Introduction
Chronic myeloid leukemia (CML) is a malignant
disease in which hematopoietic stem cells proliferate continuously,
and it is the first tumor that is confirmed to be related to
chromosome aberration (1).
Epidemiology shows that the incidence of CML is about 1–2
cases/100,000 people, and most patients of CML are elderly people
(median age on diagnosis, 65 years) (2). The prevalence of CML in males is higher
than that in females, but the overall survival rate for females is
higher than that for males (3). The
incidence of CML in China is 0.36 case/100,000 people (4).
Imatinib mesylate (IM) is among the first generation
of tyrosine kinase inhibitors (TKIs) that are authorized by the
Food and Drug Administration of the USA in the treatment of chronic
myelogenous leukemia in the chronic phase (CML-CP) (5). With the use of IM, CML-CP patients have
achieved a survival rate longer than 5 years and it is predicted
that their life span is close to or equivalent to that of
non-leukemia patients (2,6–8). In the
course of long-term follow-up of CML, some patients are found to be
resistant to IM (9). The incidence
of drug resistance in each year of the first 3 years of treatment
is 2–4% for CML-CP patients, and the drug resistance rate is
gradually increasing with the prolongation of drug use (10,11).
The mechanism of IM drug resistance is very complex,
and the expression of multidrug transporters has attracted much
attention (12,13). The most intensively studied mechanism
is multidrug resistance (MDR) mediated by P-glycoprotein (PGP) and
multidrug resistance-associated protein-1 (MRP1) (14–16). PGP
is encoded by multidrug resistance gene (adenosine triphosphate
binding cassette subfamily B member 1 (ABCB1), also known as MDR1),
and involved in drug absorption, distribution, metabolism, and
excretion (17). Therefore, the
regulation of ABCB1 has become an important research direction for
IM resistance therapy.
microRNA (miRNA or miR) molecules are small-molecule
non-encoding RNA molecules that widely exist in eukaryotic cells,
and they regulate protein expression at mRNA level (18–20).
Studies show that miRNA plays important biological roles in
imatinib resistance (21,22). This process is also accompanied by
alterations in the expression of multiple miRNA and proteins. The
microRNA that is upstream of ABCB1 and regulates ABCB1 expression
in CML has not been reported yet. In the present study, we
determine the expression of ABCB1 and its upstream miRNA, and try
to understand the mechanism of regulation between them.
Materials and methods
Patients
A total of 26 CML patients with IM resistance were
included into the present study between December 2013 and June
2017. In addition, 31 CML patients who do not have IM resistance
were included into control group (Table
I). Bone marrow was collected from all subjects. Among the 26
patients with IM resistance, 15 were males and 11 were females (age
range, 17–66 years; median age, 52.6 years). In addition, 20
patients with IM resistance were in chronic phase, while 6 patients
were in accelerated phase. Among the 31 patients in control group,
19 were males and 12 were females (age range, 15–69 years; median
age, 53.5 years). Moreover, 28 patients in control group were in
chronic phase, while 3 patients in control group were in
accelerated phase. All patients received treatments for IM. The
standard for the diagnosis of IM resistance in CML patients
includes: i) no hematologic remission was achieved within 3 months;
ii) no complete hematologic remission or cytogenetic remission was
achieved within 6 months; iii) failure to achieve major cytogenetic
remission within 12 months or complete cytogenetic remission within
18 months and iv) loss of prior hematologic or cytogenetic
remission, or the emergence of Abl kinase point mutations with high
IM resistance (23). All procedures
were approved by the Ethics Committee of Jining No. 1 People's
Hospital (Shandong, China). Written informed consents were obtained
from all patients or their families.
 | Table I.Clinical characteristics of CML and
IM-resistant patients. |
Table I.
Clinical characteristics of CML and
IM-resistant patients.
|
Characteristics | CML patients | IM-resistant
patients |
|---|
| Sex (n) |
|
|
|
Male | 19 | 15 |
|
Female | 12 | 11 |
| Age range
(years) | 15–69 | 17–66 |
| Median age
(years) | 53.5 | 52.6 |
| Treatment duration
(months) | 1–2 | 3–18 |
| White blood cell
count (×109/l) | 78±33 | 108±56 |
Cells
K562 cell line was a kind of CML cell line, while
K562R cell line was K562 cell line with IM resistance. Both types
of cell lines were originally purchased from American Type Culture
Collection (ATCC, Manassas, VA, USA) and kept at our lab. One day
before transfection, K562R cells in log-phase growth were seeded
into 24-well plates (3×105 in each well) containing
antibiotics-free F12/DMEM medium supplemented with 10% fetal bovine
serum and cultured at 37°C and 5% CO2. When reaching 70%
confluency, transfection began. In the first vial, 1 µl agomiR-214
(20 pmol/µl; miR-214 mimics group; Sangon Biotech Co., Ltd.,
Shanghai, China) was mixed with 50 µl Opti Mem medium (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Here, agomiR-214 was an
artificially synthesized modified double-chain miR-214 with the
same function with mature form of miR-214 (24). In the second vial, 1 µl Lipofectamine
2000 (Thermo Fisher Scientific, Inc.) was mixed with 50 µl Opti
Memi medium. After standing still for 5 min, the two vials were
combined before incubation at room temperature for 20 min. Then,
the mixtures were added onto cells in respective groups. Six hour
later, the medium was replaced with F12/DMEM medium containing 10%
fetal bovine serum. After cultivation at 37°C and 5% CO2
for 48 h, the cells were collected for further assays.
To obtain mononuclear cells in bone marrow, bone
marrow samples were first mixed with equal amount of
phosphate-buffered saline (v/v, 1:1). Then, the mixture was added
gently onto Ficoll-Paque Premium lymphocyte separation medium (GE
Healthcare, Chicago, IL, USA) with a ratio of 2:1 before
centrifugation at 2,000 rpm for 20 min. After centrifugation, the
middle layer was aspirated and thoroughly mixed with 5 volumes of
phosphate-buffered saline before centrifugation at 1,500 rpm for 10
min. After discarding supernatant, 5 volumes of phosphate-buffered
saline were added again followed by thorough mixing. After
centrifugation at 1,500 rpm for 10 min, the supernatant was
discarded again. The cells that were attached on the bottom were
mononuclear cells in bone marrow.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extract from cells using TRIzol
reagent following the manufacturer's manual (Yeasen, Shanghai,
China). Then, total RNA was extracted using phenol chloroform
method. The concentration and quality of RNA was measured using
ultraviolet spectrophotometry (Nanodrop ND2000; Thermo Fisher
Scientific, Inc.). Then, cDNA was obtained by reverse transcription
from 1 µg RNA and stored at −20°C. Reverse transcription of mRNA
was performed using TIANScript II cDNA First Strand Synthesis kit
(Tiangen Biotech Co., Ltd., Beijing, China), and reverse
transcription of miRNA was carried out using miRcute miRNA cDNA
First Strand Synthesis kit (Tiangen Biotech Co., Ltd.).
