Introduction
Immunoglobulin A nephropathy (IgAN) is the most
common form of primary glomerulonephritis globally (1). According to the International Kidney
Biopsy Survey, IgAN was diagnosed in 22% of all glomerular diseases
in Europe and in up to 39% in Asia (2). The clinical manifestations of IgAN are
variable, ranging from isolated hematuria to rapidly progressive
renal dysfunction. Correspondingly, the histological lesions are
diverse, ranging from mild mesangial proliferative
glomerulonephritis to crescentic glomerulonephritis. Approximately
50% of patients with IgAN develop end-stage renal disease (ESRD)
within 30 years, which indicates that IgAN is an important cause of
ESRD (3).
The pathological lesions of IgAN are characterized
by mesangial IgA (primarily IgA1) deposits, viewed by
immunofluorescence, frequently concomitant with the deposition of
IgG and complement factor 3 (1).
Therefore, IgAN is considered to be an immune-mediated kidney
disease and is treated through administration of immunosuppressive
treatments, including mycophenolate mofetil (MMF) (4). Mycophenolic acid (MPA) is an active
compound derived from MMF, which inhibits inosine 5′-monophosphate
dehydrogenase reversibly and non-competitively and is essential for
de novo biosynthesis of guanine nucleotides and lymphocyte
proliferation (5,6). MMF has been commonly used in patients
undergoing solid organ transplantation (7,8) and in
recent years it has been applied in IgAN. However, the efficacy and
safety of MMF in IgAN remains controversial. Randomized controlled
trials (RCTs) and previous meta-analyses led to inconsistent
conclusions (9–21). Various studies have determined that
there were no differences between therapeutic regimens that did and
did not utilize MMF in patients with IgAN (10,12,15,16).
However, other studies have demonstrated that the therapeutic
regimens with MMF were superior to those without (11,13,14,17). The
previously published meta-analyses also demonstrated different
results; some demonstrated the superiority of MMF for IgAN
(19,21), but others failed to exhibit any
difference (18,20). Therefore, the efficacy and safety of
MMF for IgAN is yet to be fully elucidated. To examine the efficacy
and safety of MMF for IgAN, a meta-analysis of RCTs was performed
in the present study on the basis of the most complete
evidence.
Materials and methods
Data sources and searches
Two independent assessments of the literature were
performed using three computerized databases, PubMed/MEDLINE
(https://www.ncbi.nlm.nih.gov/pubmed/), EMBASE
(https://www.embase.com/login) and the
Cochrane Central Register of Controlled Trials (CCRCT; http://www.cochranelibrary.com), prior to October
2017. The following medical subject heading terms and text words
were used: Mycophenolate mofetil, mycophenolic acid and IgA
nephropathy. The searches were restricted to clinical trials in the
three databases. No language restriction was applied.
Study selection and outcome
measures
Two independent assessments evaluated all retrieved
titles and abstracts for eligibility and a detailed evaluation by
full-text review of the publications that were likely to meet the
inclusion criteria was performed. The present meta-analysis
included RCTs, which investigated efficacy and/or safety of MMF for
patients with IgAN and reported at least one outcome measure,
including primary endpoint of renal remission (i.e., complete and
partial remission) and secondary endpoints of ESRD and adverse
events. The original definitions of complete and partial remission
were very similar among the included trials and were therefore
applied in the present meta-analysis. Any disagreement or
uncertainty was discussed for consensus.
Study quality assessment
The methodological quality of the included RCTs was
assessed twice, according to Cochrane recommendations and the
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
guidelines (22,23). The following six items were
considered for the assessment of methodological quality: Adequate
generation of randomization, blinding, allocation concealment,
incomplete outcome data, selective outcome reporting and possible
sources of other bias. The procedure of assessment was performed
according to the following criteria: ‘Yes’ (low risk) or adequate,
if the item was described clearly and adequately; ‘no’ (high risk)
or inadequate, if the item was not described adequately; or
‘unclear’ if the information was insufficient to judge the risk of
bias as ‘low’ or ‘high.’ The quality of assessment was evaluated
using the κ statistic. κ statistic was calculated with the
following formula:
κ=(p0-pe)/(1-pe),
p0=(a+d)/(a+b+c+d),
pe=pyes+pno, pyes=
(a+b)/(a+b+c+d)·(a+c)/(a+b+c+d),
pno=(c+d)/(a+b+c+d)·(b+d)/ (a+b+c+d). Where a, b, c, and
d represent the data in the 4-fold table.
Data extraction and synthesis
Two independent data extractions of the
characteristics of the included RCTs (country, sample size, study
period, regimens of the treatment and control groups, definitions
of complete and partial remission and duration of follow-up), study
population (age, sex, population setting, clinical and laboratory
parameters) and outcome measures (renal remission, ESRD and adverse
events) were performed.
The pooled risk ratio (RR) with 95% confidence
interval (CI) was estimated for all dichotomous outcome measures.
Heterogeneity was assessed with Cochran's Q test (heterogeneity
χ2) and I2 and H statistics. Since the
estimated intervention effects in the included trials were not
consistent, data synthesis was performed using the random effects
model (Knapp-Hartung method). Random effects meta-regression was
performed to examine the sources of heterogeneity by evaluating the
correlations between logRRs of the primary endpoint and
co-variables [characteristics of the population and the included
trials that may be associated with outcomes (sex, age, population
settings, country, laboratory parameters at baseline and the
periods of follow-up)]. Subgroup analysis was applied when the
source of heterogeneity was identified. Publication bias was
investigated using Egger's regression analysis and visual
examination of funnel plots.
