Introduction
As a common type of arrhythmia, atrial fibrillation
(AF) occurs in 1–2% of individuals (1–3). In
patients with non-valvular AF, the risk of ischemic stroke and
systemic embolism is 5-fold increased compared with that in the
general population (4), leading to a
yearly incidence of ischemic stroke of 5% in patients with
non-valvular AF and 15% in high-risk patients (5). In patients with AF, stroke is mainly
caused by atrial thrombi formed in the left atrial appendage (LAA),
and according to a previous study, ~90% of the thrombi formed in
patients with AF were arising from the LAA (6). According to the European Society of
Cardiology (ESC) guidelines, all AF patients with a high risk of
thrombosis are required to take oral anti-coagulants (OACs) to
prevent thromboembolic events (7).
Although it is well established that patients with AF at a high
risk of stroke benefit from warfarin or the recently introduced
novel (N)OACs, these medications have several disadvantages,
including severe hemorrhage and non-compliance (8–10). While
catheter ablation (CA) for the treatment of AF is recommended by
the 2016 ESC guidelines and is efficacious in rhythm control, its
long-term efficacy is poor, and its role in stroke prevention
remains unproven. Randomized clinical trials have demonstrated that
percutaneous mechanical LAA occlusion (LAAO) is effective in
preventing thromboembolism in AF patients (11–13).
Combining CA and LAAO in a single procedure is an efficacious
strategy for the treatment of patients at high risk of stroke. The
present study reported on the rationale and feasibility of the
technique of combining of CA and percutaneous LAAO in a single
procedure, which is a novel procedure compared to various previous
approaches, providing an optimized surgical route.
Materials and methods
Study group
Patients aged ≥18 years with documented paroxysmal
or (longstanding) persistent, non-valvular AF, a CHADS2
score (14) of ≥1 and/or a
CHA2DS2-VASc score (15) ≥2, a HAS-BLED score (16) ≥1, who were subjected to CA and
percutaneous LAAO in a single procedure at Yantai Yuhuangding
Hospital (Yantai, China) between July 2016 and June 2017 were
included in the present study (Table
I). Written informed consent was obtained from all participants
included in the study. The Ethics Committee of Yantai Yuhuangding
Hospital (Yantai, China) approved the protocol of the present
study.
 | Table I.Baseline characteristics of the study
population (n=25). |
Table I.
Baseline characteristics of the study
population (n=25).
| Characteristics | Value |