SuperReal PreMix (SYBR-Green) RT-qPCR kit (Tiangen
Biotech Co., Ltd.) was used to detect mRNA expression of Abcb1a,
using GAPDH as internal reference. The sequences of Abcb1a were
5′-TGGGGCTGGACTTCCTCTCATGATGC-3′ (sense) and
5′-GCAGCAACCAGCACCCCAGCACCAAT-3′ (anti-sense). The sequences of GAP
DH were 5′-AGAAGGCTGGGGCTCATTTG-3′ (sense) and
5′-GGAACGCTTCACGAATTTG-3′ (anti-sense). The reaction system (25 µl)
was composed of 12.5 µl SYBR Premix EXTaq, 0.5 µl upstream primer,
0.5 µl downstream primer, 1 µl cDNA and 10.5 µl ddH2O.
PCR condition was: Initial denaturation at 95°C for 3 min; 40
cycles of denaturation at 95°C for 10 sec, annealing at 60°C for 30
sec, and elongation at 72°C for 20 sec (iQ5; Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The 2−ΔΔCq method (25) was used to calculate the relative
expression of Abcb1a mRNA against GAPDH. Each sample was tested in
triplicate.
The expression of miR-214 was determined by miRcute
miRNA RT-PCR kit (Tiangen Biotech Co., Ltd.), using U6 as internal
reference. The sequences of miR-214 primers were
5′-AGCATAATACAGCAGGCACAGAC-3′ (upstream) and
5′-AAAGGTTGTTCTCCACTCTCTCAC-3′ (downstream). The sequences of U6
were 5′-ATTGGAACGATACAGAGAAGATT-3′ (upstream) and
5′-GGAACGCTTCACGAATTTG-3′ (downstream). The reaction system (20 µl)
contained 10 µl RT-qPCR-Mix, 0.5 µl upstream primer, 0.5 µl
downstream universal primer, 2 µl cDNA and 7 µl ddH2O.
The reaction protocol was: Initial denaturation at 95°C for 3 min;
40 cycles of denaturation at 95°C for 12 sec, annealing at 62°C for
40 sec and 72°C for 20 sec (iQ5; Bio-Rad Laboratories, Inc.). The
2−ΔΔCq method (25) was
used to calculate the relative expression of miR-214 against U6.
Each sample was tested in triplicate.
Western blot analysis
Cells (1×106) in each group were
collected and precooled Radio-Immunoprecipitation Assay (RIPA)
lysis buffer (1,000 µl; 50 mM Tris-base, 1 mM EDTA, 150 mM NaCl,
0.1% sodium dodecyl sulfate, 1% TritonX-100, 1% sodium
deoxycholate; Beyotime Institute of Biotechnology, Shanghai, China)
was added to the cells. After lysis for 40 min on ice, the mixture
was centrifuged at 12,000 rpm and 4°C for 10 min. The supernatant
was used to determine protein concentration by bicinchoninic acid
(BCA) protein concentration determination kit (RTP7102, Real-Times
Biotechnology Co., Ltd., Beijing, China). Protein samples were then
mixed with 2× sodium dodecyl sulfate loading buffer before
denaturation in boiling water bath for 10 min. Afterwards, the
samples (20 µg) were subjected to 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (100 V). The resolved
proteins were transferred to polyvinylidene difluoride membranes on
ice (100 V, 2 h) and blocked with 5% skimmed milk at room
temperature for 1 h. Then, the membranes were incubated with rabbit
anti-human PGP (1:3,000; Abcam, Cambridge, UK) and rabbit
anti-human β-actin (1:5,000; Abcam) polyclonal primary antibodies
at 4°C overnight. After extensive washing with phosphate-buffered
saline with Tween 20 for 5 times of 5 min, the membranes were
incubated with goat anti-rabbit horseradish peroxidase-conjugated
secondary antibodies (1:3,000; Abcam) for 1 h at room temperature
before washing with phosphate-buffered saline with Tween 20 for 5
times of 5 min. Then, the membrane was developed with enhanced
chemiluminescence detection kit (Abcam) for imaging. Image Lab v3.0
software (Bio-Rad Laboratories, Inc.) was used to acquire and
analyze imaging signals. The relative content of target protein was
expressed against β-actin.
Bioinformatics
Bioinformatics prediction is a powerful tool for the
study of the functions of miRNAs. To understand the regulatory
mechanism of ABCB1, we used miRanda (http://www.microrna.org/microrna/home.do), TargetScan
(http://www.targetscan.org), PiTa
(http://genie.weizmann.ac.il/pubs/mir07/mir07_data.html),
RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/)
and PICTA (http://pictar.mdc-berlin.de/) to predict miRNA
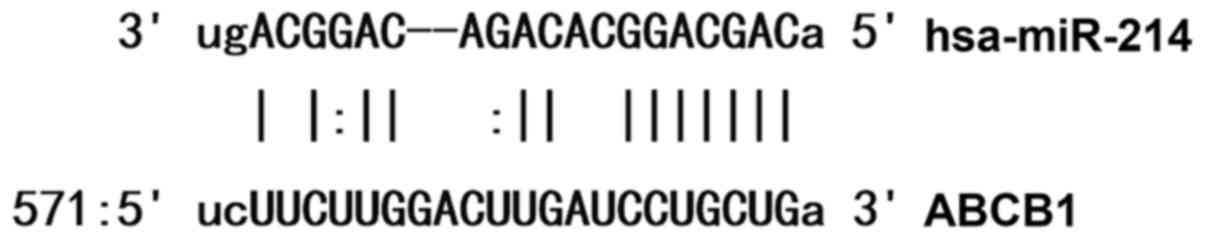
molecules that might regulate ABCB1, and found that miR-214 was
able to potentially regulate ABCB1 (Fig.
1).