Stata 11.0 (StataCorp LLC, College Station, TX, USA)
was used for all analytical procedures. Two-sided P-values <0.05
were considered to indicate a statistically significant difference
and P<0.10 was applied for heterogeneity tests.
Results
Description of included trials
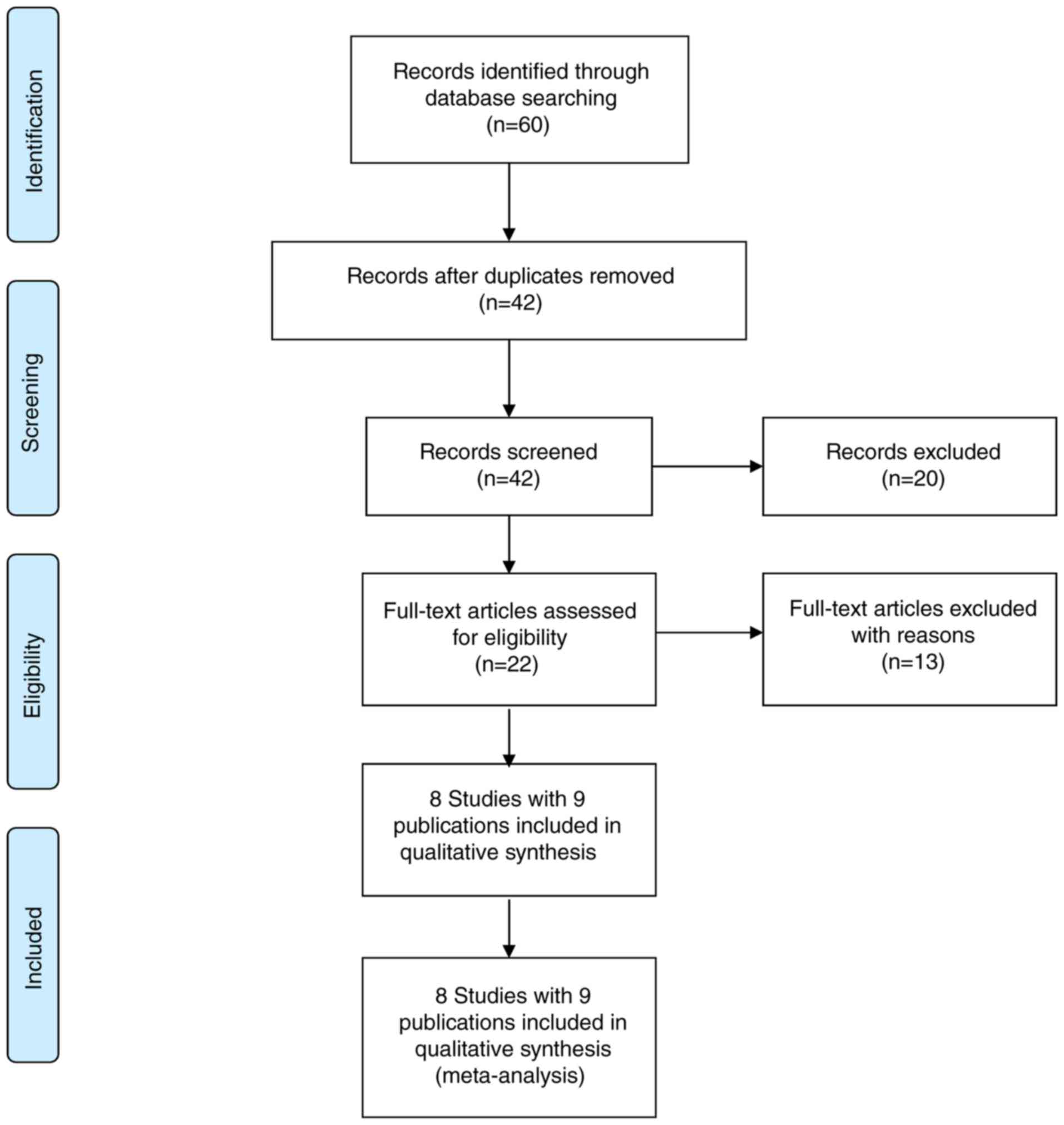
A total of 42 publications were identified in the
trial selection process following exclusion of duplications from
the three electronic databases (PubMed/MEDLINE, EMBASE and CCRCT).
The screening process is summarized in Fig. 1. A total of 20 articles were excluded
in the first round, including 16 irrelevant articles and four
reviews, and 22 full-text articles were further evaluated for
eligibility. A further 13 publications were excluded for the
following reasons: One as non-RCT, six as study protocols of
clinical trials and six as multiple publications. Consequently,
eight RCTs with nine publications (9–17) (510
participants) were included in the present meta-analysis to
evaluate the efficacy and safety of MMF for patients with IgAN.
Tang et al (13,14) reported short-term and long-term
outcomes of the same trial in two publications.
The baseline characteristics of trials and study
populations are listed in Table I.