|---|
| Mean age (years) | 64.2±3.5 |
| Females | 10 (40) |
| Hypertension | 18 (72) |
| Type of AF |
|
|
Paroxysmal | 3 (12) |
|
Persistent | 8 (32) |
|
Long-standing persistent | 14 (56) |
| CHADS2 score |
|
| 1 | 0 (0) |
| 2 | 3 (12) |
| 3 | 8 (32) |
| 4 | 11 (44) |
| 5 | 3 (12) |
| CHA2DS2-VASc
score | 4.5 (2–6) |
| HAS-BLED score | 3.17 (1–7) |
| Drug use |
|
| Vitamin K
antagonist | 14 (56) |
| NOAC | 9 (36) |
|
Aspirin | 2 (8) |
| Stroke during use of
(N)OAC | 19 (76) |
Pre-procedure preparation
Patients with an international normalized ratio of
2.0–3.0 were required to take vitamin K antagonist prior to the
procedure or to take (N)OACs, which was discontinued from the day
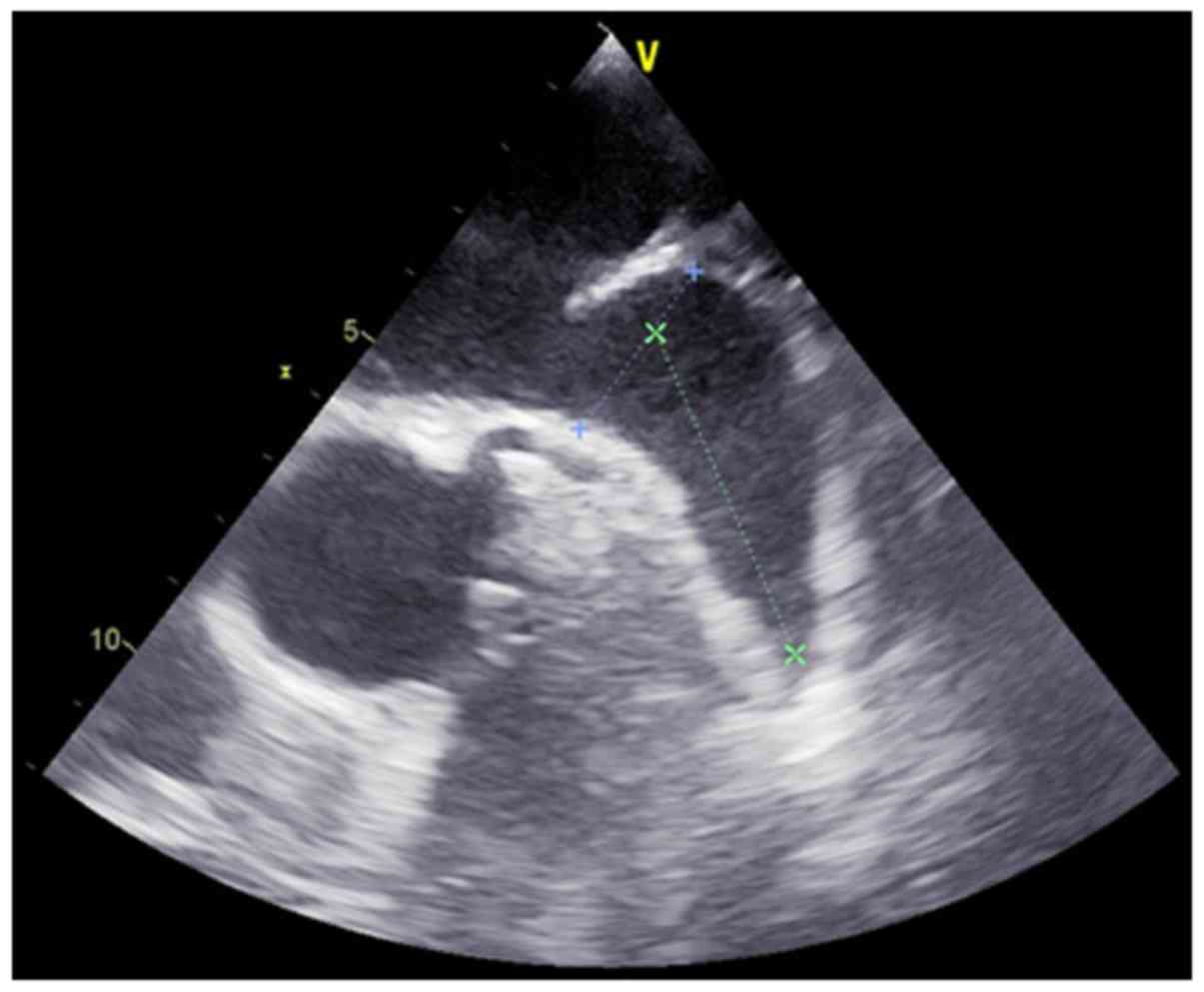
of the procedure. Prior to the procedure, a transesophageal
echocardiogram (TEE) was recorded to exclude thrombi within the
LAA, assess the anatomy of the LAA and to determine the appropriate
occlusion device size (Fig. 1). All
of the procedures were performed without general anesthesia.
Electrophysiological and CA procedure
in AF patients
Local anesthesia was performed by administration of
lidocaine in the groin and left subclavian region, and fentanyl was
given as an analgesic. Through the left subclavian venous access, a
decapolar catheter was placed in the coronary vein. Two transseptal
punctures were performed through the right femoral vein using a
Brockenbrough (BRK) needle (Baylis Medical Company Inc.,
Sainy-Laurent, Canada) to ensure co-axial alignment with the
appendage. One inferior and posterior transseptal puncture was
required. An unfractioned heparin bolus of 7,500 international
units (IU) was given after transseptal puncture, and thereafter
1,000 IU were administered at 1-h intervals. Angiography of LAA and
pulmonary veins (PVs) was performed via the sheath. A circular
mapping catheter (Lasso NAV; Biosense Webster; Johnson &
Johnson, New Brunswick, NJ, USA) was used to map PV potentials and
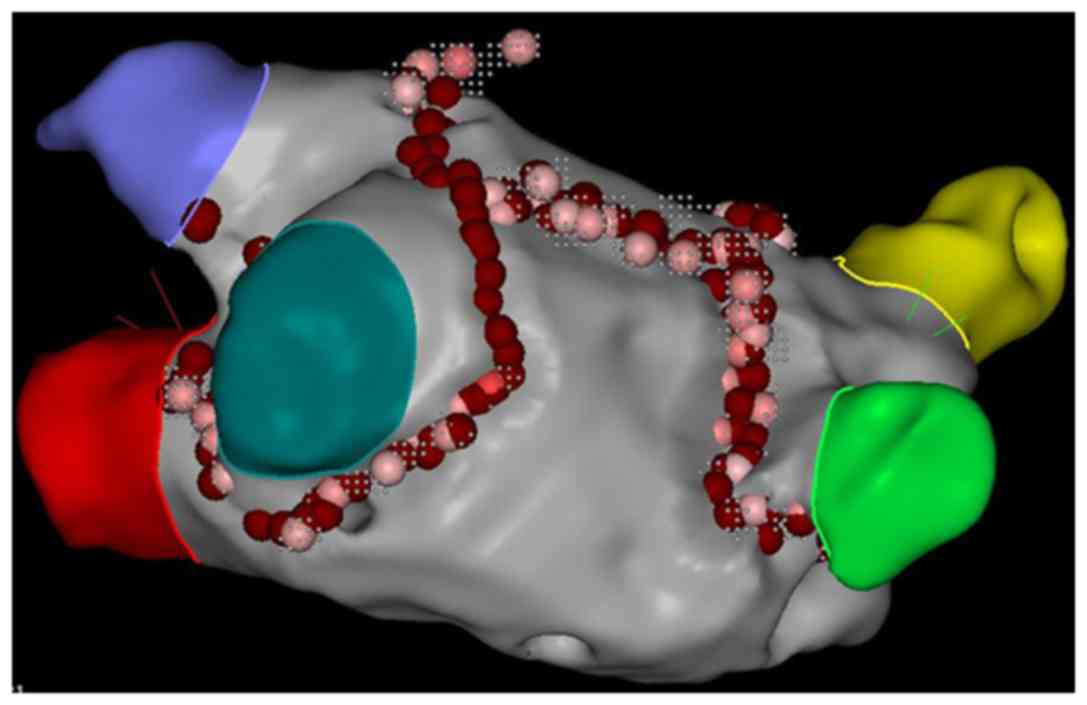
construct the model of the left atrium and PVs. PV isolation was
guided by a 3-dimensional mapping system (CARTO3 system; Biosense
Webster; Johnson & Johnson) and performed point-by-point using
a ThermoCool Smart Touch Catheter (Biosense Webster; Johnson &
Johnson) with the power and temperature limited to 30–35 W and
43°C, respectively. Bidirectional conduction block between the LA
and PVs was the end-point of catheter ablation. Only PV isolation
was performed for patients with paroxysmal AF. A roofline was also
performed for patients with persistent AF (Fig. 2).