Dual luciferase reporter assay
According to bioinformatics results, wild-type (WT)
and mutant seed regions of miR-214 in the 3′-UTR (150 bp upstream
and downstream of CCUGCUG) of ABCB1 gene were chemically
synthesized in vitro, added with Spe-1 and HindIII
restriction sites, and then cloned into pMIR-REPORT luciferase
reporter plasmids. Plasmids (0.8 µg) with WT or mutant 3′-UTR DNA
sequences were co-transfected with agomiR-214 (100 nM; Sangon
Biotech Co., Ltd.) into 293T cells. After cultivation for 24 h, the
cells were lysed using dual luciferase reporter assay kit (Promega
Corporation, Madison, WI, USA) according to the manufacturer's
manual, and luminescence intensity was measured using GloMax 20/20
luminometer (Promega Corporation). Using Renilla luminescence
activity as internal reference, the luminescence values of each
group of cells were measured.
MTT assay
After transfection, cells were seeded into 96-well
plates at a density of 2×103 cells per well and cultured
in the presence of l µM IM. Each condition was tested in triplicate
wells according to a previously published method (26). At 24, 48, and 72 h, 20 µl MTT (5 g/l,
JRDC000003; JRDUN Biotechnology, Shanghai, China) was added into
each well. On the last day, DMSO (150 µl per well) was added to
dissolve purple crystals after incubation at 37°C for 4 h. Then,
absorbance of each well was measured at 490 nm with a microplate
reader (Bio-Rad Laboratories, Inc.). Cell survival curves were
plotted.
Statistical analysis
The results were analyzed using SPSS v.18.0
statistical software (SPSS, Inc., Chicago, IL, USA). The data were
expressed as the mean ± standard deviation. Data were tested for
normality. Multigroup measurement data were analyzed using one-way
analysis of variance. In case of homogeneity of variance, the Least
Significant Difference and Student-Newman-Keuls post hoc methods
were used; in case of heterogeneity of variance, Tamhane's T2 or
Dunnett's T3 post hoc methods were used. P<0.05 was considered
to indicate a statistically significant difference.
Results
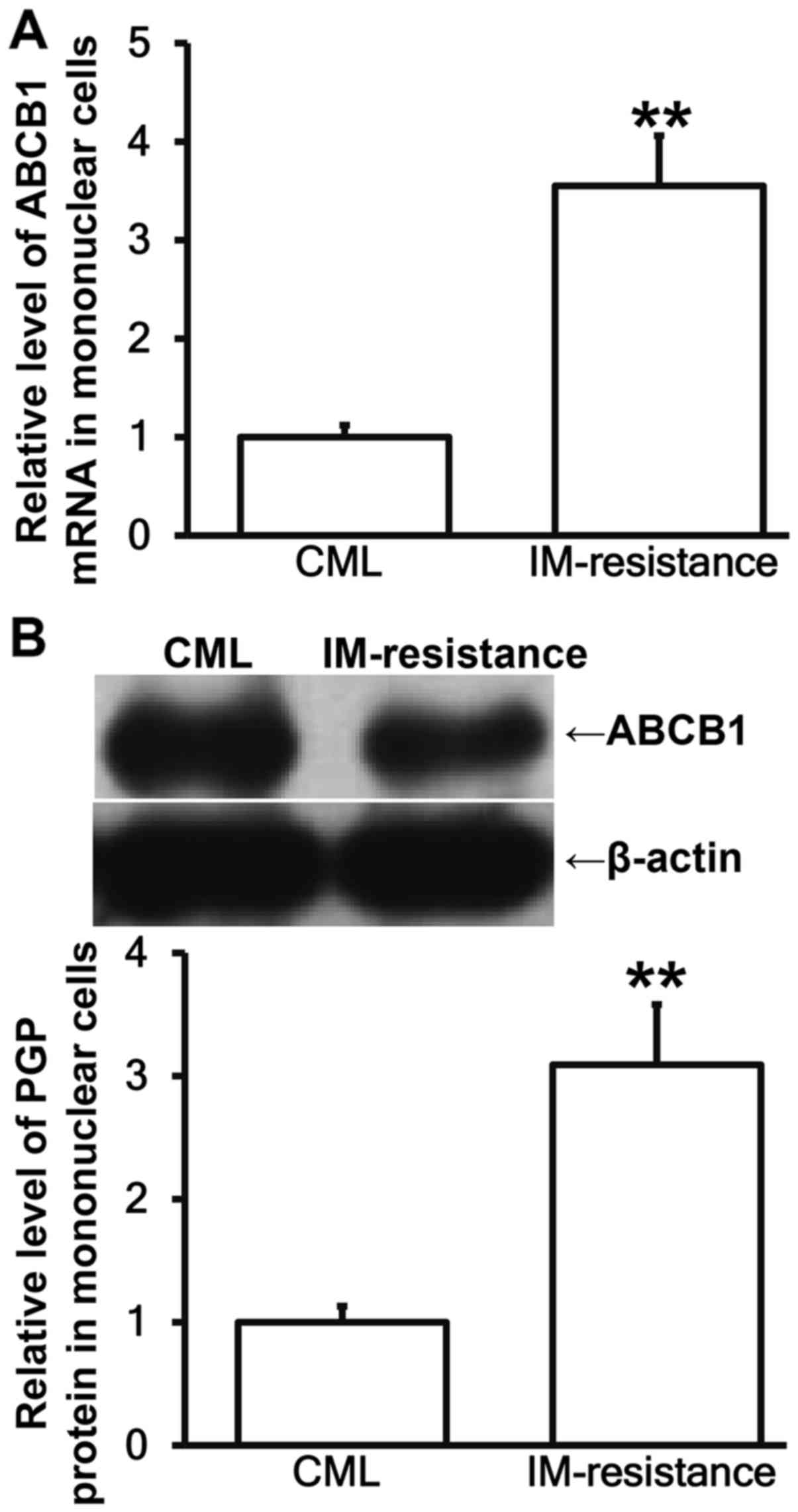
ABCB1 mRNA and PGP expression is
up-regulated in bone marrow mononuclear cells from CML patients
with IM resistance
To measure the expression of ABCB1 mRNA and its
protein PGP in bone marrow mononuclear cells, RT-qPCR and western
blotting were performed. The data showed that the levels of ABCB1
mRNA and PGP in mononuclear cells from patients with IM resistance
were significantly higher than those from patients without IM
resistance (P<0.05; Fig. 2A and
B). The result suggests that ABCB1 mRNA and PGP expression is
up-regulated in bone marrow mononuclear cells from CML patients
with IM resistance.
