, Hashimoto I and
Kamada H: Histone deacetylase inhibitor, FK228, induces apoptosis
and suppresses cell proliferation of human glioblastoma cells in
vitro and in vivo. Acta Neuropathol. 107:523–531. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ziegler DS, Wright RD, Kesari S, Lemieux
ME, Tran MA, Jain M, Zawel L and Kung AL: Resistance of human
glioblastoma multiforme cells to growth factor inhibitors is
overcome by blockade of inhibitor of apoptosis proteins. J Clin
Invest. 118:3109–3122. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
DeAngelis T, Ferber A and Baserga R:
Insulin-like growth factor I receptor is required for the mitogenic
and transforming activities of the platelet-derived growth factor
receptor. J Cell Physiol. 164:214–221. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coppola D, Ferber A, Miura M, Sell C,
D'Ambrosio C, Rubin R and Baserga R: A functional insulin-like
growth factor I receptor is required for the mitogenic and
transforming activities of the epidermal growth factor receptor.
Mol Cell Biol. 14:4588–4595. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pollak M: Insulin and insulin-like growth
factor signalling in neoplasia. Nat Rev Cancer. 8:915–928. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sell C, Dumenil G, Deveaud C, Miura M,
Coppola D, DeAngelis T, Rubin R, Efstratiadis A and Baserga R:
Effect of a null mutation of the insulin-like growth factor I
receptor gene on growth and transformation of mouse embryo
fibroblasts. Mol Cell Biol. 14:3604–3612. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zeigler ME, Krause S, Karmiol S and Varani
J: Growth factor-induced epidermal invasion of the dermis in human
skin organ culture: Dermal invasion correlated with epithelial cell
motility. Invasion Metastasis. 16:3–10. 1996.PubMed/NCBI
|
|
25
|
Long L, Rubin R, Baserga R and Brodt P:
Loss of the metastatic phenotype in murine carcinoma cells
expressing an antisense RNA to the insulin-like growth factor
receptor. Cancer Res. 55:1006–1009. 1995.PubMed/NCBI
|
|
26
|
Arcaro A: Targeting the insulin-like
growth factor-1 receptor in human cancer. Front Pharmacol.
4:302013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang L, Zhou F and ten Dijke P: Signaling
interplay between transforming growth factor-β receptor and
PI3K/AKT pathways in cancer. Trends Biochem Sci. 38:612–620. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Edwards LA, Thiessen B, Dragowska WH,
Daynard T, Bally MB and Dedhar S: Inhibition of ILK in PTEN-mutant
human glioblastomas inhibits PKB/Akt activation, induces apoptosis,
and delays tumor growth. Oncogene. 24:3596–3605. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shingu T, Yamada K, Hara N, Moritake K,
Osago H, Terashima M, Uemura T, Yamasaki T and Tsuchiya M:
Synergistic augmentation of antimicrotubule agent-induced
cytotoxicity by a phosphoinositide 3-kinase inhibitor in human
malignant glioma cells. Cancer Res. 63:4044–4047. 2003.PubMed/NCBI
|