LAAO with Watchman device
Immediately after the ablation procedure, the
implantation of a Watchman device was performed by using
fluoroscopy and TEE guidance.
Cefazolin sodium pentahydrate was administered as a
prophylactic antibiotic at the end of the ablation procedure. A
Watchman device (Boston Scientific, Marlborough, MA, USA) was then
implanted using the following procedure: A 14F transseptal access
sheath for delivering the pigtail catheter replaced the more
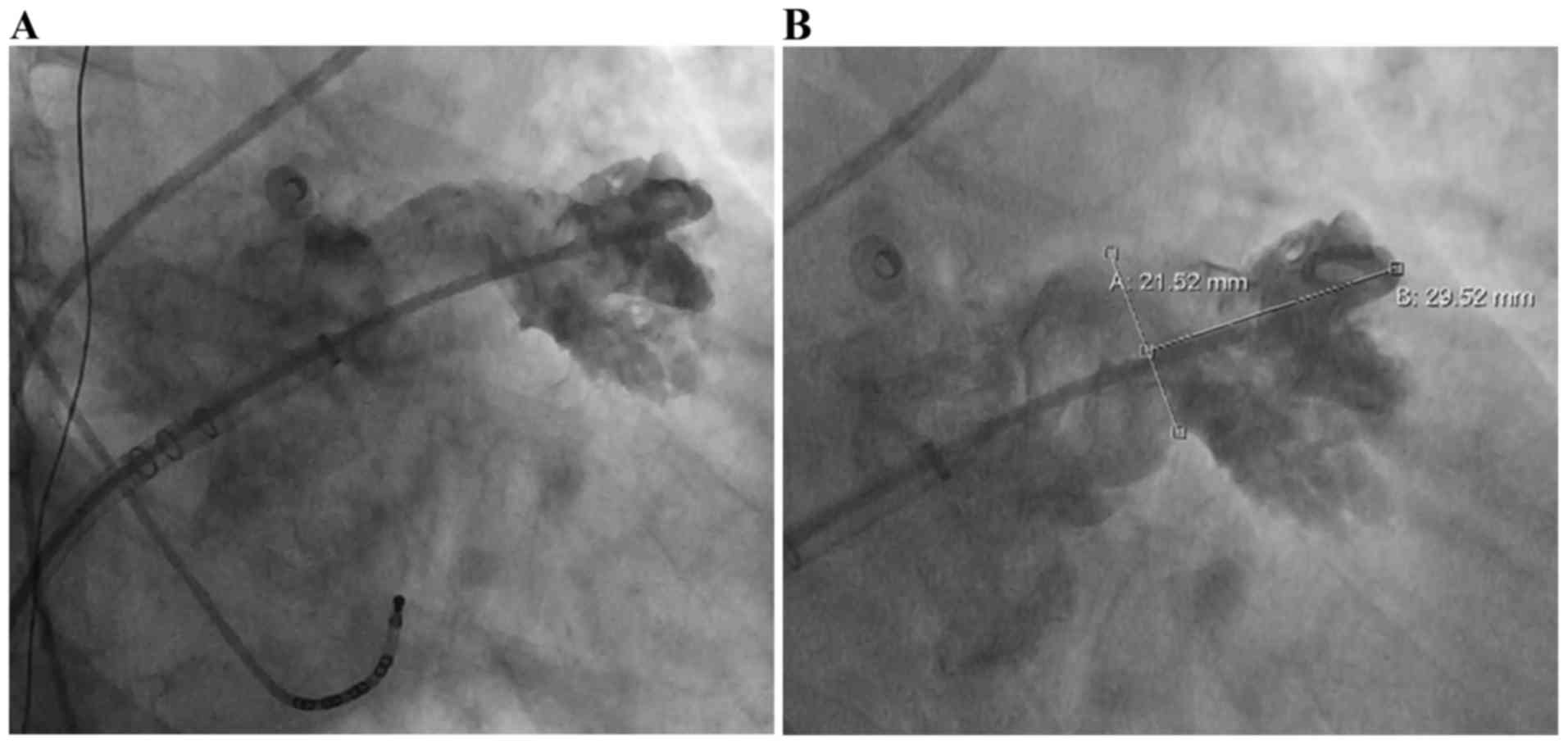
inferior and posterior transseptal sheath. A pigtail catheter was
positioned in the LAA to perform an angiography of the LAA at a
right anterior oblique angle of 20–30° and a caudal angle 20–30°,
delineating the shape and size of the LAA (Fig. 3). The device size was selected to be
10–20% larger than the largest diameter of the LAA measured by
angiography and TEE guidance was applied for stable
positioning.