The population refers to patients with overt proteinuria; patients
with severe renal dysfunction were excluded. Renal pathological
lesions were considered as inclusion criteria in six of the
included trials. Four of the eight included trials compared MMF
with a placebo and the other four trials compared immunosuppressive
regimens with and without MMF. Mean ages and percentage of male
patients ranged from 28.5 to 42.7 years and from 35.0 to 84.4%,
respectively. The periods of follow-up ranged between 6 and 72
months. Furthermore, the definitions of renal remission (i.e.,
complete remission and partial remission) in the included trials
are described in Table II,
illustrating similarities.
 | Table I.Characteristics of the included
trials. |
Table I.
Characteristics of the included
trials.
| Author, year | Country | Population
setting | No. of patients | Male, % | Mean age, years | eGFR, ml/min/1.73
m2 | SCr, µmol/l | UPE, g/day | Regimens in MMF
groups (treatment time) | Regimens in control
groups (treatment time) | months | Follow-up,
(Refs.) |
|---|
| Hou et al,
2017 | China | IgAN with active | 174 | 44.3 | 31.5 | MMF: 90.2 | MMF: 85.4 | MMF: | MMF 1.5 g/day + | Prednisone | 12 | (9) |
|
|
| proliferative
lesions; |
|
|
| (64.4–109.6); | (68.1–109.6); | 2.37±1.23; | prednisolone
(6m) | (6m) |
|
|
|
|
| UPE ≥1.0 g/day;
eGFR |
|
|
| Control: 94.3 | Control: 80.4 | Control: |
|
|
|
|
|
|
| ≥30 ml/min/1.73
m2 |
|
|
| (72.2–111.4) | (64.1–105.7) | 2.47±2.01 |
|
|
|
|
| Hogg et al,
2015 | USA | IgAN with UPCR ≥0.6
g/day | 44 | 62.0 | 32.0 | MMF: | NS | MMF: | MMF 25–36 | Placebo | 12 | (10) |
|
|
| (males) or ≥0.8
g/day (females); |
|
|
| 95.3±36.5; |
| 1.59±0.90; | mg/kg/day
(12m) |
|
|
|
|
|
| and eGFR ≥50
ml/min/1.73 m2 | |
|
| Control: |
| Control: |
|
|
|
|
|
|
| or ≥40 ml/min/1.73
m2 in those already receiving RASI | |
|
| 105.6±49.0 |
| 1.40±0.56 |
|
|
|
|
| Liu et al,
2014 | China | IgAN ≥Lee III, UPE
≥1.0 g/day, | 84 | 60.7 | 38.6 | MMF: | MMF: | MMF: | MMF 1.5 g/day
+ | CTX + | 18 | (11) |
|
|
| and SCr <267
µmol/l |
|
|
| 56.5±17.4; | 134.7±35.2; | 2.83±0.65; | prednisone | prednisone |
|
|
|
|
|
|
|
|
| Control: | Control: | Control: | (12m); | (12m) |
|
|
|
|
|
|
|
|
| 52.9±18.8 | 127.3±31.4 | 2.77±0.81 |
|
|
|
|
| Liu et al,
2010 | China | IgAN with
nephrotic | 40 | 52.5 | 32.0 | NS | MMF: | MMF: | MMF 1.5 g/day
+ | LEF + | 6 | (12) |
|
|
| syndrome |
|
|
|
| 92.8±26.1; | 4.9±2.4; | prednisone
(6m) | prednisone |
|
|
|
|
|
|
|
|
|
| Control: | Control: |
| (6m) |
|
|
|
|
|
|
|
|
|
| 96.4±24.6 | 4.8±2.6 |
|
|
|
|
| Tang et al,
2010; | China | IgAN with UPE >1
g/day, | 40 | 35.0 | 42.7 | MMF: | MMF: | MMF: | MMF 1.5–2.0 | Placebo (6m) | 72 | (13,14) |
| Tang et al,
2005 |
| SCr ≤300 µmol/l,
and Haas |
|
|
| 75±7.3; | 135.25±15.03; | 1.8±0.21; | g/day (6m) |
|
|
|
|
|
| subclass II–IV |
|
|
| Control: | Control: | Control: |
|
|
|
|
|
|
|
|
|
|
| 69±7.1 | 145.86±20.33 | 1.87±0.28 |
|
|
|
|
| Frisch et
al, 2005 | USA | IgAN with UPE >1
g/day | 32 | 84.4 | 38.1 | MMF: | MMF: | MMF: | MMF 2.0 g/day | Placebo (12m) | 24 | (15) |
|
|
| plus ≥2 of the
following risk |
|
|
| 38±22.2; | 229.84±106.08; | 2.7±1.6; | (12m) |
|
|
|
|
|
| factors: male sex,
hypertension, |
|
|
| Control: | Control: | Control: |
|
|
|
|
|
|
| CrCl <80 ml/min
and severe lesions on biopsy |
|
|
| 41±26.3 | 194.48±63.65 | 2.7±1.4 |
|
|
|
|
| Maes et al,
2004 | Belgium | IgAN with CCr 20–70
ml/min/ | 34 | 70.6 | 40.5 | MMF: | MMF: | MMF: | MMF 2 g/day | Placebo (36m) | 36 | (16) |
|
|
| 1.73 m2
and/or >1 g/day/1.73 m2 |
|
|
| 60±7; | 129.06±7.07; | 1.9±0.3; | (36m) |
|
|
|
|
|
| and/or hypertension
and/or |
|
|
| Control: | Control: | Control: |
|
|
|
|
|
|
| histologic
unfavorable criteria |
|
|
| 69±7 | 122.88±8.84 | 1.3±0.4 |
|
|
|
|
| Chen et al,
2002 | China | IgAN with Lee
IV–V, | 62 | 75.8 | 28.5 | NS | NS | MMF: 3.2±1.7; | MMF 1.0–1.5
g/day | Prednisone | 18 | (17) |
|
|
| interstitial area
with inflammatory cell infiltration, UPE ≥2.0 g/day and SCr <355
µmol/l |
|
|
|
|
| Control:
2.9±1.5 | (>6m) | 0.8 mg/kg/day
(>6m) |
|
|
 | Table II.Definitions of renal remission in the
included trials. |
Table II.
