Introduction
Hepatic steatosis [fatty liver disease (FLD)]
significantly affects morbidity and mortality of patients
undergoing major liver surgery (1–7). The
prevalence is increased in the developed world (20–30% of the
general population), with obese patients showing frequencies above
95% with increased markers of incidence of steatohepatitis
(2,7–13). The
etiology of hepatic steatosis has not been fully elucidated and
etiological factors include dietary habits, obesity, dyslipidemia,
alcoholism, hepatitis and metabolic disorders (2,14–18).
In recent years, liver surgery presents excellent
growth by reducing morbidity and postoperative mortality (14,18,19).
However, postoperative mortality in surgical patients with hepatic
steatosis remains in high rates (>14%) (14,19–23). The
surgical technique in these procedures involves manipulations of
prolonged hepatic ischemia followed by reperfusion
[ischemia/reperfusion (IR)], which cause a significant degree of
inflammation and ischemic damage by greatly aggravating survival
(14,20,24–27).
Various models of hepatic steatosis in rodents are
presented in the literature aiming to investigate both
pathophysiology and the study of the effect of various
pharmaceutical agents and/or interventional procedures in livers
with severe fatty infiltration. The aim of the present study is to
investigate the time frame for the development of a severe hepatic
steatosis model in rats after choline-free diet and the assessment
of histopathological changes of the hepatic parenchyma, such as
hepatic steatosis, microvesicular, macrovesicular and mixed
steatosis, necrosis, ischemia and inflammation, compared to normal
rats (1–27).
Materials and methods
Animals
Male Wistar rats (n=96) aged 12–14 weeks and
weighing 250–300 g were randomly divided into 2 groups. The first
group of 48 rats was characterized as control group C and freely
received the standard laboratory diet (concentrated feed with a
protein concentration of 20–27%), while the remaining 48 rats were
group A and received choline-free diet (Mucedola, PF1877) for 14
weeks. Rats had free access to food and water (daily water
consumption was about 10 ml/100 g of weight) while living
conditions included constant ambient temperature (22°C and 60%
humidity) and a 12-h light-dark cycle. This experimental study was
implemented at the Experimental Research Center of ELPEN (Athens,
Greece) and was approved by Veterinary Authority of East Attika
Prefecture (Protocol ref. no. 1633 directive 609/1986) and
performed complying with the rules of experimentation and 3Rs
(Replace, Reduce and Refine).
Experimental design
In the pilot phase of the experiment the type of
food and the induction time of severe hepatic steatosis (>66%)
were investigated. After reviewing the literature on liver
steatosis models the choline-free diet was selected (2). From the existing data, 8–10 week
feeding with choline-free diet leads to moderate hepatic steatosis,
a 12–14 week feeding achieves a steatosis rate of >66%, whereas
after this time, rats significantly reduce food intake and show a
severe steatohepatitis and cirrhosis over the next 3–4 weeks
(2). From this test feeding of rat
animals, a significant steatosis development occurred after 8 weeks
during the pilot phase. Therefore, in order to confirm the existing
literature, it was decided to feed the rats with choline-free diet
(group A) and to randomly divide them into subgroups of 12 rats
named A1, A2, A3 and
A4. Every two weeks after the 8th week to the 14th (8th,
10th, 12th, 14th week) euthanasia was performed in each subgroup,
in order to determine the time and the degree of hepatic steatosis
reaching a rate >66% of the hepatocytes. Similarly, to compare
with control group C rats fed with a standard laboratory diet were
randomly divided into 4 subgroups of 12 rats and named
C1, C2, C3 and C4.
Euthanasia was performed in each control subgroup every 2 weeks
from 8th week to week 14th (8th, 10th, 12th, 14th week) to study
possible differences with the corresponding subgroups receiving
choline-free diet for hepatic steatosis. Specifically, the
experimental groups were: Group A, 48 rats received choline-free
diet for the induction of hepatic steatosis and were divided into
subgroups: A1, (n=12) were subjected to euthanasia at 8
week after the start of feeding; A2, (n=12) were
subjected to euthanasia at 10 week after the start of feeding;
A3, (n=12) were subjected to euthanasia at 12 week after
the start of feeding; A4, (n=12) were subjected to
euthanasia at 14 week after the start of feeding. Control group C,
with 48 rats that received a standard laboratory diet after their
birth and at the age of 12–14 weeks (experimental start time) were
selected to continue to receive the same diet and to be studied
comparatively after 8 to 14 weeks with rats of group A. In
particular, the subgroups of control group C were: C1,
(n=12) were subjected to euthanasia at 8 week after the start of
the experiment; C2, (n=12) were subjected to euthanasia
at 10 week after the start of the experiment; C3, (n=12)
were subjected to euthanasia at 12 week from the start of the
experiment; C4, (n=12) were subjected to euthanasia at
14 week after the start of the experiment.
Hepatic steatosis
The duration of steatosis for male rats (initial
body weight 250–300 g) was ultimately determined by the control
group at 12–14 weeks using the recombined Mucedola-PF1877
choline-free diet. The mean consumption of choline-free diet is
amounted to 20.41 g/animal with an average gain of body weight
(BWt) daily of 3.49 g/24 h. For example, the corresponding average
daily standard consumption of typical laboratory food in normal
rats was about 16.78 g/animal, with an average daily increase of
1.18 g/24 h. The daily BWt increase for rats with hepatic steatosis
ranged from 4.09 g/24 h to 1.714 g/24 h (in the last week), with a
final weight ranging from 528 to 636 g, in contrast with the
age-matched animals without hepatic steatosis, whose weight ranged
from 340 to 424 g. The percentage of liver weight in rats with
hepatic steatosis after 12–14 week feeding with choline-free diet
corresponded to 4.61±0.43% of total body weight. On the contrary,
the weight percentage of normal liver in the control group was only
3.8±0.25% of the total BWt of rats of the same age without hepatic
steatosis. After 12–14 weeks, fatty infiltration of hepatic
parenchyma >66% was achieved, causing moderate to severe FLD
(2,28–30).
Histopathological examination
The liver specimens to be studied for
histopathological changes were dipped in a 10% Formol solution (10
ml of formol 100% in 90 ml of ddH2O). During their
processing, tissue sections of 10 µm were taken, stained with the
combination of hematoxylin and eosin and then at ×200 visual
magnification were studied. The main features of the confirmation
of the extent of hepatic fatty filtration are mentioned, as well as
the ratio between the microvesicular (microlacunar) and the
macrovesicular (macrolacunar) form of hepatic steatosis in the
experimental groups.
Statistical analysis
Continuous variables were described using the
average levels, standard deviations and medians, while the
categorical ones were described using the frequencies and the
corresponding rates. The control of the regularity of the
measurements distribution was done using the Kolmogorov-Smirnov
test and normal probability plot. Two-way analysis of variance
(ANOVA) without repetitive measurements was used to study factors,
including inflammation, necrosis, ischemia, body weight, time,
steatosis (microvesicular, macrovesicular and mixed), and their
interaction. Multiple comparisons were made with the post hoc
Bonferroni test. In statistically significant interaction one-way
ANOVA model was used so that multiple comparisons are made with
Bonferroni test. The non-parametric analysis, was made using the
Kruskal-Wallis test and Mann-Whitney test. All statistical analyses
were performed with the SPSS statistical package, version 17.00
(SPSS, Inc., Chicago, IL, USA). All tests are two-sided. The
P-value <0.05 was established as a statistically significant
difference level.