The access sheath for delivering the Watchman device
was carefully advanced over the pigtail catheter. The pigtail was
then slowly removed. The device was deployed by retraction of the
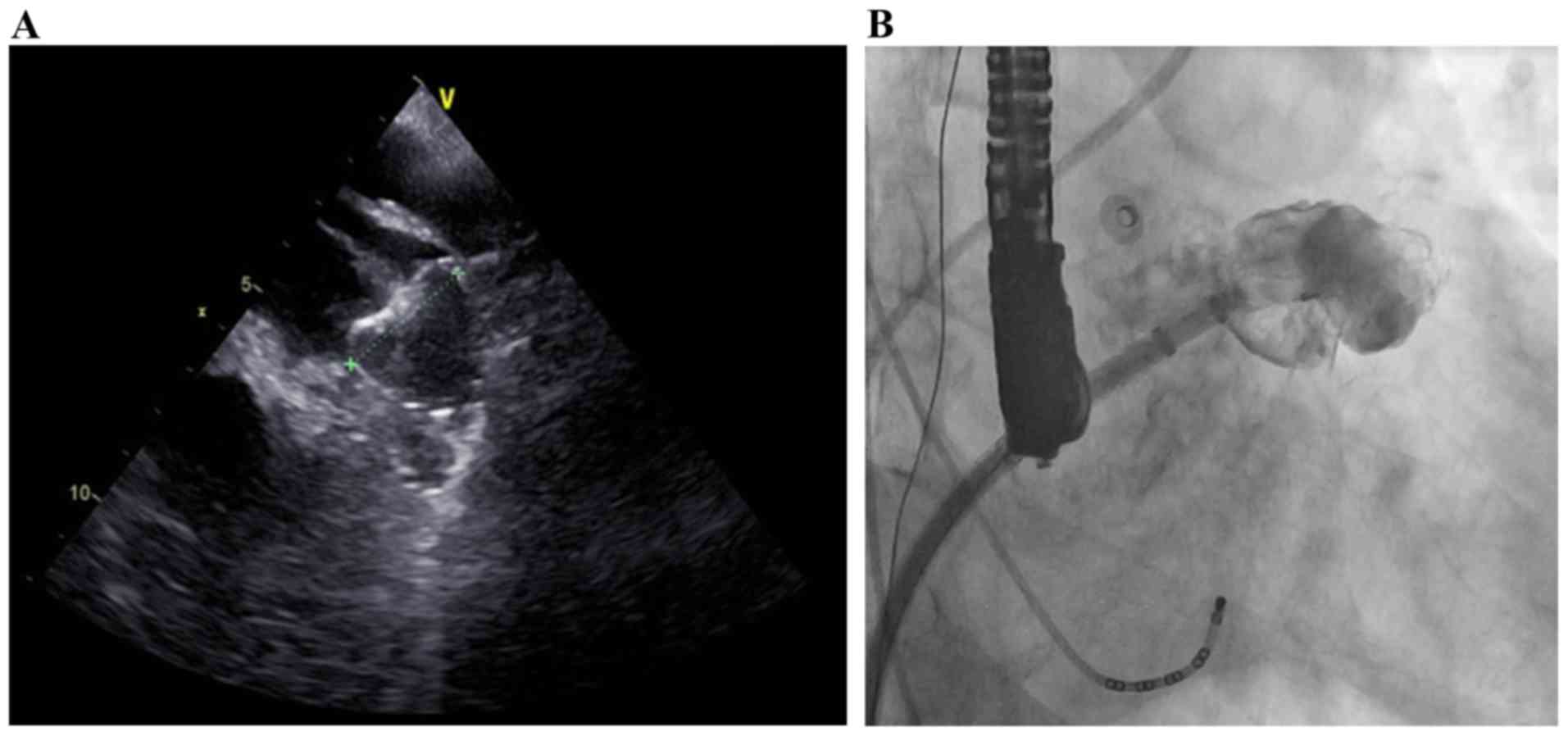
access sheath. Before the device was released, it was ensured that
it was properly positioned, with minimal (leak of ≤5 mm) or no
residual lateral flow past the device (confirmed by angiography and
TEE; Fig. 4), and a sustained tug
test for stability was performed.
Statistical analysis
The patients' characteristics were reported by
descriptive statistics. The results were expressed as the mean ±
standard deviation or the median (25–75th percentiles). Categorical
variables were reported as n (%). All statistical data were
analyzed by using SPSS software 19.0 (IBM Corp., Armonk, NY,
USA).