Definitions of renal remission in the
included trials.
| Author, year | Complete
remission | Partial
remission | (Refs.) |
|---|
| Hou et al,
2017 | Undetectable
proteinuria and a stable SCr level (≤25% above the baseline) | 0.4<UPE <1.0
g/day, serum albumin level ≥35 g/l, and stable SCr (≤25% above the
baseline) | (9) |
| Hogg et al,
2015 | UPCR <0.2
g/g | UPCR <50% of
level at time of randomization | (10) |
| Liu et al,
2014 | UPE <0.4 g/day,
serum albumin >35 g/l and stable renal function | UPE declined from
≥50% of the basal value, with stable renal function | (11) |
| Liu et al,
2010 | UPE <0.3 g/day,
normal levels of serum albumin and SCr and <5/HP RBCs in urinary
sediment | UPE 0.3–3.0 g/day
or reduced to ≥50% of that before therapy, serum albumin level ≥30
g/l, stable or improved renal function and the decreased RBC count
in the urinary sediment | (12) |
| Tang et al,
2005 | UPE <0.3
g/day | Decline of UPE ≥50%
over baseline value, but UPE >0.3 g/day | (14) |
| Frisch et
al, 2005 | NS | ≥50% decrease in
proteinuria | (15) |
| Chen et al,
2002 | UPE <0.2 g/day
and normal renal function | ≥50% decrease in
proteinuria and SCr decreased to normal level or declined ≥50% of
basal level | (17) |
Methodological quality assessment was accomplished
with a high estimated level of consistence (κ statistic=1.0), which
is described in detail in Table
III. The sequence generation was adequate in three trials and
unclear in five trials due to insufficient information. Allocation
concealment was adequate in two trials and unclear in the other
six. Blinding was performed adequately in two trials, inadequately
in two and was unclear in four. Incomplete outcome data were
addressed adequately in six trials and inadequately in two.