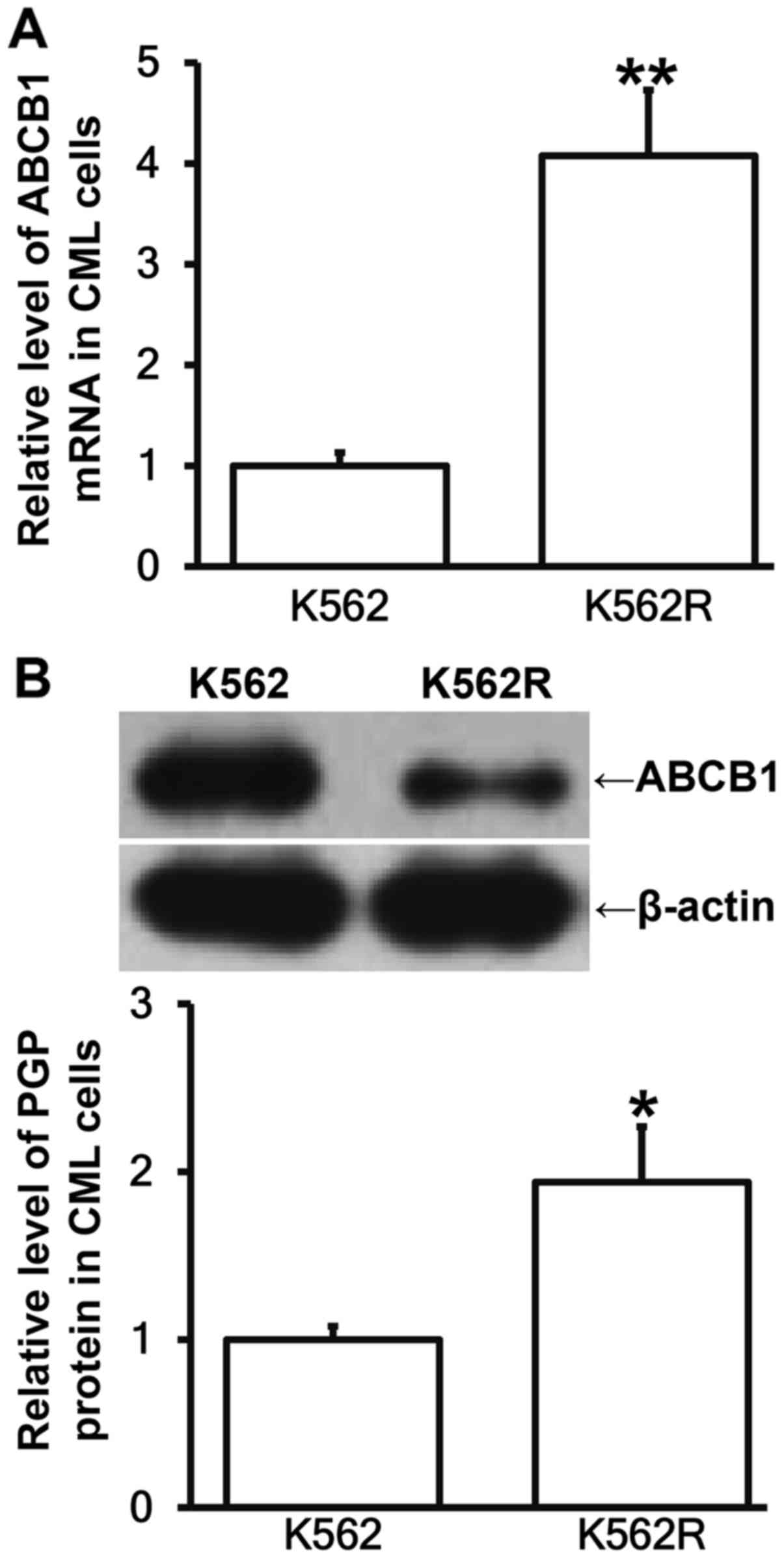
K562R cells have higher ABCB1 and PGP
expression than K562 cells, probably due to their different
sensitivity to IM
To determine the expression of ABCB1 mRNA and its
protein PGP in K562 and K562R cells, RT-qPCR and western blotting
were employed. The data showed that the levels of ABCB1 mRNA and
PGP in K562R cells were significantly increased than those in K562
cells (P<0.05; Fig. 3A and B).
The result indicates that K562R cells have higher ABCB1 and PGP
expression than K562 cells, probably due to their different
sensitivity to IM.
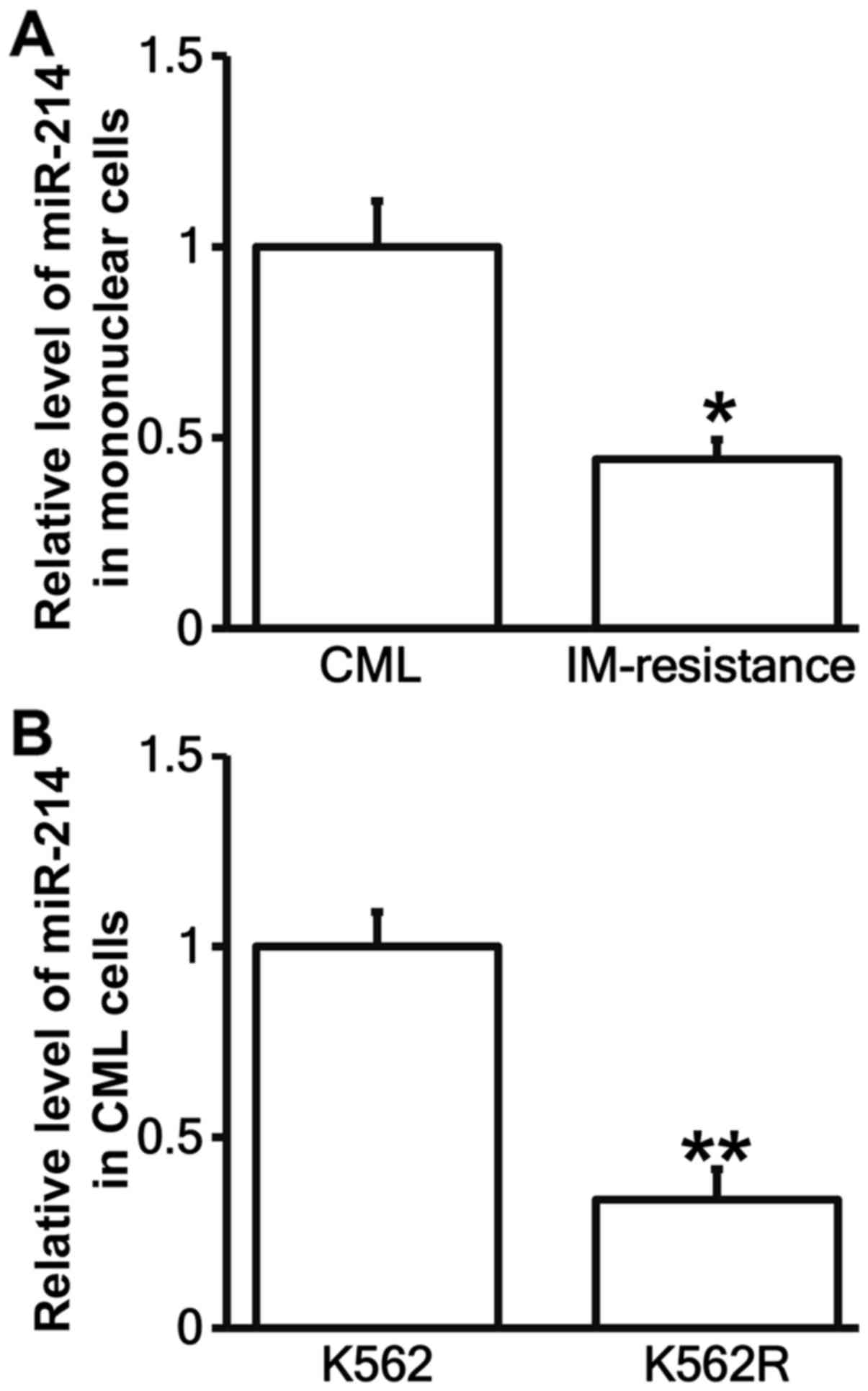
Expression miR-214 is decreased in
bone marrow mononuclear cells from patients with IM resistance and
K562R cells
To test the levels of miR-214 in bone marrow
mononuclear cells and K562R cells, RT-qPCR was carried out. The
data showed that miR-214 levels in bone marrow mononuclear cells
from patients with IM resistance were significantly lower than that
from patients without IM resistance (P<0.05; Fig. 4A). Similarly, miR-214 expression in
K562R cells was significantly decreased than that in K562 cells
(P<0.05; Fig. 4B). The results
suggest that the expression miR-214 is decreased in bone marrow
mononuclear cells from patients with IM resistance and K562R
cells.
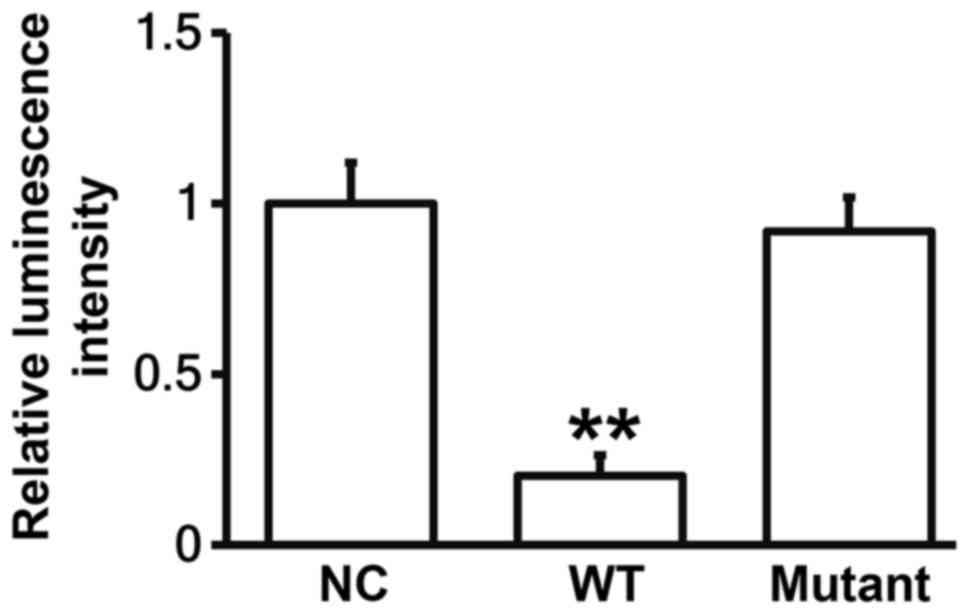
miR-214 can bind with the 3′-UTR seed
region of ABCB1 mRNA to regulate its expression
To identify the interaction between miR-214 and the
3′-UTR of ABCB1 mRNA, dual luciferase reporter assay was performed.
The luminescence value of cells co-transfected with agomiR-214 and