Results
Between July 2016 and June 2017, all 25 patients
(40% females; mean age, 64.2±3.5 years) successfully underwent a
combination of CA and LAA occlusion with Watchman device
(short-term success rate, 100%). In 1 patient, due to the large
residual lateral flow (leak of >5 mm), the device required
replacing with a larger-diameter one, which was successfully
deployed in this second procedure. The LAAO procedure was therefore
completed using a mean of 1.04±0.20 devices per patient in the
present cohort. After implantation, 3 patients had a small
peri-device leak (≤5 mm), while complete closure of the LAA was
achieved in all other cases. The median time for the combined
procedure was 185.58 min (85.67 min for LAAO; Table II). No serious complications
occurred during the procedure, and afterwards, only 2 patients had
minor complications, including a slightly elevated temperature and
a small groin hematoma, and no intervention was performed. No
serious peri-procedural complications, including cardiac tamponade,
dislodgement of the LAA closure, thrombus formation on the Watchman
device and coronary artery air embolism occurred in any of the
patients.
 | Table II.Procedural characteristics in the
study population (n=25). |
Table II.
Procedural characteristics in the
study population (n=25).
| Characteristics | Value |
|---|
| Successful
implantation of LAA occlusion device | 25 (100) |
| LAA measurements
(mm) |
|
|
Width | 28.76
(21.4–31.9) |
|
Length | 30.98
(26.29–34.1) |
| Morphology type of
LAA |
|
| Chicken
wing | 5 (20) |
|
Cauliflower | 14 (56) |
| Wind
sock | 6 (24) |
| Device size
(mm) |
|
| 27 | 4 (16) |
| 30 | 14 (56) |
| 33 | 7 (28) |
| LAA occlusion time
(min) | 85.67 (60–120) |
| Total procedural
time (min) | 185.58
(123–295) |
| Total contrast dose
(ml) | 162 (80–260) |
| Mean no. of devices
deployed per patient | 1.04±0.20 |
| Hospitalization
time (days) | 7.67 (2–13) |
On the day after the procedure, all patients were
discharged from hospital (Table
III). The median hospital stay was 7.67 days (range, 2–13
days).
 | Table III.Procedure-associated complications
and post-procedure TEE results. |
Table III.
Procedure-associated complications
and post-procedure TEE results.
|
Characteristics | Value |
|---|
| Complications |
|
| Catheter
thrombus | 0 (0) |
|
Hematoma | 1 (4) |
|
Pericardial effusion | 0 (0) |
| Post-procedure TEE
observations |
|
|
Successful implantation | 25 (100) |
| Minimal
residual flow | 3 (12) |
| Hospitalization
time (days) | 7.67 (2–13) |
Follow-up
All of the 25 patients (100%) underwent TEE at 60
days after the procedure and an optimal sealing performance of the
LAA was observed in 92% of the cases. Complete sealing of the LAA
was achieved in 23 patients (92%) at the 6-month follow-up. A
minimal residual flow (leak size, <5 mm) was detected in 2
patients (8%). In 24 patients (96%), the administration of (N)OACs
was discontinued and aspirin treatment was initiated at the 6-month
follow-up. (N)OACs treatment was maintained in 1 patient (4%) on
the basis of transient ischemic attack (Table IV). During the 6-month follow-up, 3
patients who had a recurrence of AF received a repeated ablation.
In this second ablation, the Watchman device was stable and did not
interfere with the procedure. Furthermore, no thrombus formation on
the device was detected during the follow-up. Prior to the paper
being finished, a 1-year follow-up was performed for 18 patients
(Table V). During the 1-year
follow-up, 17 patients (95%) achieved a complete sealing and only 1
patient (5%) had minimal residual flow (<2 mm). The NOACs was
interrupted in all patients and aspirin was taken. No mortality,
stroke or transient ischemic events occurred. A total of 4 patients
(22%) who had a recurrence of AF received a redo-ablation
successfully.
 | Table IV.Characteristics of the patients
(n=25) at 6-month follow-up. |
Table IV.
Characteristics of the patients
(n=25) at 6-month follow-up.
|
Characteristics | Value |
|---|
| TEE
observations |
|
| Minimal
residual flow | 2 (8) |
| Device
embolism | 0 (0) |
|
Thrombus on device | 0 (0) |
| No atrial
fibrillation recurrent | 11 (44) |
| (N)OAC use | 1 (4) |
| Repeated
ablation | 2 (12) |
| Complications
during follow-up |
|
|
Stroke | 0 (0) |
|
Transient ischemic attack | 1 (4) |
| Major
bleeding | 0 (0) |
 | Table V.Characteristics at 1-year follow-up
(n=18). |
Table V.
Characteristics at 1-year follow-up
(n=18).
|
Characteristics | Value |
|---|
| TEE
observations |
|
| Minimal
residual flow | 1 (5) |
| Device
embolism | 0 (0) |
|
Thrombus on device | 0 (0) |
| No atrial
fibrillation recurrent | 9 (50) |
| (N)OAC use | 0 (0) |
| Redo ablation | 4 (22) |
Discussion
The present study reports that the combination of
LAAO and CA is a feasible and safe strategy for the treatment of AF
in patients at high risk of stroke.