Selective reporting was adequate in six trials and inadequate in
two. A total of six trials were free of other bias, one trial was
unclear and one trial had a high risk of bias due to early
termination.
 | Table III.Risk analysis of potential bias. |
Table III.
Risk analysis of potential bias.
| Author, year | Adequate sequence
generation | Adequate allocation
concealment | Blinding | Address incomplete
outcome data | Selective outcome
report | Free of other
bias | (Refs.) |
|---|
| Hou et al,
2017 | Yes | Yes | Yes | Yes | Yes | Yes | (9) |
| Hogg et al,
2015 | Yes | Unclear | Unclear | No | No | Yes | (10) |
| Liu et al,
2014 | Unclear | Unclear | Unclear | Yes | Yes | Yes | (11) |
| Liu et al,
2010 | Unclear | Unclear | Unclear | Yes | Yes | Yes | (12) |
| Tang et al,
2005 | Unclear | Unclear | No | Yes | Yes | Yes | (14) |
| Frisch et
al, 2005 | Yes | Yes | Yes | Yes | Yes | Noa | (15) |
| Maes et al,
2004 | Unclear | Unclear | Unclear | No | No | Unclear | (16) |
| Chen et al,
2002 | Unclear | Unclear | No | Yes | Yes | Yes | (17) |
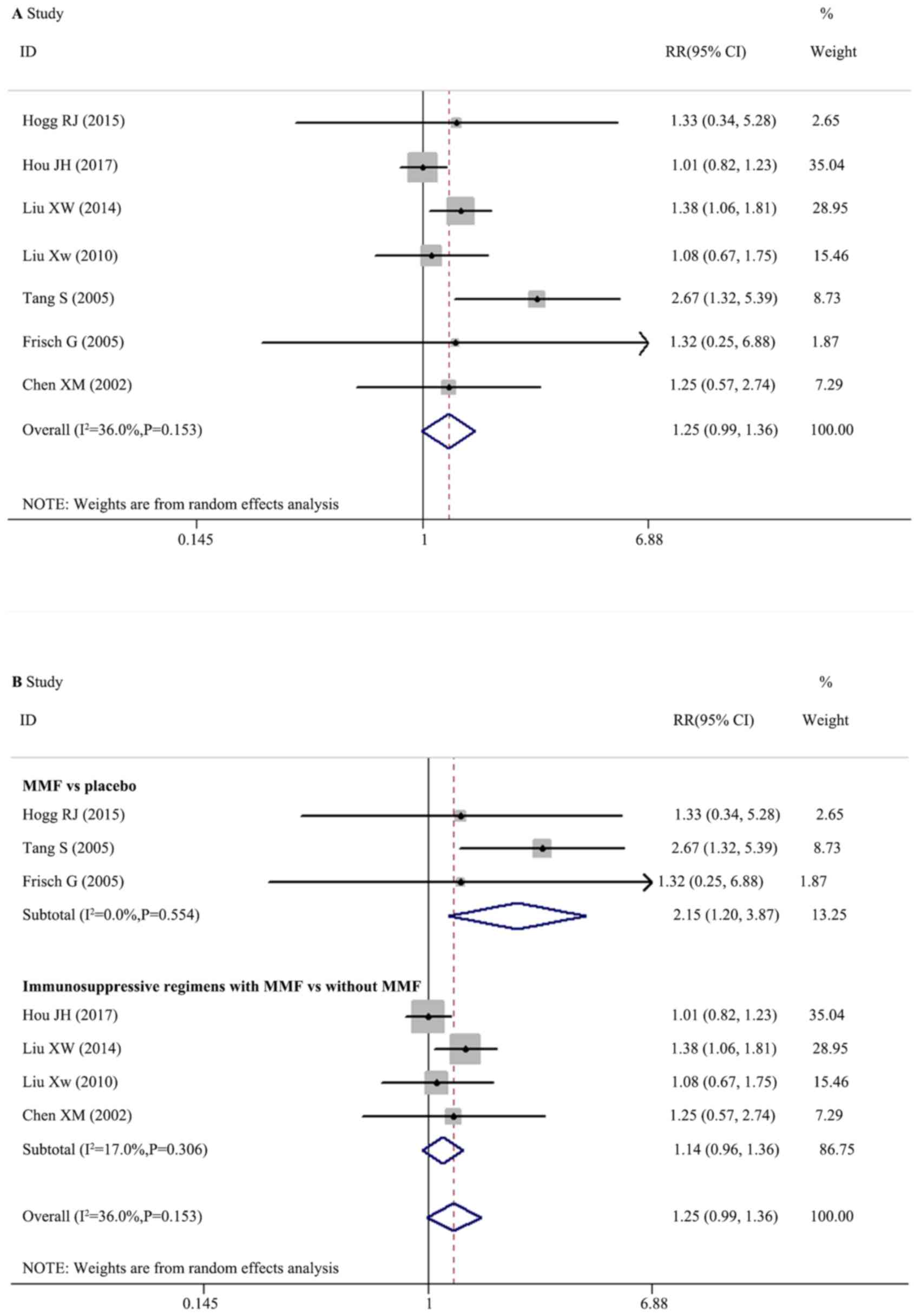
Effect of MMF on renal remission
A total of seven trials with 478 participants
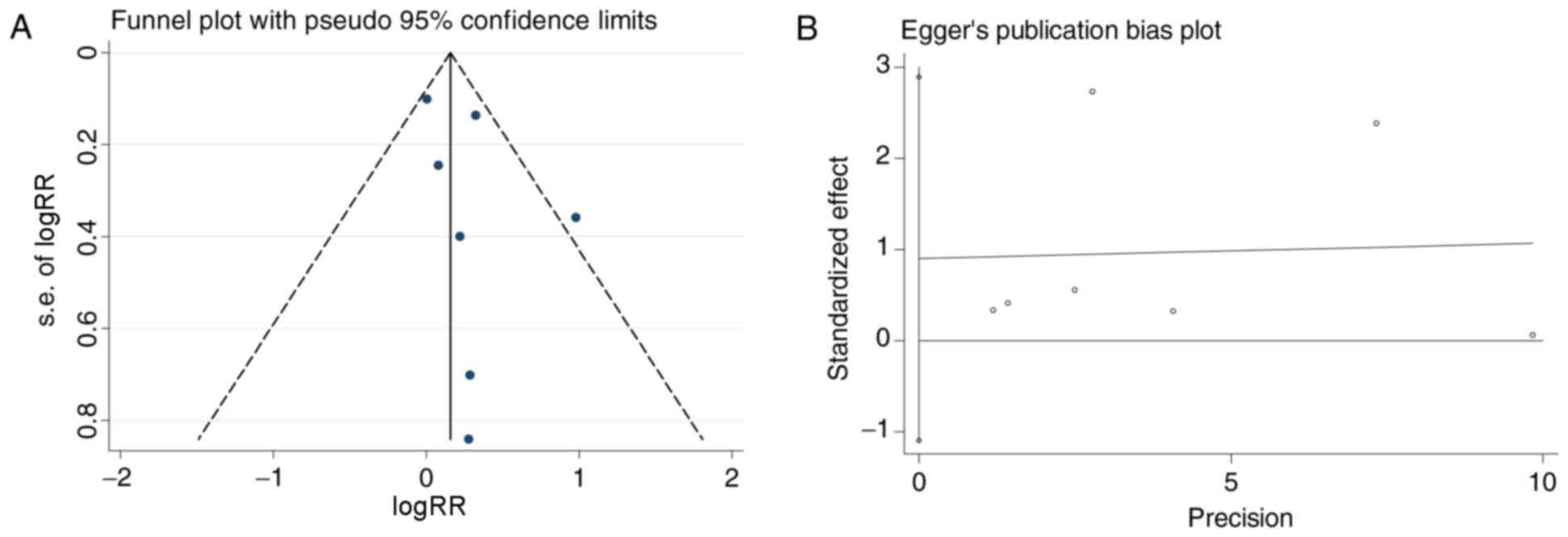
reported data on renal remission. The funnel plots exhibited
asymmetry (Fig. 2A). Egger's
regression analysis demonstrated no significant publication bias
(P=0.297; Fig. 2B). The pooled
effects (RRs) were homogeneous (I2=36.0%; Q=9.38,
degrees of freedom, df=6; P=0.153; H=1.3; 95% CI, 1.0–1.9)
according to the random effects model and there was no significant
difference between therapeutic regimens with and without MMF for
renal remission in patients with IgAN (Fig. 3A; RR, 1.250; 95% CI, 0.993–1.574;
P=0.057).