Results
From the parametric and non-parametric study of the
percentage of fatty infiltration between groups A and C was
confirmed a strong statistically significant difference
(P<0.0005). In control group C lean rats in which the liver
parenchymal fatty infiltration rate ranged from 0 to 4.5% for all
four subgroups (C1-4), while in rats with hepatic
steatosis in group A ranged from 43.3% (subgroup A1), up
to 68.4% (subgroup A4) with maximum values observed
after free choline feeding for 14 weeks for the A4. The
percentage of macrovesicular fatty infiltration in subgroup
A4 reached 54.7% of total fatty infiltration
(corresponding to 37.4% of total liver parenchyma), the equivalent
of microvesicular fatty infiltration averaged 35.8% (corresponding
to 24.5% of total hepatic parenchyma), while mixed fatty
infiltration was 9.5% (corresponding to 6.5% of total hepatic
parenchyma).
Histopathological findings
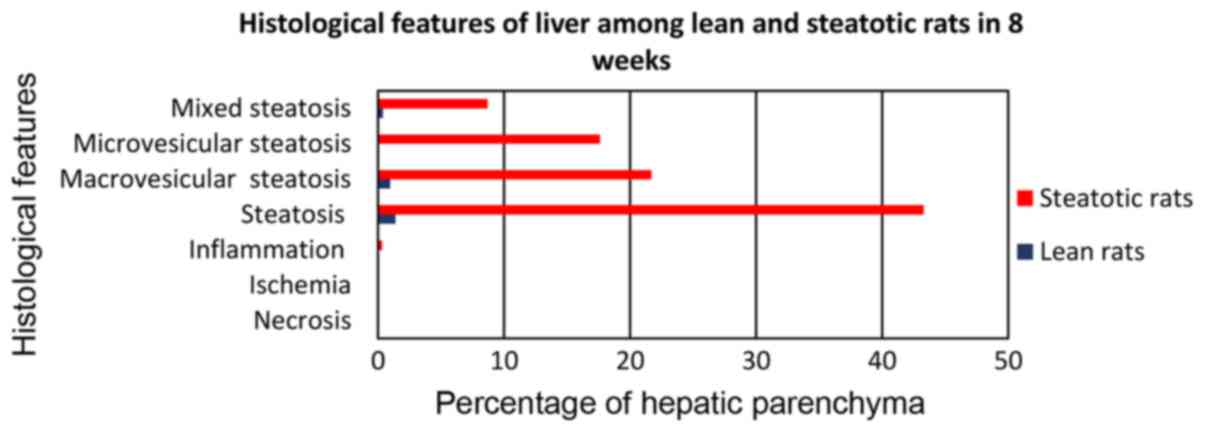
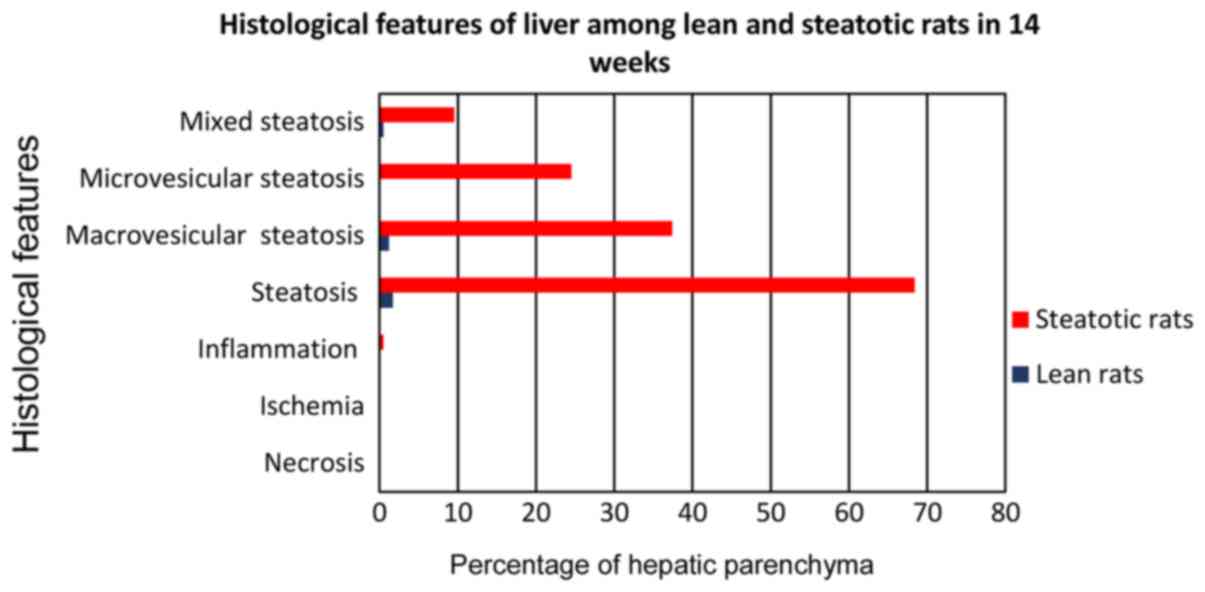
The control group C, fed with the standard
laboratory diet, did not show fatty infiltration greater than 4.5%
in any of the C1-4 subgroups (Fig. 1). Therefore, hepatic steatosis cannot
be considered. This percentage is considered to be normal,
especially when it comes to the form of microvesicular
(microlacunar) fatty infiltration, which was the most common form
of fatty liver degeneration in all subgroups of the control group.
No necrosis, ischemia or inflammation was developed in any subgroup
of the control group. The steatosis did not develop in any subgroup
greater than 4.5%. Microvesicular (microlacunar) hepatic fatty
infiltration was developed in a percentage of 68.9–70.2%,
macrovesicular in a percentage of 1.7 to 2.5% and the mixed one
from 27.3–29.4%. Under no circumstances was observed a
statistically significant difference between the control group
subgroups for any of the histological parameters (Fig. 1).
The subgroups of group A (A1-4) showed no
necrosis or ischemia. Therefore, they showed no difference from the
control group. All subgroups of group A showed mild inflammation
(rarely one leukocyte infiltration focus in 20–30% of the studied
optical fields). There was no statistically significant difference
among subgroups of A, while a statistically significant difference
(P<0.01) was observed in the corresponding subgroups of control
group C.