The Watchman device has been proved to be safe and
effective in numerous randomized clinical trials. A previous
multicenter randomized clinical trial, the ‘PROETCT AF’ trial,
evaluated the efficacy and safety of LAAO with a Watchman device
compared with warfarin treatment (17). This trial reported that LAAO with the
Watchman device was not inferior compared with standard warfarin
therapy in patients with non-valvular AF.
Furthermore, the ‘PREVAIL’ trial (18) proved that compared with the PROETCT
AF study, complications associated with the LAAO procedure were
infrequent, providing a significant improvement. The PREVAIL trial
also indicated that LAAO with the Watchman device for the
prevention of stroke was not inferior to standard warfarin
therapy.
Alli et al (19), performed a quality of life (QOL)
assessment in the cohort of the PROTECT AF trial, revealing that in
non-valvular AF patients treated with LAAO with the Watchman
device, the QOL was improved compared to that in patients treated
with warfarin.
CA is a well-established treatment to prevent
recurrent AF (20,21), and in maintaining the sinus rhythm,
it is more effective, while the complication rate is similar
compared with that associated with anti-arrhythmic drugs (22,23). The
optimal surgical outcome for CA is complete bidirectional
conduction block between the LA and PVs (24–26),
which is achieved by lesions encircling PVs caused by
radiofrequency ablation or cryoballoon ablation (27,28).
Reynolds et al (29) reported
that the risk of stroke and transient ischemic attack is decreased
more significantly in patients treated with ablation than in those
receiving anti-arrhythmic drug therapy. The efficacy of the hybrid
procedure of CA and LAAO with the Watchman device to maintain the
sinus rhythm and prevent stroke in patients with AF is supported by
the data of previous studies (30,31).
Certain studies reasoned that the longer duration of the procedure
and fluoroscopy, as well as the application of general anesthesia
are disadvantages of the combined procedure (30,32). In
the present study, all of the procedures were performed under local
anesthesia for the first time. In the present cohort, the Watchman
device was successfully implanted in 96% of patients under local
anesthesia on the first attempt.
Apart from the abovementioned limited single-center
studies, a consensus statement from the European Heart Rhythm
Association/European Association of Percutaneous Cardiovascular
Interventions (33) suggests that
the hybrid procedure of CA and LAAO is beneficial for patients with
a contraindication for OACs and a high risk of thromboembolic
events.
The feasibility and techniques of combining CA and
LAAO with the Watchman device in a single procedure have been
reported by few studies (30,31). The
avoidance of a second vascular access and transseptal puncture may
make the combined strategy safer. It is important to note that a
combined procedure of Watchman placement and CA does not interfere
with the electrical isolation of the LAA. Di Biase et al
(34) revealed that in 27% of
patients with recurrent AF after initial ablation, foci arising
from the LAA were observed, and electrical isolation of the LAA
should ideally be performed in these patients. LAA may be more
thrombogenic due to LAA stasis caused by electrical isolation of
the LAA. LAAO may be particularly beneficial for these
patients.
In the present study, 100.0% of the patients had a
successful occlusion, and 12.0% (3/25) had small residual leak
immediately after the release of the device. During the 6-months
follow-up, the stroke rate in the present study was 0. However, 1
patient had a transient ischemic attack at 3 months after the
procedure. Device-associated thrombus formation remains a concern,
but was not observed in the present study. A limitation of the
present study is that there is no other treatment that the group
was compared with.
In conclusion, the present study indicated that
combination of CA and LAAO in a single procedure is feasible, safe
and efficacious for patients with non-valvular AF at a high risk of
stroke, and a contraindication regarding the use of (N)OACs.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL and JL contributed to the conception and design
of the study and wrote this manuscript. HC, LW and LS collected and
analyzed clinical data. GW and XW contributed to the patient
follow-ups and revised the manuscript for intellectual content. The
final version of the manuscript has been read and approved by all
authors, and each author believes that the manuscript represents
honest work.
Ethical approval and consent to
participate
Written informed consent was obtained from all
participants included in the study. The Ethics Committee of Yantai
Yuhuangding Hospital (Yantai, China) approved the protocol of the
present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Camm AJ, Kirchhof P, Lip GY, Schotten U,
Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G,
Prendergast B, et al: Guidelines for the management of atrial
fibrillation: The Task Force for the Management of Atrial
Fibrillation of the European Society of Cardiology (ESC). Europace.
12:1360–1420. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Go AS, Hylek EM, Phillips KA, Chang Y,
Henault LE, Selby JV and Singer DE: Prevalence of diagnosed atrial
fibrillation in adults: National implications for rhythm management
and stroke prevention: The AnTicoagulation and risk factors in
atrial fibrillation (ATRIA) study. JAMA. 285:2370–2375. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stewart S, Hart CL, Hole DJ and McMurray
JJ: Population prevalence, incidence, and predictors of atrial
fibrillation in the Renfrew/Paisley study. Heart. 86:516–521. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lloyd-Jones D, Adams R, Carnethon M, De
Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund
K, et al: Heart disease and stroke statistics - 2009 update: A
report from the American Heart Association Statistics Committee and
Stroke Statistics Subcommittee. Circulation. 119:480–486. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lloyd-Jones DM, Wang TJ, Leip EP, Larson
MG, Levy D, Vasan RS, D'Agostino RB, Massaro JM, Beiser A, Wolf PA
and Benjamin EJ: Lifetime risk for development of atrial
fibrillation: The Framingham Heart Study. Circulation.