Random effects meta-regression was performed, which
indicated no significant interactions between treatment effect,
trial and population characteristics. Further subgroup analysis was
performed according to the therapeutic regimens in the control
groups of a placebo or other immunosuppressants, which demonstrated
that MMF was significantly superior compared with a placebo
(Fig. 3B; three trials; RR, 2.152;
95% CI, 1.198–3.867; P=0.010). The immunosuppressive regimens with
MMF had no significantly different effects compared with
immunosuppressive regimens without MMF (Fig. 3B; four trials; RR, 1.140; 95% CI,
0.955–1.361; P=0.146).
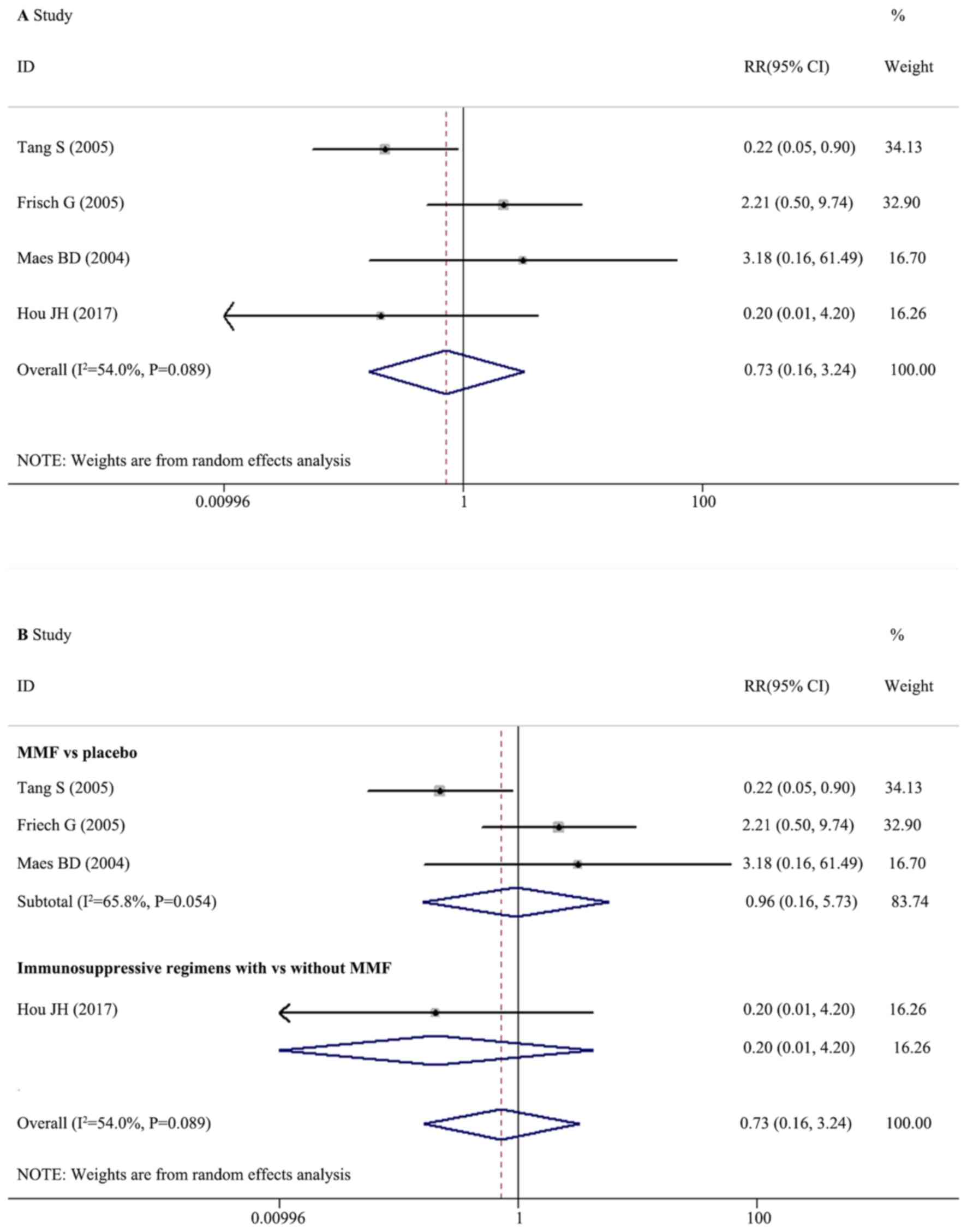
Effect of MMF on ESRD
A total of four trials with 282 participants
reported data on ESRD. The included effects (RRs) were
heterogeneous (I2=54.0%; Q=6.53, df=3; P=0.089; H=1.35;
95% CI, 1.0–2.6) according to the random effects model. The pooled
RR was 0.728 (95% CI, 0.164–3.236), which demonstrated that the
therapeutic regimens with MMF had no significantly different
effects on the ESRD of patients with IgAN compared with regimens
without MMF (Fig. 4A; P=0.676).