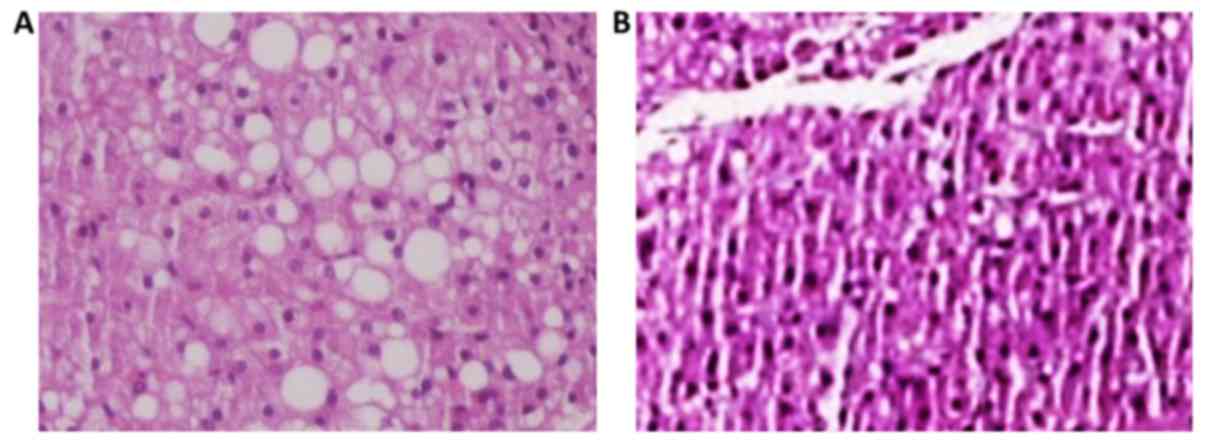
In particular, subgroup A1 (Fig. 2) of rats receiving choline-free diet
for 8 weeks (from the age of 12–14 weeks) developed fatty liver
infiltration of 43.3%, macrovesicular to 21.9%, microvesicular at
17.6%, and mixed at 8.7% (Fig. 3).
In each case, compared to the control subgroup C1, there
was a strong statistically significant difference (P<0.0001) for
the fatty infiltration parameters (macrovesicular, microvesicular
and mixed), with subgroup A1 showing the highest
values.
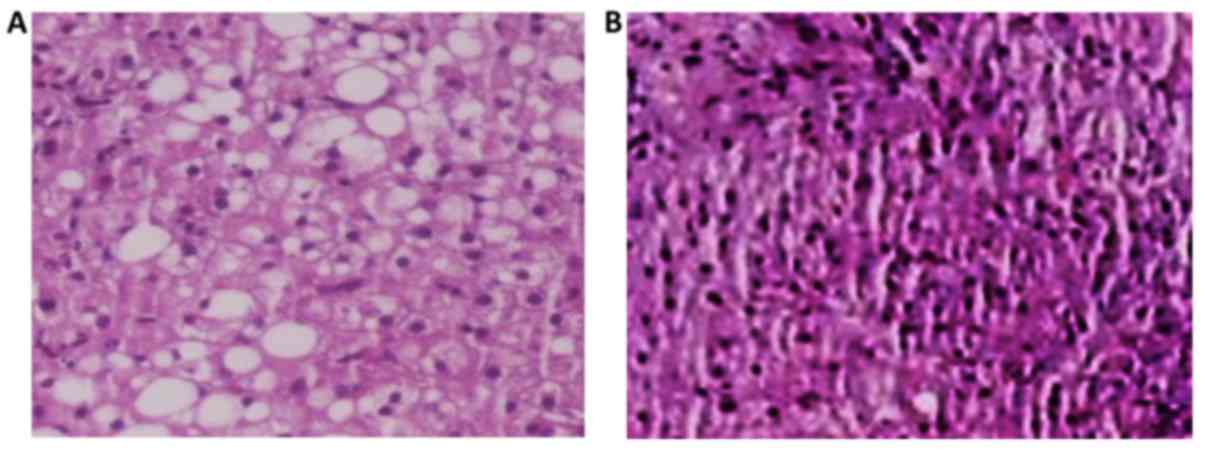
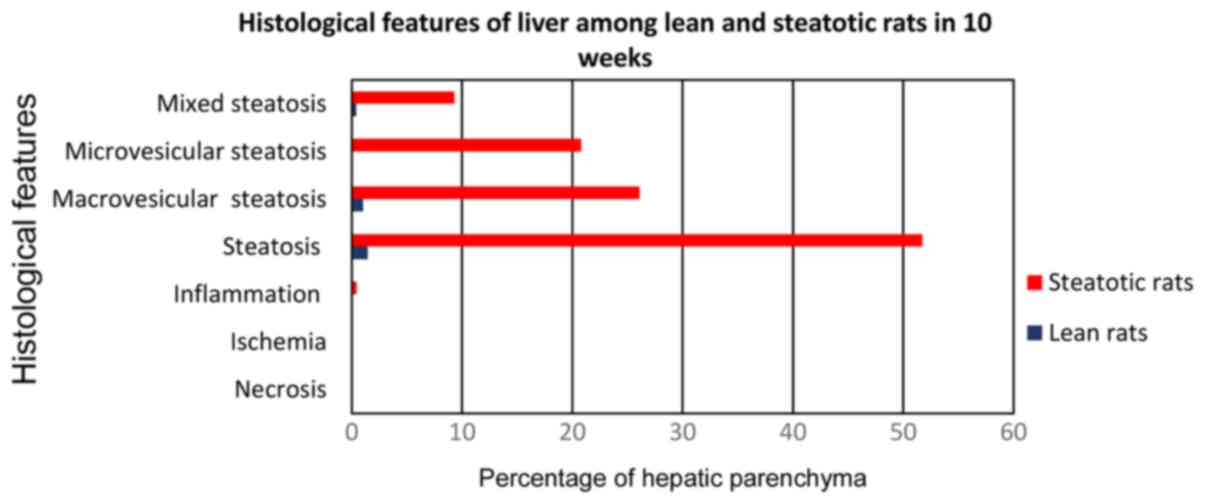
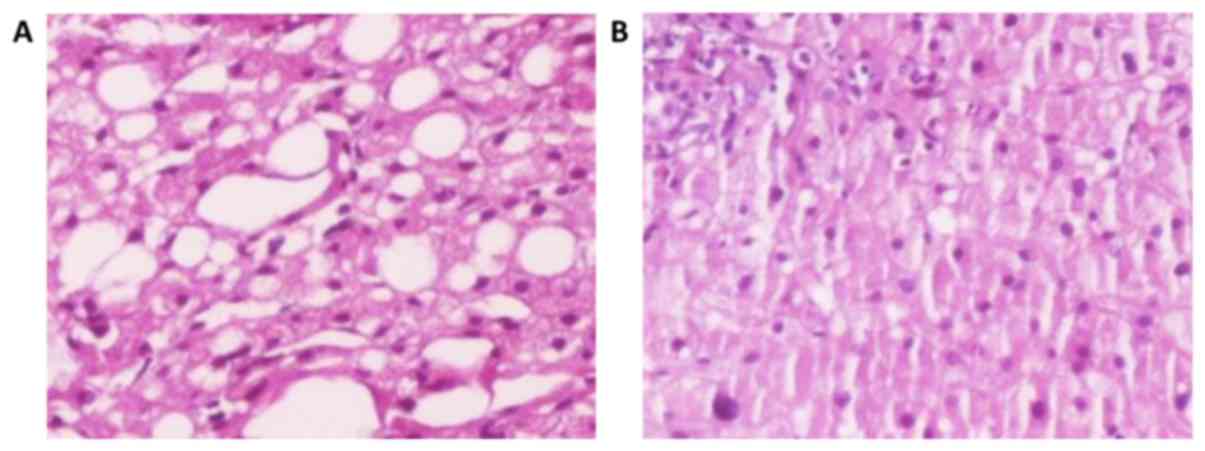
The A2 subgroup of rats (Fig. 4) which received choline-free diet for
10 weeks (from the age of 12–14) developed 51.7% fatty liver
infiltration, 26.5% macrovesicular, 20.8% microvesicular, and mixed
in the 9.3% (Fig. 5). In each case,
compared to the control subgroup C2, there was a strong
statistically significant difference (P<0.0001) for the
parameters of macrovesicular, microvesicular and mixed fatty
infiltration, with subgroup A2 showing the highest
values.