110:1042–1046. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goldman ME, Pearce LA, Hart RG,
Zabalgoitia M, Asinger RW, Safford R and Halperin JL:
Pathophysiologic correlates of thromboembolism in nonvalvular
atrial fibrillation: I. Reduced flow velocity in the left atrial
appendage (The stroke prevention in atrial fibrillation (SPAF-III)
study). J Am Soc Echocardiogr. 12:1080–1087. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kirchhof P, Benussi S, Kotecha D, Ahlsson
A, Atar D, Casadei B, Castellá M, Diener HC, Heidbuchel H, Hendriks
J, et al: 2016 ESC guidelines for the management of atrial
fibrillation developed in collaboration with EACTS. Rev Esp Cardiol
(Engl Ed). 70:502017.(In English, Spanish). PubMed/NCBI
|
|
8
|
Baker WL, Cios DA, Sander SD and Coleman
CI: Meta-analysis to assess the quality of warfarin control in
atrial fibrillation patients in the United States. J Manag Care
Pharm. 15:244–252. 2009.PubMed/NCBI
|
|
9
|
Hylek EM, Evans-Molina C, Shea C, Henault
LE and Regan S: Major hemorrhage and tolerability of warfarin in
the first year of therapy among elderly patients with atrial
fibrillation. Circulation. 115:2689–2696. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lip GY, Andreotti F, Fauchier L, Huber K,
Hylek E, Knight E, Lane DA, Levi M, Marin F, Palareti G, et al:
Bleeding risk assessment and management in atrial fibrillation
patients: A position document from the European Heart Rhythm
Association, endorsed by the European Society of Cardiology Working
Group on Thrombosis. Europace. 13:723–746. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Holmes DR, Reddy VY, Turi ZG, Doshi SK,
Sievert H, Buchbinder M, Mullin CM and Sick P: PROTECT AF
Investigators: Percutaneous closure of the left atrial appendage
versus warfarin therapy for prevention of stroke in patients with
atrial fibrillation: A randomised non-inferiority trial. Lancet.
374:534–542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reddy VY, Holmes D, Doshi SK, Neuzil P and
Kar S: Safety of percutaneous left atrial appendage closure:
Results from the Watchman left atrial appendage system for embolic
protection in patients with AF (PROTECT AF) clinical trial and the
continued access registry. Circulation. 123:417–424. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reddy VY, Doshi SK, Sievert H, Buchbinder
M, Neuzil P, Huber K, Halperin JL and Holmes D: PROTECT
AFInvestigators: Percutaneous left atrial appendage closure for
stroke prophylaxis in patients with atrial fibrillation: 2.3-Year
follow-up of the PROTECT AF (Watchman left atrial appendage system
for embolic protection in patients with atrial fibrillation) trial.
Circulation. 127:720–729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gage BF, Waterman AD, Shannon W, Boechler
M, Rich MW and Radford MJ: Validation of clinical classification
schemes for predicting stroke: Results from the National Registry
of Atrial Fibrillation. JAMA. 285:2864–2870. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lip GY, Nieuwlaat R, Pisters R, Lane DA
and Crijns HJ: Refining clinical risk stratification for predicting
stroke and thromboembolism in atrial fibrillation using a novel
risk factor-based approach: The euro heart survey on atrial
fibrillation. Chest. 137:263–272. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lip GY, Frison L, Halperin JL and Lane DA:
Comparative validation of a novel risk score for predicting
bleeding risk in anticoagulated patients with atrial fibrillation:
The HAS-BLED (hypertension, abnormal renal/liver function, stroke,
bleeding history or predisposition, labile INR, elderly,
drugs/alcohol concomitantly) score. J Am Coll Cardiol. 57:173–180.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fountain RB, Holmes DR, Chandrasekaran K,
Packer D, Asirvatham S, Van Tassel R and Turi Z: The PROTECT AF
(WATCHMAN Left Atrial Appendage System for Embolic PROTECTion in
patients with atrial fibrillation) trial. Am Heart J. 151:956–961.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Belgaid DR, Khan Z, Zaidi M and Hobbs A:
Prospective randomized evaluation of the watchman left atrial
appendage closure device in patients with atrial fibrillation
versus long-term warfarin therapy: The PREVAIL trial. Int J
Cardiol. 219:177–179. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alli O, Doshi S, Kar S, Reddy V, Sievert
H, Mullin C, Swarup V, Whisenant B and Holmes D Jr: Quality of life
assessment in the randomized PROTECT AF (percutaneous closure of
the left atrial appendage versus warfarin therapy for prevention of
stroke in patients with atrial fibrillation) trial of patients at
risk for stroke with nonvalvular atrial fibrillation. J Am Coll
Cardiol. 61:1790–1798. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arbelo E, Brugada J, Hindricks G, Maggioni
AP, Tavazzi L, Vardas P, Laroche C, Anselme F, Inama G, Jais P, et
al: The atrial fibrillation ablation pilot study: A European Survey
on Methodology and results of catheter ablation for atrial
fibrillation conducted by the European Heart Rhythm Association.