pMIR-REPORT-WT luciferase reporter plasmids was significantly lower
than that in negative control group (P<0.05). By contrast, the
luminescence value of cells co-transfected with agomiR-214 and
pMIR-REPORT-mutant luciferase reporter plasmids was not
significantly different from that in negative control group
(P>0.05; Fig. 5). The result
suggests that miR-214 can bind with the 3′-UTR seed region of ABCB1
mRNA to regulate its expression.
Elevated expression of miR-214
restores IM-sensitivity to K562R cells possibly by affecting ABCB1
expression
To test how miR-214 affects the expression of ABCB1
mRNA and PGP protein, we used RT-qPCR, western blotting and MTT
assay. The data showed that expression of miR-214 in K562R cells
after transfection with agomiR-214 was significantly enhanced
(P<0.05; Fig. 6A). In addition,
the expression of ABCB1 mRNA in K562R cells transfected with
agomiR-214 was significantly lower than that in K562R cells
transfected with negative control (P<0.05; Fig. 6B). Similarly, PGP expression in K562R
cells transfected with agomiR-214 was significantly reduced than
that in K562R cells transfected with negative control (P<0.05;
Fig. 6C). Of note, MTT assay showed
that the survival rate of K562R cells transfected with agomiR-214
was significantly reduced than those of K562R cells transfected
with negative control or K562 cells at 24, 48 and 72 h in the
presence of IM (P<0.05 for all points; Fig. 6D). The results indicate that elevated
expression of miR-214 restores IM sensitivity to K562R cells
possibly by affecting ABCB1 expression.