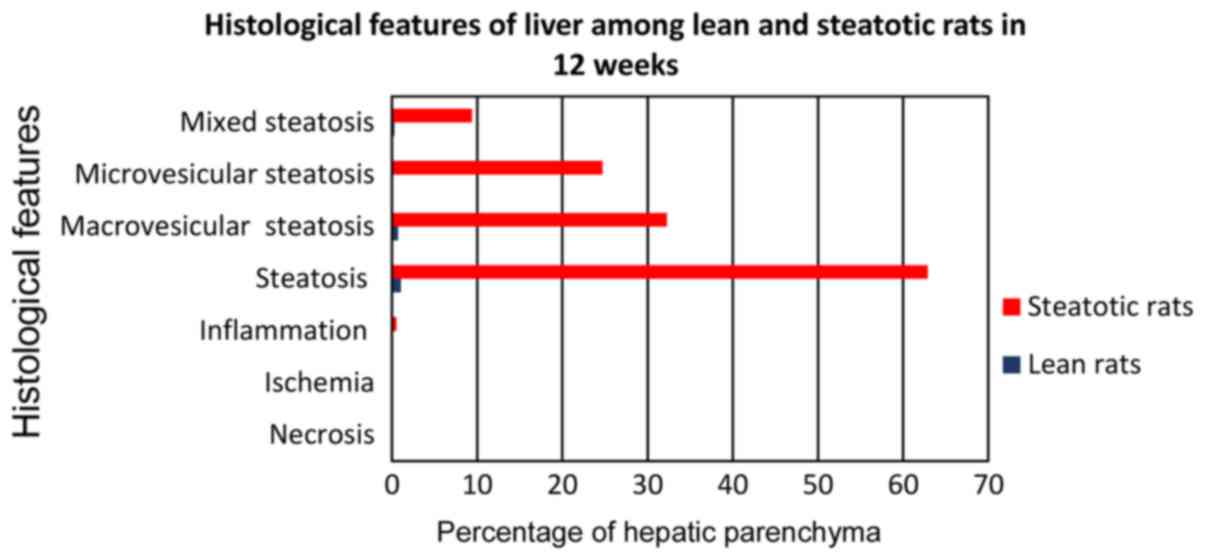
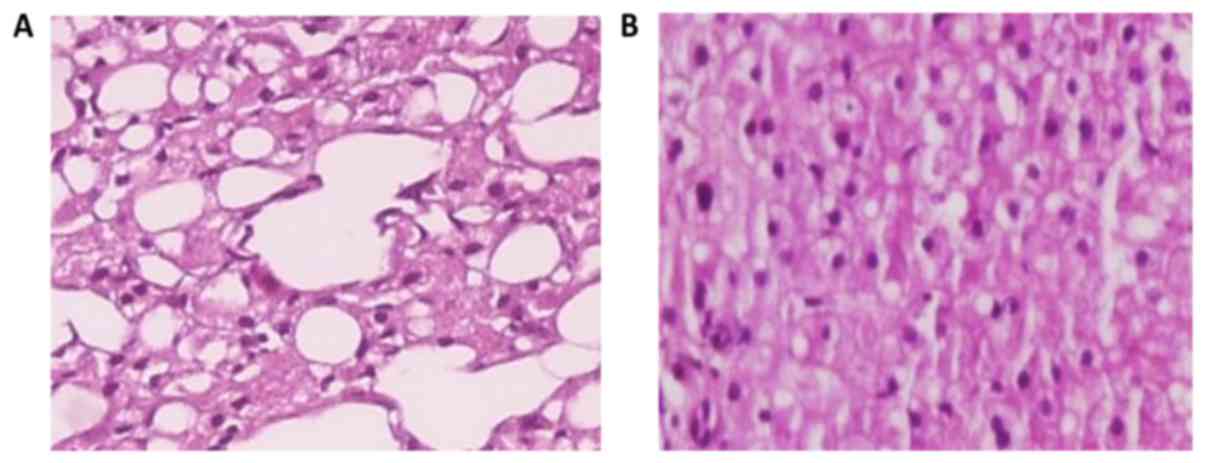
The A3 subgroup of rats (Fig. 6) which received choline-free rats
diet for 12 weeks (from the age of 12–14 weeks) developed fatty
liver infiltration 62.9%, with macrovesicular corresponding to
32.3%, microvesicular corresponding to 24.7%, and mixed to 9.4%
(Fig. 7). In each case, compared to
the control subgroup C3, there was a strong
statistically significant difference (P<0.0001) for the
parameters of macrovesicular, microvesicular and mixed fatty
infiltration, with subgroup A3 showing the highest
values.
The A4 subgroup of rats (Fig. 8) which received choline-free diet for
14 weeks (from the age of 12–14 weeks) developed fatty liver
infiltration of 68.4%, macrovesicular corresponding to 37.4%,
microvesicular corresponding to 24.5%, and mixed to 9.5% (Fig. 9). In each case, compared to the
control subgroup C4, there was a strong statistically
significant difference (P<0.0001) for the parameters of
macrovesicular, microvesicular and mixed fatty infiltration, with
subgroup A4 showing the highest values.
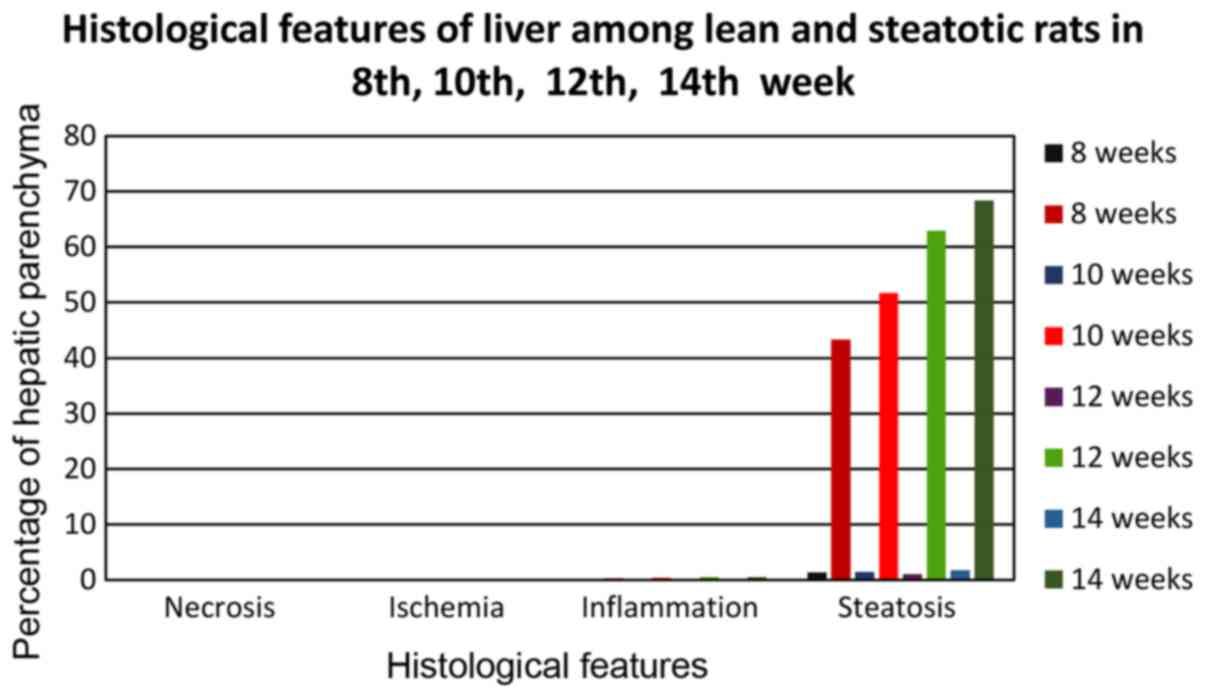
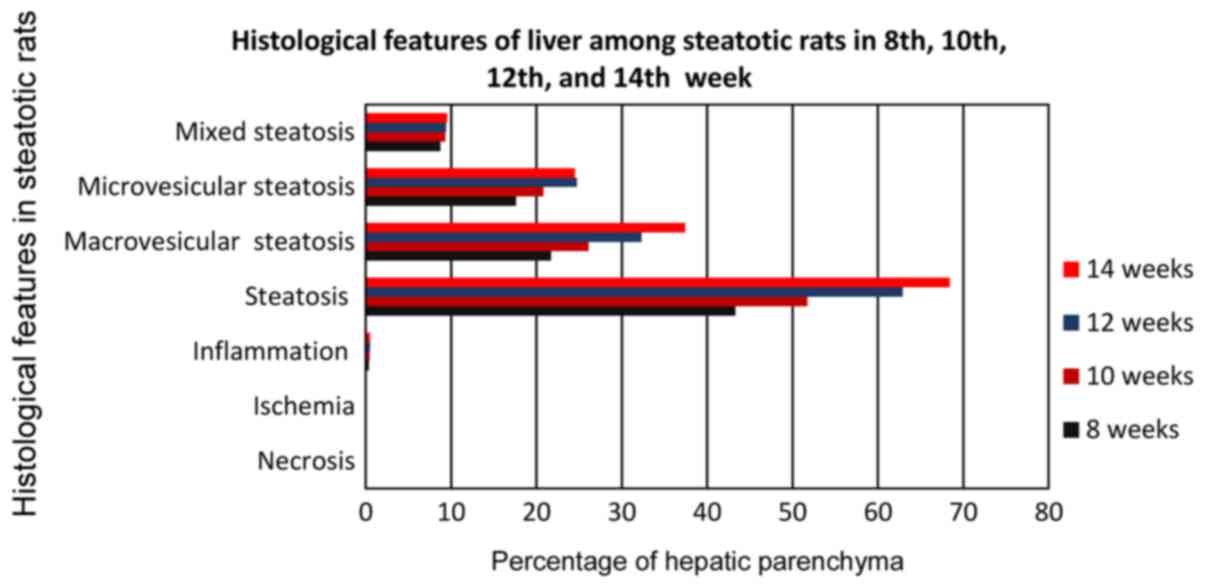
From the statistical analysis of the studied
parameters of the model studying the optimal feeding time with a
choline-free diet (Fig. 10), for
the development of hepatic steatosis, for the key parameter of
hepatic fatty infiltration a strong statistically significant
(P<0.0001) difference of all subgroups of group A occurred
compared to the corresponding subgroups of control group C, at each
of the studied time periods (8th, 10th, 12th and 14th week), with
subgroups of group A, showing very high levels of fatty liver
infiltration compared to the subgroups of group C, which was in
each case <4.5%. Group A4 showed the highest average
infiltration rate (68.4%), indicating a high degree of hepatic
steatosis. High percentages were also shown by the remaining
subgroups of group A, but below the 66% limit, in order to consider
serious steatosis. In particular, subgroup A1 (8-week
diet with choline-free diet) showed a percentage of 43.3%,
A2 (10-week diet with choline-free diet) 51.7% and
A3 (12-week diet with choline-free diet) with a
percentage of 62.9%. Subgroups A1 and A2
showed a statistically significant (p <0.0001) difference
compared to subgroup A4, while subgroup A3
showed only statistically significant difference (P<0.01)
compared to A4.
Regarding the parameter of macrovesicular steatosis
(Fig. 10), again subgroup
A4 showed the highest percentage (37.4%) which is