Eur Heart J. 35:1466–1478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Calkins H, Kuck KH, Cappato R, Brugada J,
Camm AJ, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, DiMarco J,
et al: 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter
and Surgical Ablation of Atrial Fibrillation: Recommendations for
patient selection, procedural techniques, patient management and
follow-up, definitions, endpoints, and research trial design.
Europace. 14:528–606. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mont L, Bisbal F, Hernández-Madrid A,
Pérez-Castellano N, Viñolas X, Arenal A, Arribas F,
Fernández-Lozano I, Bodegas A, Cobos A, et al: Catheter ablation
vs. antiarrhythmic drug treatment of persistent atrial
fibrillation: A multicentre, randomized, controlled trial (SARA
study). Eur Heart J. 35:501–507. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Walfridsson H, Walfridsson U, Nielsen JC,
Johannessen A, Raatikainen P, Janzon M, Levin LA, Aronsson M,
Hindricks G, Kongstad O, et al: Radiofrequency ablation as initial
therapy in paroxysmal atrial fibrillation: Results on
health-related quality of life and symptom burden. The MANTRA-PAF
trial. Europace. 17:215–221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kuck KH, Hoffmann BA, Ernst S, Wegscheider
K, Treszl A, Metzner A, Eckardt L, Lewalter T, Breithardt G and
Willems S: Gap-AF-AFNET 1 Investigators*: Impact of complete versus
incomplete circumferential lines around the pulmonary veins during
catheter ablation of paroxysmal atrial fibrillation: Results from
the gap-atrial fibrillation-German atrial fibrillation competence
network 1 trial. Circ Arrhythm Electrophysiol. 9:e0033372016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McLellan AJ, Ling LH, Azzopardi S, Lee GA,
Lee G, Kumar S, Wong MC, Walters TE, Lee JM, Looi KL, et al: A
minimal or maximal ablation strategy to achieve pulmonary vein
isolation for paroxysmal atrial fibrillation: A prospective
multi-centre randomized controlled trial (the Minimax study). Eur
Heart J. 36:1812–1821. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Verma A, Sanders P, Macle L, Deisenhofer
I, Morillo CA, Chen J, Jiang CY, Ernst S and Mantovan R: Substrate
and trigger ablation for reduction of atrial fibrillation
trial-part II (STAR AF II): Design and rationale. Am Heart J.
164:1–6.e6. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luik A, Radzewitz A, Kieser M, Walter M,
Bramlage P, Hörmann P, Schmidt K, Horn N, Brinkmeier-Theofanopoulou
M, Kunzmann K, et al: Cryoballoon versus open irrigated
radiofrequency ablation in patients with paroxysmal atrial
fibrillation: The prospective, randomized, controlled,
noninferiority freezeaf study. Circulation. 132:1311–1319. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schmidt M, Dorwarth U, Andresen D,
Brachmann J, Kuck KH, Kuniss M, Lewalter T, Spitzer S, Willems S,
Senges J, et al: Cryoballoon versus RF ablation in paroxysmal
atrial fibrillation: Results from the German Ablation Registry. J
Cardiovasc Electrophysiol. 25:1–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reynolds MR, Gunnarsson CL, Hunter TD,
Ladapo JA, March JL, Zhang M and Hao SC: Health outcomes with
catheter ablation or antiarrhythmic drug therapy in atrial
fibrillation: Results of a propensity-matched analysis. Circ
Cardiovasc Qual Outcomes. 5:171–181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Phillips KP, Walker DT and Humphries JA:
Combined catheter ablation for atrial fibrillation and
Watchman® left atrial appendage occlusion procedures:
Five-year experience. J Arrhythm. 32:119–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Walker DT, Humphries JA and Phillips KP:
Combined catheter ablation for atrial fibrillation and
watchman® left atrial appendage occlusion procedures: A
single centre experience. J Atr Fibrillation. 5:6872012.PubMed/NCBI
|
|
32
|
Bolao IG, Calvo N, Macias A, Barba J,
Salterain N, Ramos P, Ballesteros G and Neglia R: Ablation of
atrial fibrillation in combination with left atrial appendage
occlusion in a single procedure. Rationale and technique. J Atr
Fibrillation. 8:13462016.PubMed/NCBI
|
|
33
|
Meier B, Blaauw Y, Khattab AA, Lewalter T,
Sievert H, Tondo C and Glikson M: EHRA/EAPCI expert consensus
statement on catheter-based left atrial appendage occlusion.
EuroIntervention. 10:1109–1125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Di Biase L, Burkhardt JD, Mohanty P,
Sanchez J, Mohanty S, Horton R, Gallinghouse GJ, Bailey SM,
Zagrodzky JD, Santangeli P, et al: Left atrial appendage: An
underrecognized trigger site of atrial fibrillation. Circulation.
122:109–118. 2010. View Article : Google Scholar : PubMed/NCBI
|