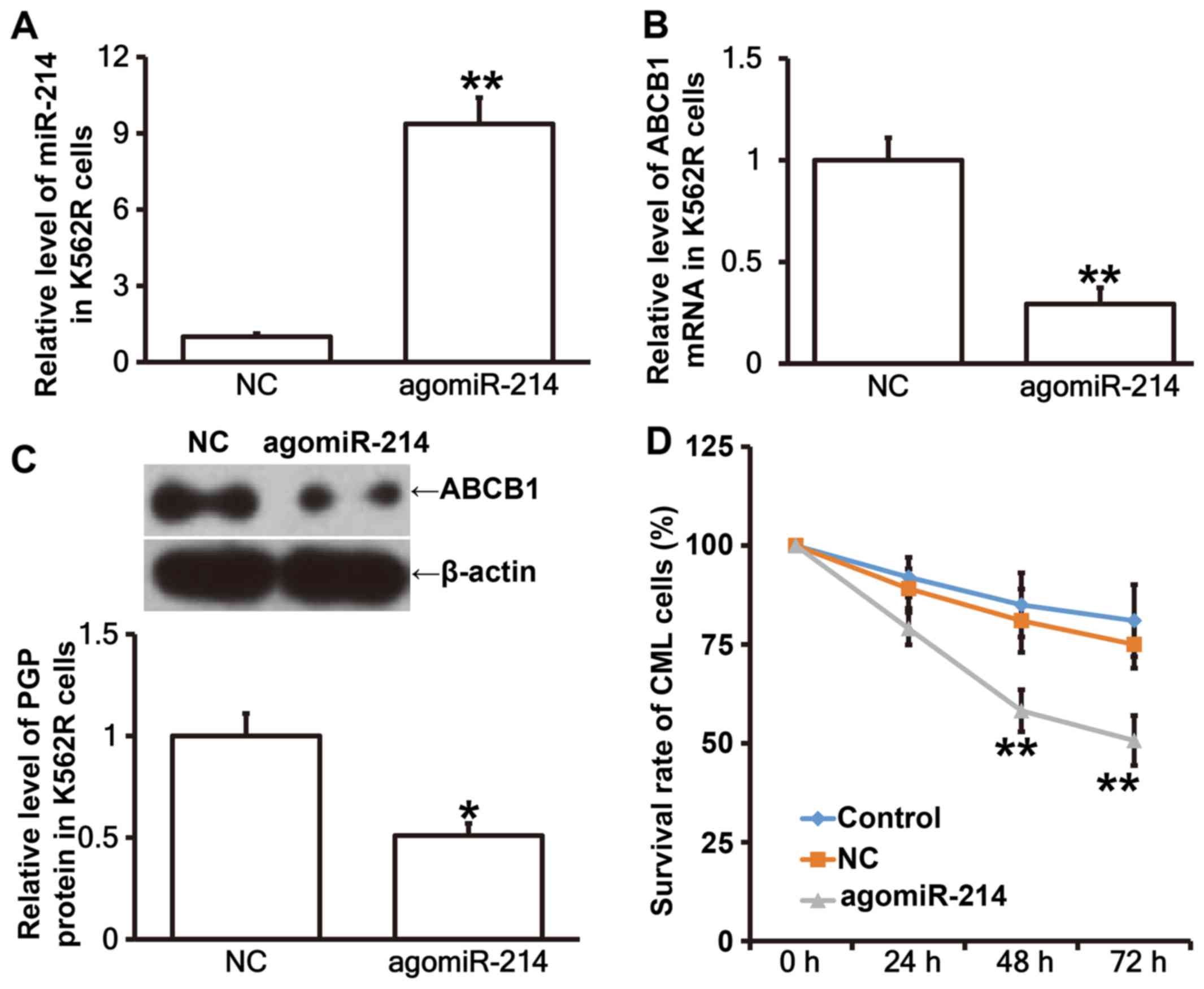 | Figure 6.Effect of miR-214 expression on the
expression of ABCB1 mRNA and PGP protein, as well as the survival
of CML cells. (A) Expression of miR-214 in K562R cells transfected
with NC or agomiR-214. Expression of (B) ABCB1 mRNA and (C) PGP in
K562R cells transfected with NC or agomiR-214. (D) Survival rate of
K562 cells (control), and K562R cells transfected with NC or
agomiR-214 in the presence of IM (1 µM). Reverse
transcription-quantitative polymerase chain reaction was employed
to measure the expression of miR-214 and ABCB1 mRNA, western
blotting was used to determine PGP expression, and MTT assay was
performed to determine cell survival rate. *P<0.05 and
**P<0.01 vs. NC group. miR, microRNA; ABCB1, adenosine
triphosphate binding cassette subfamily B member 1; PGP,
P-glycoprotein; CML, chronic myeloid leukemia; NC, negative
control; IM, imatinib mesylate. |
Discussion
CML is the first tumor that has targeted
therapeutics drugs designed according to its disease-causing gene
types (2). Discontinuation of IM
treatment results in high frequency of disease recurrence,
indicating that only a small proportion of patients may receive
curative or prolonged remission after treatment (27,28). IM
resistance has become an important issue in the treatment of CML.
Studies show that overexpression of multidrug transporter and drug
target enzyme may be involved in IM resistance of CML (29–31).
Multidrug transporters, such as PGP, MRPS, LRP and BCRP, can
increase intracellular drug efflux or vesicle isolation, resulting
in decreased intracellular drug concentration or altered drug
distribution. The best studied is MDR mediated by P-GP/MDRI and
MRPI. It is first discovered in colchicine-resistant Chinese
hamster ovary cells (32), and later
found in all tissues of the organism (33). MDR prevents the body from absorbing
harmful substances, mediates the output of substances, protects the
brain and testis (34). It is
reported that PGP expressed by ABCB1 gene in human body is closely
related to drug distribution and translocation (34). PGP utilizes ATP in the organism to
transport exogenous drugs and toxins outside the cells, reduces the
concentration of intracellular drugs and toxins, decreases effect
of drug treatment, and produces drug resistance (35). In the present study, we discover that
expression of ABCB1 mRNA and PGP in mononuclear cells from CML
patients with IM resistance is up-regulated, and similar trend is
also observed for K562R that has IM resistance. This suggests that
ABCB1 gene and its encoded protein play regulatory roles in IM
resistance.
Our further study is then focused on the upstream
miRNA that may regulate ABCB1 expression. It is reported that miRNA
molecules cut mRNA and inhibits its translation to achieve a
negative feedback regulation (36,37).
miRNA molecules are important regulators in development, normal
physiology, and diseases. Moreover, some miRNA molecules have
become biomarkers for diseases (38,39).