statistically significant (P<0.01) higher than A3
(32.3%) and statistically much higher than subgroups A2
(26.1%) and A1 (21.7%). All A1-4 subgroups
showed statistically significant (P<0.0001) much higher values
for macrovesicular steatosis than the corresponding subgroups of
the control group C which showed rates <1% in each case.
Regarding the parameter of macrovesicular steatosis, subgroup
A4 with a percentage of (24.5%) and A3 with a
similar percentage (24.7%) showed strong statistically significant
(P<0.01) higher values than the subgroups A2 (20.8%)
and A1 (17.6%). All A1-4 subgroups showed
statistically significant (P<0.0001) much higher values for
microvesicular steatosis than the corresponding subgroups of the
control group C, which showed rates <0.05% in each case.
Regarding the parameter of mixed steatosis, all subgroups of group
A showed a similar variation of the rate from 8.7 to 9.5%, without
any statistically significant difference. On the contrary, all
A1-4 subgroups showed statistically significantly
(P<0.0001) much higher values for mixed steatosis than the
corresponding subgroups of the control group C, which showed rates
<0.5% in each case.
From the statistical analysis of the studied
parameters of the model studying the optimal feeding time with a
choline-free diet, for the development of hepatic steatosis, no
data on the necrosis and ischemia of the hepatic parenchyma were
obtained since they did not develop at any stage of the study
(Fig. 10). As for the inflammation
parameter, all subgroups of group A exhibited mild inflammatory
symptoms (periportal polymorphonuclear infiltration) without any
statistically significant difference. On the contrary, all
subgroups A1-4 exhibited statistically significant
(P<0.0001) higher values for the inflammation parameter than the
corresponding subgroups of the control group C, where almost no
outbreaks of inflammation (extremely rare mononuclear random
infiltrations) were observed.
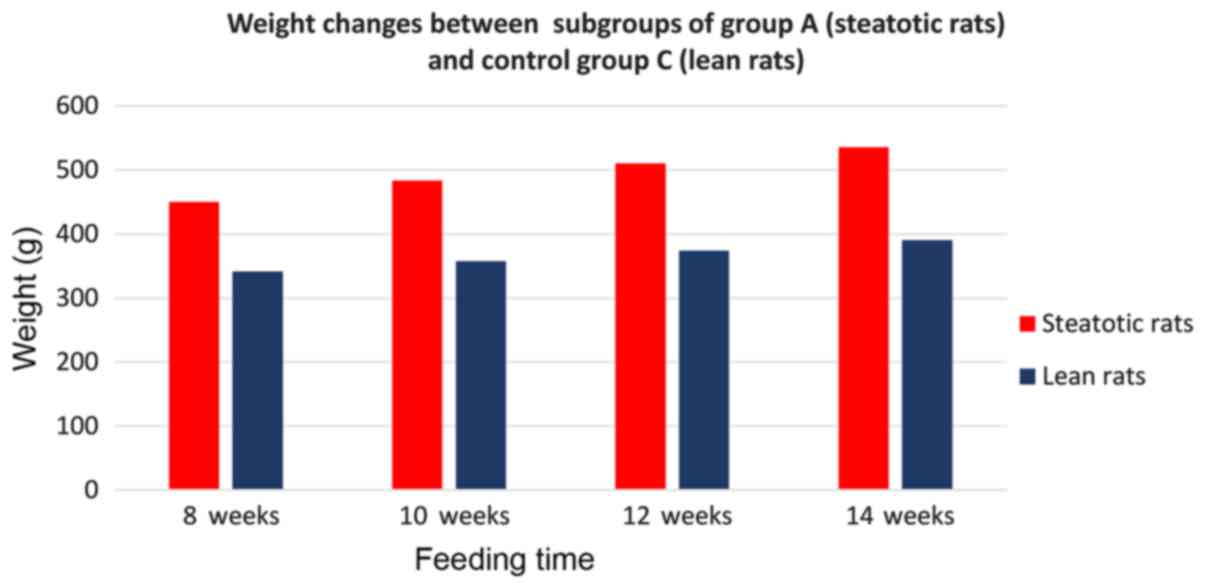
Finally, from the study of weight changes between
the subgroups of group A and the control group C (Fig. 11), there was a strong statistically
significant (P<0.0001) correlation, with subgroups of group A
(which received a choline-free diet) developing a higher body
weight compared to the corresponding subgroups of control groups
(which received standardized laboratory diet) for the corresponding
time intervals. By comparing subgroups of group A, only
A4 showed a statistically significant increase in weight
(535.6 g) compared with only A1 (450 g) and
A2 (483.7 g).