Subgroup analysis based on therapeutic regimens illustrated that
there were no significantly different effects on the risk of ESRD
between MMF and a placebo (three trials; RR, 0.957; 95% CI,
0.160–5.726; P=0.962) and between the immunosuppressive regimens
with MMF and without MMF (Fig. 4B;
one trial; RR, 0.205; 95% CI, 0.010–4.200; P=0.303).
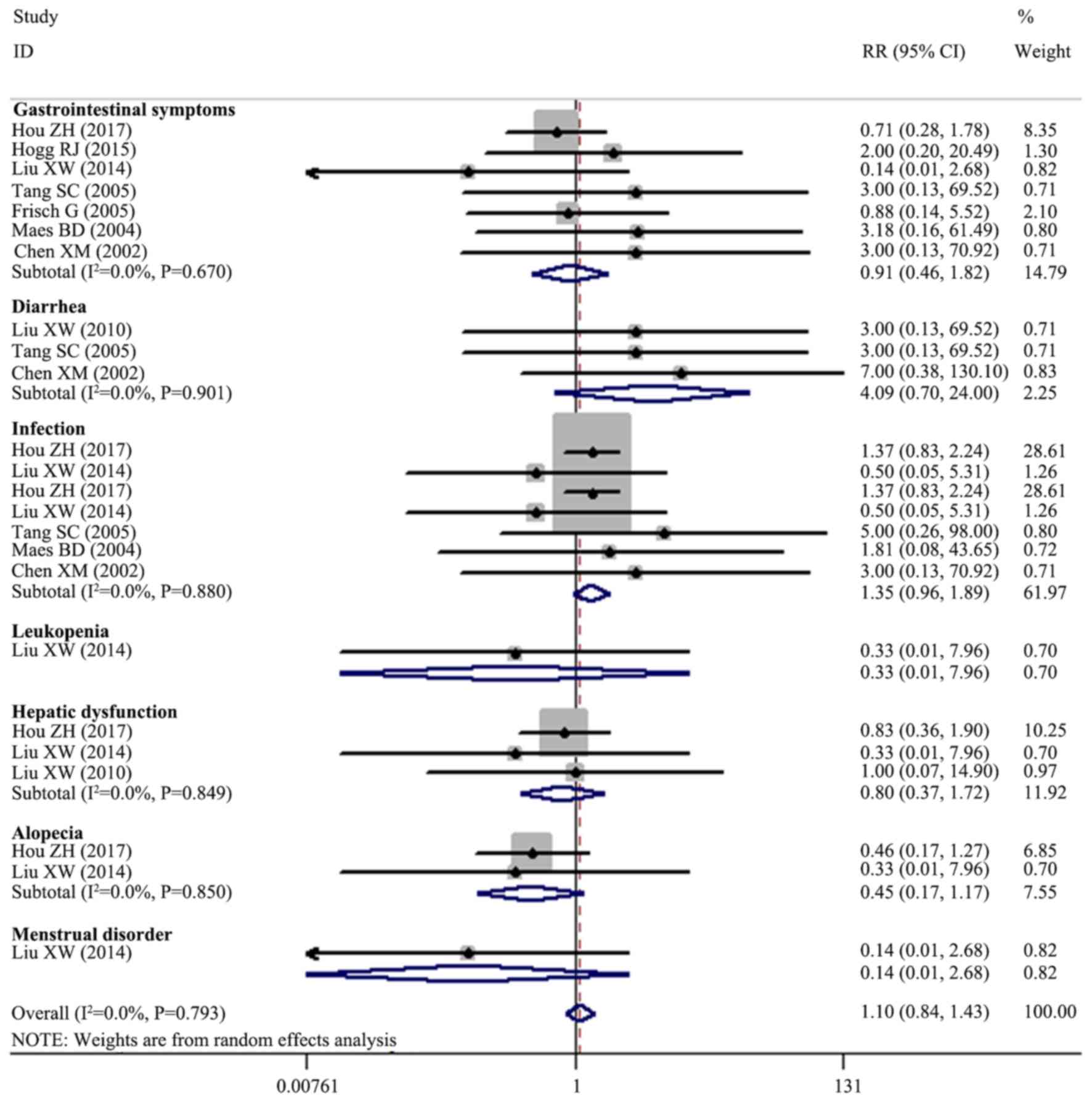
Adverse events
Data on adverse events were analyzed (Fig. 5), indicating no significant
difference between the therapeutic regimens with and without MMF in
terms of the risk of gastrointestinal symptoms (seven trials;
n=471; RR, 0.913; 95% CI, 0.458–1.821; P=0.796), diarrhea (three
trials; n=142; RR, 4.092; 95% CI, 0.698–24.000; P=0.118),
infections (seven trials; n=654; RR, 1.350; 95% CI, 0.963–1.892;
P=0.081), leukopenia (one trial; n=84; RR, 0.333; 95% CI,
0.014–7.956; P=0.497), hepatic dysfunction (three trials; n=299;
RR, 0.797; 95% CI, 0.369–1.719; P=0.562), alopecia (two trials;
n=259; RR, 0.446; 95% CI, 0.170–1.173; P=0.102) and menstrual
disorders (one trial; n=84; RR, 0.143; 95% CI, 0.008–2.683;
P=0.193).