Using bioinformatics, we have discovered that miR-214 is an
upstream regulator gene of ABCB1. It is reported that miR-214 can
be used as a predictive factor for the diagnosis of gastric cancer,
and it can also affect the proliferation and invasion of tumor
cells (40). In addition, miR-214
can affect the proliferation and invasion of breast cancer through
P53 (41). miRNA-214 can also
inhibit bladder cancer growth by targeting PDRG1 gene (42). Moreover, Wan et al (43) report that miR-214 has a protective
role in the post treatment for myocardial ischemia, and Izawa et
al (44) discover that miR-214
effectively alleviates thioacetamide-induced cirrhosis and may have
an anti-fibrotic effect. In the present study, we find that
expression of miR-214 is reduced in both mononuclear cells from
IM-resistant CML patients and K562R cells. Furthermore, dual
luciferase reporter assay demonstrates that miR-214 directly binds
with the 3′-UTR seeding region of ABCB1 mRNA and regulates its
expression. Then, we have transfected agomiR-214 into K562R cells
to up-regulate the expression of miR-214 in these cells. After
culturing the cells in IM medium, we find that the survival rate of
K562R cells transfected with agomiR-214 is reduced in the presence
of IM, suggesting that the sensitivity of K562R cells to IM is
partially restored by overexpression of miR-214. The limitations of
the study include low sequence homology between miRNA-214 and the
ABCB1 gene and relatively small sample numbers in some experiments.
We will improve this in future studies.
In conclusion, the present study demonstrates that
miR-214 alters the expression of PGP by targeting ABCB1, and
elevates IM resistance of CML cells. It plays an important
biological role in IM resistance of CML patients.
Acknowledgements
The authors would like to thank the research teams
at the Department of Pharmacy, Jining No. 1 People's Hospital
(Shandong, China), and the Department of Pharmacy, Qilu Medical
University (Shandong, China) for their assistance during the
present study.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JJ and JY collaborated on the design of the present
study, performed the experiments, and analyzed the data. FY, ZJ and
DL contributed to the literature review, and assisted with the
experimental design and completion. SW was responsible for
experimental design, data collection and analysis, and writing the
manuscript. All authors collaborated to interpret the results and
develop the manuscript. The final version of the manuscript has
been read and approved by all authors.
Ethics approval and consent to
participate
All procedures were approved by the Ethics Committee
of Jining No. 1 People's Hospital (Shandong, China). Written
informed consents were obtained from all patients or their
families.
Consent for publication
Written informed consent for the publication of any
associated data and accompanying images were obtained from all
patients, or their parents, guardians or next of kin.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nowell PC: The minute chromosome (Phl) in
chronic granulocytic leukemia. Blut. 8:65–66. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hehlmann R, Hochhaus A and Baccarani M:
European LeukemiaNet: Chronic myeloid leukaemia. Lancet.
370:342–350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berger U, Maywald O, Pfirrmann M, Lahaye
T, Hochhaus A, Reiter A, Hasford J, Heimpel H, Hossfeld DK, Kolb
HJ, et al: Gender aspects in chronic myeloid leukemia: Long-term
results from randomized studies. Leukemia. 19:984–989. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang JX, Huang XJ, Wu DP, Hu JD, Liu T, Hu
Y, Meng FY, Chen XQ, Hou M, Li Y, et al: Overview of chronic
myelogenous leukemia and its current diagnosis and treatment
patterns in 15 hospitals in China. Zhonghua Xue Ye Xue Za Zhi.
30:721–725. 2009.(In Chinese). PubMed/NCBI
|
|
5
|
Cohen MH, Williams G, Johnson JR, Duan J,
Gobburu J, Rahman A, Benson K, Leighton J, Kim SK, Wood R, et al:
Approval summary for imatinib mesylate capsules in the treatment of
chronic myelogenous leukemia. Clin Cancer Res. 8:935–942.
2002.PubMed/NCBI
|
|
6
|
Eskazan AE, Ar MC and Soysal T: Critical
appraisal of European LeukemiaNet (ELN) 2013 recommendations for
the management of chronic myeloid leukemia: Is it early for a
warning? Expert Rev Hematol. 9:919–921. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Höglund M, Sandin F, Hellström K, Björeman
M, Björkholm M, Brune M, Dreimane A, Ekblom M, Lehmann S, Ljungman
P, et al: Tyrosine kinase inhibitor usage, treatment outcome, and
prognostic scores in CML: Report from the population-based Swedish
CML registry. Blood. 122:1284–1292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kantarjian H, O'Brien S, Jabbour E,
Garcia-Manero G, Quintas-Cardama A, Shan J, Rios MB, Ravandi F,
Faderl S, Kadia T, et al: Improved survival in chronic myeloid
leukemia since the introduction of imatinib therapy: A
single-institution historical experience. Blood. 119:1981–1987.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shah NP: Loss of response to imatinib:
Mechanisms and management. Hematology Am Soc Hematol Educ Program.
183–187. 2005.PubMed/NCBI
|
|
10
|
La Rosée P and Hochhaus A: Resistance to
imatinib in chronic myelogenous leukemia: Mechanisms and clinical
implications. Curr Hematol Malig Rep. 3:72–79. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hochhaus A and Hughes T: Clinical
resistance to imatinib: Mechanisms and implications. Hematol Oncol
Clin North Am. 18(641–656): ix2004.
|
|
12
|
Vine J, Cohen SB, Ruchlemer R, Goldschmidt
N, Levin M, Libster D, Gural A, Gatt ME, Lavie D, Ben-Yehuda D and
Rund D: Polymorphisms in the human organic cation transporter and
the multidrug resistance gene: Correlation with imatinib levels and
clinical course in patients with chronic myeloid leukemia. Leuk
Lymphoma. 55:2525–2531. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gromicho M, Dinis J, Magalhães M,
Fernandes AR, Tavares P, Laires A, Rueff J and Rodrigues AS:
Development of imatinib and dasatinib resistance: Dynamics of
expression of drug transporters ABCB1, ABCC1, ABCG2, MVP, and
SLC22A1. Leuk Lymphoma. 52:1980–1990. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mlejnek P, Kosztyu P, Dolezel P, Bates SE
and Ruzickova E: Reversal of ABCB1 mediated efflux by imatinib and
nilotinib in cells expressing various transporter levels. Chem Biol
Interact. 273:171–179. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou ZY, Wan LL, Yang QJ, Han YL, Li D, Lu
J and Guo C: Nilotinib reverses ABCB1/P-glycoprotein-mediated
multidrug resistance but increases cardiotoxicity of doxorubicin in
a MDR xenograft model. Toxicol Lett. 259:124–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Negi LM, Jaggi M, Joshi V, Ronodip K and
Talegaonkar S: Hyaluronan coated liposomes as the intravenous
platform for delivery of imatinib mesylate in MDR colon cancer. Int
J Biol Macromol. 73:222–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leslie EM, Deeley RG and Cole SP:
Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2,
and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol.
204:216–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Graziano A, Monte Lo G, Piva I, Caserta D,
Karner M, Engl B and Marci R: Diagnostic findings in adenomyosis: A
pictorial review on the major concerns. Eur Rev Med Pharmacol Sci.