Discussion
In the present study, the choline-free diet led to
66% fatty liver infiltration at 12–13 weeks, and after 14 weeks the
rate of infiltration exceeded 68%. Before 8 weeks, the fatty
infiltration rate reached 43%, with a gradual increase, showing a
stronger rate from 8 to 12 weeks and a gradual decline in the rate
until 14 weeks. After 12 weeks, and in particular after the 13th
week, the percentage of fatty infiltration satisfies the evidence
of severe hepatic steatosis. Macrovesicular fatty infiltration
showed a significant increase at a steady rate between the 8th and
14th week. Microvesicular fatty infiltration showed a lower growth
rate between the 8th and 12th week while maintaining a steady rate
between 12th and 14th week. Mixed fatty infiltration maintained its
steady-state percentage of hepatic parenchyma from 8.8–9.5%. The
highest incidence of macrovesicular fatty infiltration, along with
the presence of mild neutrophilic inflammatory infiltration,
confirms the gradual progression of severe hepatic steatosis to
steatohepatitis, after 14 weeks, in the choline-free diet group. In
terms of body weight, obesity has been developed in rats with
choline-free diet (subgroup A1) as early as 8 weeks and
at 14 weeks obesity is severely impaired (subgroup A4)
compared to both the control group and the other subgroups of group
A (A1-3).
The development of experimental models mimicking
non-alcoholic fatty liver disease (NAFLD) is the key objective for
investigating the etiopathology of the disease and it is treatment
with therapeutic interventions (2,28–30).
Unfortunately, to date no ideal model of hepatic steatosis has been
developed simulating the same histopathological lesions with humans
and with the same timing of progression of the lesions (2). Existing experimental models mimic
different stages and pathologoanatomical lesions with different
clinical signs. Key problems of the existing models are the
inability to develop a sufficient extent of macrovesicular fatty
liver infiltration, the failure to develop a sufficient extent of
inflammation of the hepatic parenchyma and its progression to a
sufficient degree of fibrosis (2,28–30).
Also in several cases, the inability of development of metabolic
disorders is observed (obesity, insulin resistance, hyperglycemia,
hyperlipidemia, metabolic syndrome), despite the moderate or severe
development of hepatic parenchymal steatosis (2,28–30).
They have been used as animal models, mice and rats
in genetic, nutritional and mixed models for the development of
NAFLD (2,29–32).
NAFLD developmental dietary models are rather more reliable
research tools. Nutrition with data leading to gradual development
of hepatic steatosis due to complicated metabolic pathogenetic
disorders and its progression to steatohepatitis is a basic feature
of all dietary models (2,31). Clearly, the differences in gene
background, physiology, nutrition, metabolism, endocrine system,
habits and lifestyle, exercise and medication, change the organic
reaction, the development of hepatic steatosis and its effect
between humans and different animal models. In humans, the various
pathogenic agents act spontaneously over many years, while in
animal models, they have a violent effect over a short period of
time, irrespective of their small biological life cycle. Only these
differences significantly reduce the credibility of existing
experimental models (2,30). NAFLD's genetic models usually fail to
simulate the polygenic pathogenic disorder that causes the disease.
Activation of all multiple gene disorders usually fails, while
animal models are extremely difficult to replicate and are costly
to research (2,28–34).
In particular, Zucker rats (fa/fa rats) which
develop obesity, hyperglycemia, hyperinsulinemia and hyperlipidemia
have been used. Their disadvantage is that they develop mild
macrolacunar and mild to moderate microlacunar liver hepatic
steatosis but are unable to develop into steatohepatitis. In
addition, in cases of extensive hepatectomy (>60–70%), this
model presents severe disorders in liver regeneration and survival
(2,30). Transgenic models overexpressing the
SREBP-1a gene lead to the development of metabolic syndrome and
mild hepatic steatosis (2,30). Otsuka Long-Evans Tokushima obese rats
show between 28–38 weeks an automatic development of microvesicular
and macrovesicular hepatic steatosis, along with metabolic syndrome
and type II diabetes. They do not have a similar cause pathogenesis
of NAFLD, such as in humans, and should receive a special diet in
order to develop hepatic steatosis and steatohepatitis (2,30,35,36).
In most cases, it is necessary to feed genetically
modified models with special nutrition in order to achieve the
development of severe hepatic steatosis and its progression to
steatohepatitis, which is not always successful. These models are
designated as mixed (composite). In particular, mixed models using
ZFR genetically modified animal models require the addition of a
diet rich in fats or with excess of disaccharides and a reduction
in the percentage of lipopolysaccharides, or free of choline and
methionine (2,28,30,2).
The transgenic animal models overexpressing SREBP-1a in order to
develop similar pathogenetic mechanisms with humans to induce NAFLD
should be fed a diet rich in fructose for about 16 months (2,35).
Otsuka Long-Evans Tokushima obese rats need to receive a high-fat
or choline-methionine-free diet in order to develop severe hepatic
steatosis, with lobular inflammation and fibrosis (2,35,39–41).
Finally, Wistar rats with hereditary hypercholesterolemia (Prague
hereditary hypercholesterolaemic rats) in addition to the gene
disorder, require a high cholesterol (>5%) diet to develop mild
to moderate hepatic fatty filtration (2).
Pure nutritional models of hepatic steatosis have
been used in a variety of studies, yielding satisfactory results
with relatively low development costs. Existing studies with
fat-rich diets for the induction of hepatic steatosis in rats have
failed to show a progression of simple hepatic steatosis in
steatohepatitis and also have different effects on the development
of human-like metabolic pathophysiology (insulin resistance,
hyperglycemia and dyslipidemia). The right choice of the ratio of
monounsaturated and polyunsaturated fatty acids (ω-3 to ω-6 fatty
acids) is of great importance as well as the ratio of proteins and
carbohydrates. In many cases, administration should be done by
placing a gastric catheter in order to administer the required
amount of food (2,28–30,42–45).
Also, a high carbohydrate (fructose and sucrose) diet lasting
>15–16 weeks, which may cause panlobular microvesicular hepatic
steatosis has been used (2,30).
The most frequently used experimental models of
NAFLD concern the choline or choline/ methionine- free diet
(2,30,46). The
absence of choline disrupts the synthesis of phosphatidylcholine in
the liver, leading to accumulation of triglycerides in the
hepatocyte and development of hepatic steatosis (2,14,29,2).
Feeding with a choline-free diet causes severe chronic-dependent
hepatic steatosis, characterized by inflammation, with or without
mild fibrosis, while prolonged feeding can cause even neoplasia
(2,14,28–30,46).
In the choline-free diet there are changes that
resemble metabolic syndrome, the development of obesity,
dyslipidemia and insulin resistance after 7–12 weeks of feeding.
The absence of insulin resistance is an important difference
observed in this experimental model of steatosis with choline and
methionine-free diet in rats with respect to humans (2,14,20–30,39,46,47).
The free choline and choline/methionine diet in Wistar rats causes
in a week microvesicular liver steatosis, mainly in zone III, and
after 3–5 weeks there is progressive development of inflammation.
After the 7th week, there is an intense macrovesicular hepatic
steatosis (2,39,46,47).
Mitochondrial function is maintained at reduced but
satisfactory levels despite the oxidative phosphorylation of
reduced performance whereas liver regeneration after hepatectomy is
not significantly affected in Wistar rats. These models achieve the
development of hepatic steatosis and its progression to
steatohepatitis depending on the duration of feeding. However, the
choline/ methionine- free diet does not cause a corresponding
metabolic burden (hyperglycemia, hyperlipidemia, insulin
resistance), such as the choline-free diet. The latter, ultimately,
is considered the most appropriate for the development of reliable
models for the study of the effects of hepatic steatosis,
inflammation and oxidative stress in the liver, in combination with
metabolic syndrome and FLD disorders (2,30).
In this rat model, the development of macrovesicular
severe hepatic steatosis with evidence of onset of lobular
inflammation and onset of steatohepatitis, was demonstrated after a
14-week feeding with choline-free diet. Although in the literature
there is reference of hepatic steatosis development after the
7th-8th week, however severe hepatic steatosis (fatty infiltration
>66% with predominantly macrovesicular form) develops after the
14th week. No fibrosis elements were observed during this time, as
confirmed by the literature (2,28–30,46).
The particular interest is in performing surgical manipulations and
study their effect on liver physiology with steatosis without the
synergistic effect, together with metabolic pathophysiological
mechanisms.
Developing an ideal model of NAFLD is a particular
challenge. The histopathological changes that occurred in animals
with hepatic steatosis are in many cases unable to mimic the
corresponding changes in humans. The results of this study
indicated that severe hepatic steatosis in rodents may lead to the
development of steatohepatitis after feeding with a choline-free
diet for at least 14 weeks. This model may be of interest to
researchers of experimental liver surgery and related surgical
maneuvers, as it is easily reproducible. Further studies should
focus on the search for longer periods of feeding with a
choline-free diet, beyond the 14th week and should further
investigate on the progression of steatohepatitis in order to
control possible process of hepatic fibrosis and cirrhosis.
Acknowledgements
Not applicable.
Funding
The present study was funded by Scholarship - grant
by the Experimental Research Center ELPEN Pharmaceuticals
(E.R.C.E), Athens, Greece.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TK and NS conceived and designed the study, and
drafted the manuscript. DM and AP performed the analysis and
interpretation of data. KA, LM conducted the experiments. GK
reviewed and edited the manuscript critically for important
intellectual content. All authors read and approved the
manuscript.
Ethics approval and consent to
participate
This study was approved by the Veterinary Authority
of East Attika Prefecture (approval no. 1633 directive
609/1986).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Clavien PA, Petrowsky H, DeOliveira ML and
Graf R: Strategies for safer liver surgery and partial liver
transplantation. N Engl J Med. 356:1545–1559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kucera O and Cervinkova Z: Experimental
models of non-alcoholic fatty liver disease in rats. World J
Gastroenterol. 20:8364–8376. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vernon G, Baranova A and Younossi ZM:
Systematic review: The epidemiology and natural history of
non-alcoholic fatty liver disease and non-alcoholic steatohepatitis
in adults. Aliment Pharmacol Ther. 34:274–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kogure K, Ishizaki M, Nemoto M, Kuwano H
and Makuuchi M: A comparative study of the anatomy of rat and human
livers. J Hepatobiliary Pancreat Surg. 6:171–175. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clavien PA, Selzner M, Rüdiger HA, Graf R,
Kadry Z, Rousson V and Jochum W: A prospective randomized study in
100 consecutive patients undergoing major liver resection with
versus without ischemic preconditioning. Ann Surg. 238:843–850;
discussion 851–852. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin LM, Liu YX, Zhou L, Xie HY, Feng XW,
Li H and Zheng SS: Ischemic preconditioning attenuates
morphological and biochemical changes in hepatic
ischemia/reperfusion in rats. Pathobiology. 77:136–146. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yadav SS, Sindram D, Perry DK and Clavien
PA: Ischemic preconditioning protects the mouse liver by inhibition
of apoptosis through a caspase-dependent pathway. Hepatology.
30:1223–1231. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Neuschwander-Tetri BA: Nonalcoholic
steatohepatitis and the metabolic syndrome. Am J Med Sci.
330:326–335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
DuBray BJ, Gunter K, Hassan H,
Balachandran P, Upadhya GA, Knolhoff BL, Jia J, Ramachandran S,
Hotchkiss RS, Mohanakumar T, et al: Absence of BH3-only proteins
mitigates accentuation of hepatic ischemia-reperfusion injury
caused by steatosis. J Surg Res. 172:302–303. 2012. View Article : Google Scholar
|
|
10
|
Hilden M, Christoffersen P, Juhl E and
Dalgaard JB: Liver histology in a ‘normal’ population -
examinations of 503 consecutive fatal traffic casualties. Scand J
Gastroenterol. 12:593–597. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Veteläinen R, van Vliet AK and van Gulik
TM: Severe steatosis increases hepatocellular injury and impairs
liver regeneration in a rat model of partial hepatectomy. Ann Surg.
245:44–50. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Selzner N, Selzner M, Jochum W and Clavien
PA: Ischemic preconditioning protects the steatotic mouse liver
against reperfusion injury: An ATP dependent mechanism. J Hepatol.
39:55–61. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Underwood Ground KE, . Prevalence of fatty
liver in healthy male adults accidentally killed. Aviat Space
Environ Med. 55:59–61. 1984.PubMed/NCBI
|
|
14
|
Koneru B, Reddy MC, dela Torre AN, Patel
D, Ippolito T and Ferrante RJ: Studies of hepatic warm ischemia in
the obese Zucker rat. Transplantation. 59:942–946. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Centers for Disease Control and
Prevention: Overweight and obesity. http://www.cdc.gov/nccdphp/dnpa/obesity/Dec
13–2014
|
|
16
|
Berson A, De Beco V, Lettéron P, Robin MA,
Moreau C, El Kahwaji J, Verthier N, Feldmann G, Fromenty B and
Pessayre D: Steatohepatitis-inducing drugs cause mitochondrial
dysfunction and lipid peroxidation in rat hepatocytes.