19:1146–1154. 2015.PubMed/NCBI
|
|
19
|
Jia W, Wu Y, Zhang Q, Gao GE, Zhang C and
Xiang Y: Expression profile of circulating microRNAs as a promising
fingerprint for cervical cancer diagnosis and monitoring. Mol Clin
Oncol. 3:851–858. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang XI, Luo Y, Zhao S, Chen Q, Jiang C,
Dai Y, Chen Y and Cao Z: Clinical significance and expression of
microRNA in diabetic patients with erectile dysfunction. Exp Ther
Med. 10:213–218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou H, Li Y, Liu B, Shan Y, Li Y, Zhao L,
Su Z and Jia L: Downregulation of miR-224 and let-7i contribute to
cell survival and chemoresistance in chronic myeloid leukemia cells
by regulating ST3GAL IV expression. Gene. 626:106–118. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu L, Wang S, Chen R, Wu Y, Zhang B,
Huang S, Zhang J, Xiao F, Wang M and Liang Y: Myc induced
miR-144/451 contributes to the acquired imatinib resistance in
chronic myelogenous leukemia cell K562. Biochem Biophys Res Commun.
425:368–373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baccarani M, Saglio G, Goldman J, Hochhaus
A, Simonsson B, Appelbaum F, Apperley J, Cervantes F, Cortes J,
Deininger M, et al: Evolving concepts in the management of chronic
myeloid leukemia: Recommendations from an expert panel on behalf of
the European LeukemiaNet. Blood. 108:1809–1820. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong
Q, Qin L, Wu X, Zheng Y, Yang Y, et al: Identification of miRNomes
in human liver and hepatocellular carcinoma reveals miR-199a/b-3p
as therapeutic target for hepatocellular carcinoma. Cancer Cell.
19:232–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leone G, D'Alò F, Zardo G, Voso MT and
Nervi C: Epigenetic treatment of myelodysplastic syndromes and
acute myeloid leukemias. Curr Med Chem. 15:1274–1287. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mahon FX, Réa D, Guilhot J, Guilhot F,
Huguet F, Nicolini F, Legros L, Charbonnier A, Guerci A, Varet B,
et al: Discontinuation of imatinib in patients with chronic myeloid
leukaemia who have maintained complete molecular remission for at
least 2 years: The prospective, multicentre Stop Imatinib (STIM)
trial. Lancet Oncol. 11:1029–1035. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cortes J, O'Brien S and Kantarjian H:
Discontinuation of imatinib therapy after achieving a molecular
response. Blood. 104:2204–2205. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
D'Cunha R, Bae S, Murry DJ and An G: TKI
combination therapy: Strategy to enhance dasatinib uptake by
inhibiting Pgp- and BCRP-mediated efflux. Biopharm Drug Dispos.
37:397–408. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eadie LN, Dang P, Saunders VA, Yeung DT,
Osborn MP, Grigg AP, Hughes TP and White DL: The clinical
significance of ABCB1 overexpression in predicting outcome of CML
patients undergoing first-line imatinib treatment. Leukemia.
31:75–82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Silva KL, de Souza PS, de Moraes Nestal G,
Moellmann-Coelho A, Fda Vasconcelos C and Maia RC: XIAP and
P-glycoprotein co-expression is related to imatinib resistance in
chronic myeloid leukemia cells. Leuk Res. 37:1350–1358. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou SF: Structure, function and
regulation of P-glycoprotein and its clinical relevance in drug
disposition. Xenobiotica. 38:802–832. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thiebaut F, Tsuruo T, Hamada H, Gottesman
MM, Pastan I and Willingham MC: Cellular localization of the
multidrug-resistance gene product P-glycoprotein in normal human
tissues. Proc Natl Acad Sci USA. 84:7735–7738. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Silva R, Vilas-Boas V, Carmo H,
Dinis-Oliveira RJ, Carvalho F, de Lourdes Bastos M and Remião F:
Modulation of P-glycoprotein efflux pump: Induction and activation
as a therapeutic strategy. Pharmacol Ther. 149:1–123. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fellner S, Bauer B, Miller DS, Schaffrik
M, Fankhänel M, Spruss T, Bernhardt G, Graeff C, Färber L,
Gschaidmeier H, et al: Transport of paclitaxel (Taxol) across the
blood-brain barrier in vitro and in vivo. J Clin Invest.
110:1309–1318. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Inoue K: MicroRNA function in animal
development. Tanpakushitsu Kakusan Koso. 52:197–204.
2007.PubMed/NCBI
|
|
37
|
Williams AE, Moschos SA, Perry MM, Barnes
PJ and Lindsay MA: Maternally imprinted microRNAs are
differentially expressed during mouse and human lung development.
Dev Dyn. 236:572–580. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li X, Yu Z, Li Y, Liu S, Gao C, Hou X, Yao
R and Cui L: The tumor suppressor miR-124 inhibits cell
proliferation by targeting STAT3 and functions as a prognostic
marker for postoperative NSCLC patients. Int J Oncol. 46:798–808.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lv ZC, Fan YS, Chen HB and Zhao DW:
Investigation of microRNA-155 as a serum diagnostic and prognostic
biomarker for colorectal cancer. Tumour Biol. 36:1619–1625. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang KC, Xi HQ, Cui JX, Shen WS, Li JY,
Wei B and Chen L: Hemolysis-free plasma miR-214 as novel biomarker
of gastric cancer and is correlated with distant metastasis. Am J
Cancer Res. 5:821–829. 2015.PubMed/NCBI
|
|
41
|
Wang F, Lv P, Liu X, Zhu M and Qiu X:
microRNA-214 enhances the invasion ability of breast cancer cells
by targeting p53. Int J Mol Med. 35:1395–1402. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang J, Zhang X, Wang L, Yang Y, Dong Z,
Wang H, Du L and Wang C: MicroRNA-214 suppresses oncogenesis and
exerts impact on prognosis by targeting PDRG1 in bladder cancer.
PLoS One. 10:e01180862015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wan DY, Zhang Z and Yang HH:
Cardioprotective effect of miR-214 in myocardial ischemic
postconditioning by down-regulation of hypoxia inducible factor 1,
alpha subunit inhibitor. Cell Mol Biol (Noisy-le-grand). 61:1–6.
2015.PubMed/NCBI
|
|
44
|
Izawa T, Horiuchi T, Atarashi M, Kuwamura
M and Yamate J: Anti-fibrotic role of miR-214 in
thioacetamide-induced liver cirrhosis in rats. Toxicol Pathol.
43:844–851. 2015. View Article : Google Scholar : PubMed/NCBI
|