Gastroenterology. 114:764–774. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fromenty B and Pessayre D: Impaired
mitochondrial function in microvesicular steatosis. Effects of
drugs, ethanol, hormones and cytokines. J Hepatol. 26 Suppl
2:43–53. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Burt AD, Mutton A and Day CP: Diagnosis
and interpretation of steatosis and steatohepatitis. Semin Diagn
Pathol. 15:246–258. 1998.PubMed/NCBI
|
|
19
|
Selzner M, Rüdiger HA, Sindram D, Madden J
and Clavien PA: Mechanisms of ischemic injury are different in the
steatotic and normal rat liver. Hepatology. 32:1280–1288. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Behrns KE, Tsiotos GG, DeSouza NF, Krishna
MK, Ludwig J and Nagorney DM: Hepatic steatosis as a potential risk
factor for major hepatic resection. J Gastrointest Surg. 2:292–298.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mokuno Y, Berthiaume F, Tompkins RG, Balis
UJ and Yarmush ML: Technique for expanding the donor liver pool:
Heat shock preconditioning in a rat fatty liver model. Liver
Transpl. 10:264–272. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park SW, Kang JW and Lee SM: Role of
Kupffer cells in ischemic injury in alcoholic fatty liver. J Surg
Res. 194:91–100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Selzner M and Clavien PA: Resection of
liver tumors: Special emphasis on neoadjuvant and adjuvant
therapyMalignant Liver Tumors - Current and Emerging Therapies.
Clavien PA: Blackwell Science; Malden, MA: pp. 137–149. 1999
|
|
24
|
McCormack L, Petrowsky H, Jochum W, Furrer
K and Clavien PA: Hepatic steatosis is a risk factor for
postoperative complications after major hepatectomy: A matched
case-control study. Ann Surg. 245:923–930. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Al-Humadi H, Theocharis S, Dontas I,
Stolakis V, Zarros A, Kyriakaki A, Al-Saigh R and Liapi C: Hepatic
injury due to combined choline-deprivation and thioacetamide
administration: An experimental approach to liver diseases. Dig Dis
Sci. 57:3168–3177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsukada K, Miyazaki T, Kato H, Masuda N,
Fukuchi M, Fukai Y, Nakajima M, Ishizaki M, Motegi M, Mogi A, et
al: Body fat accumulation and postoperative complications after
abdominal surgery. Am Surg. 70:347–351. 2004.PubMed/NCBI
|
|
27
|
Chu MJJ, Hickey AJR, Phillips ARJ and
Bartlett ASJR: The impact of hepatic steatosis on hepatic
ischemia-reperfusion injury in experimental studies: A systematic
review. BioMed Res Int. 2013:1920292013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Larter CZ and Yeh MM: Animal models of
NASH: Getting both pathology and metabolic context right. J
Gastroenterol Hepatol. 23:1635–1648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sikalias N, Karatzas T, Alexiou K,
Mountzalia L, Demonakou M, Kostakis ID, Zacharioudaki A, Papalois A
and Kouraklis G: Intermittent ischemic preconditioning protects
against hepatic ischemia-reperfusion injury and extensive
hepatectomy in steatotic rat liver. J Invest Surg. 23:1–12. 2017.
View Article : Google Scholar
|
|
30
|
Kanuri G and Bergheim I: In vitro and in
vivo models of non-alcoholic fatty liver disease (NAFLD). Int J Mol
Sci. 14:11963–11980. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schattenberg JM and Galle PR: Animal
models of non-alcoholic steatohepatitis: Of mice and man. Dig Dis.
28:247–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
London RM and George J: Pathogenesis of
NASH: Animal models. Clin Liver Dis. 11(55–74): viii2007.
|
|
33
|
Anstee QM and Day CP: The genetics of
NAFLD. Nat Rev Gastroenterol Hepatol. 10:645–655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nagarajan P, Kumar Mahesh MJ, Venkatesan
R, Majundar SS and Juyal RC: Genetically modified mouse models for
the study of nonalcoholic fatty liver disease. World J
Gastroenterol. 18:1141–1153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Malínská H, Oliyarnyk O, Hubová M, Zídek
V, Landa V, Simáková M, Mlejnek P, Kazdová L, Kurtz TW and Pravenec
M: Increased liver oxidative stress and altered PUFA metabolism
precede development of non-alcoholic steatohepatitis in SREBP-1a
transgenic spontaneously hypertensive rats with genetic
predisposition to hepatic steatosis. Mol Cell Biochem. 335:119–125.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song YS, Fang CH, So BI, Park JY, Lee Y,
Shin JH, Jun DW, Kim H and Kim KS: Time course of the development
of nonalcoholic Fatty liver disease in the Otsuka long-evans
Tokushima Fatty rat. Gastroenterol Res Pract. 2013:3426482013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fukunishi S, Nishio H, Fukuda A, Takeshita
A, Hanafusa T, Higuchi K and Suzuki K: Development of fibrosis in
nonalcoholic steatosis through combination of a synthetic diet rich
in disaccharide and low-dose lipopolysaccharides in the livers of
Zucker (fa/fa) rats. J Clin Biochem Nutr. 45:322–328. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang YY, Tsai TH, Huang YT, Lee TY, Chan
CC, Lee KC and Lin HC: Hepatic endothelin-1 and
endocannabinoids-dependent effects of hyperleptinemia in
nonalcoholic steatohepatitis-cirrhotic rats. Hepatology.
55:1540–1550. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ota T, Takamura T, Kurita S, Matsuzawa N,
Kita Y, Uno M, Akahori H, Misu H, Sakurai M, Zen Y, et al: Insulin
resistance accelerates a dietary rat model of nonalcoholic
steatohepatitis. Gastroenterology. 132:282–293. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yeon JE, Choi KM, Baik SH, Kim KO, Lim HJ,
Park KH, Kim JY, Park JJ, Kim JS, Bak YT, et al: Reduced expression
of peroxisome proliferator-activated receptor-alpha may have an
important role in the development of non-alcoholic fatty liver
disease. J Gastroenterol Hepatol. 19:799–804. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Uno M, Kurita S, Misu H, Ando H, Ota T,
Matsuzawa-Nagata N, Kita Y, Nabemoto S, Akahori H, Zen Y, et al:
Tranilast, an antifibrogenic agent, ameliorates a dietary rat model
of nonalcoholic steatohepatitis. Hepatology. 48:109–118. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fellmann L, Nascimento AR, Tibiriça E and
Bousquet P: Murine models for pharmacological studies of the
metabolic syndrome. Pharmacol Ther. 137:331–340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jump DB, Tripathy S and Depner CM: Fatty
acid-regulated transcription factors in the liver. Annu Rev Nutr.
33:249–269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zou Y, Li J, Lu C, Wang J, Ge J, Huang Y,
Zhang L and Wang Y: High-fat emulsion-induced rat model of
nonalcoholic steatohepatitis. Life Sci. 79:1100–1107. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tschöp M and Heiman ML: Rodent obesity
models: An overview. Exp Clin Endocrinol Diabetes. 109:307–319.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Veteläinen R, van Vliet A and van Gulik
TM: Essential pathogenic and metabolic differences in steatosis
induced by choline or methione-choline deficient diets in a rat
model. J Gastroenterol Hepatol. 22:1526–1533. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tahan V, Eren F, Avsar E, Yavuz D, Yuksel
M, Emekli E, Imeryuz N, Celikel C, Uzun H, Haklar G, et al:
Rosiglitazone attenuates liver inflammation in a rat model of
nonalcoholic steatohepatitis. Dig Dis Sci. 52:3465–3472. 2007.
View Article : Google Scholar : PubMed/NCBI
|