Discussion
The present meta-analysis of RCTs demonstrated that
therapeutic regimens with or without MMF did not have significantly
different effects on the rates of renal remission, ESRD or adverse
events for patients with IgAN. Subgroup analyses indicated that MMF
was superior to the placebo for renal remission, although not
different for ESRD. In addition, immunosuppressive regimens with
MMF had no significantly different effects for renal remission or
ESRD compared with immunosuppressive regimens without MMF.
MMF is an immunosuppressive agent, which was first
introduced into clinical practice to prevent allograft rejection
(24). Subsequently, the clinical
application of MMF broadened substantially and it has become part
of the first line of treatment in certain types of
glomerulonephritis, including lupus nephritis (25,26). MMF
is hydrolyzed by esterases in the intestine and blood to release
MPA. MPA is able to deplete the pool of deoxyguanosine triphosphate
and decrease T and B lymphocyte proliferation by inhibiting inosine
5′-monophosphate dehydrogenase, the rate-limiting enzyme of de
novo purine synthesis (27,28). It
is additionally able to induce apoptosis in immune cells and
inhibit the synthesis of fucose- and mannose-containing membrane
glycoproteins, altering the surface expression and binding ability
of adhesion molecules (6). MPA
additionally exerts a direct effect on nonimmune cells, including
inhibiting the activation of mesangial cells (28–30).
IgAN is an immune-mediated kidney disease and the activation of
mesangial cells is a vital part of the pathogenesis of IgAN
(31,32). A recent study reported that MMF was
able to improve endocapillary hypercellularity and
cellular/fibrocellular crescents (33). However, RCTs and previous
meta-analyses failed to reach consistent results on the efficacy
and safety of MMF in IgAN. At present, this question remains a hot
topic in clinical research.
The present meta-analysis of all RCTs, which
analyzed the efficacy and safety of MMF in IgAN, including recently
published trials (9–11), may supply comprehensive evidence on
the use of MMF in IgAN. MMF was superior to placebo for renal
remission, but not for ESRD. The majority of included RCTs in the
present meta-analysis had short periods of follow-up (6–72 months),
which may influence the evaluation of the effects on ESRD.
Furthermore, immunosuppressive regimens with MMF exhibited no
significant difference on renal remission or ESRD compared with the
immunosuppressive regimens without MMF, suggesting that MMF may
have a similar therapeutic effect to other immunosuppressants.
Additionally, therapeutic regimens with MMF exhibited no
significantly different risk of adverse events compared with those
without MMF, indicating that MMF did not add to the risk of adverse
events.
There are limitations to the present meta-analysis.
Risks of biases within the included RCTs may limit the credibility
of the present results. Furthermore, the immunosuppressive regimens
in the trials varied, including leflunomide plus steroids,
cyclophosphamide plus steroids and steroids alone. Further research
is required to compare MMF with other immunosuppressant regimens.
Additionally, the present study investigated publication bias using
funnel plots and Egger's regression analysis, which may have low
power to distinguish bias when the included number of trials is
small.
In conclusion, the present meta-analysis of the most
recent evidence demonstrated that MMF was superior to placebo and
may have similar efficacy compared with other immunosuppressants
for inducing renal remission in patients with IgAN. MMF may not add
to the risks of adverse events. The effect of MMF on the long-term
prognosis of patients with IgAN requires verification in further
research.
Acknowledgements
The authors would like to thank all the researchers
of the original trials included in the present meta-analysis.
Funding
This study was funded by the National Natural
Science Foundation Young Investigator Grant Program (grant no.
81500525) and the Natural Science Foundation of Liaoning Province
(grant no. 2014021046).
Availability of data and materials
The analyzed data generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
LL designed, supervised and mentored the present
study; JZ and TB acquired the data; and LL, JZ and LZ performed
statistical analysis. The final version of the manuscript was read
and approved by all the authors.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wyatt RJ and Julian BA: IgA Nephropathy. N
Engl J Med. 368:2402–2414. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Shaughnessy MM, Hogan SL, Thompson BD,
Coppo R, Fogo AB and Jennette JC: Glomerular disease frequencies by
race, sex and region: Results from the international kidney biopsy
survey. Nephrol Dial Transplant. Jul 2–2017.(Epub ahead of
print).
|
|
3
|
Moriyama T, Tanaka K, Iwasaki C, Oshima Y,
Ochi A, Kataoka H, Itabashi M, Takei T, Uchida K and Nitta K:
Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at
a single center in Japan. PLoS One. 9:e917562014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rasche FM, Keller F, Rasche WG, Schiekofer
S, Boldt A, Sack U and Fahnert J: Why, when and how should
immunosuppressive therapy considered in patients with
immunoglobulin A nephropathy? Clin Exp Immunol. 186:115–133. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jonsson CA and Carlsten H: Mycophenolic
acid inhibits inosine 5′-monophophate dehydrogenase and suppresses
immunoglobulin and cytokine production of B cells. Int
Immunopharmacol. 3:31–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Allison AC and Eugui EM: Mycophenolate
mofetil and its mechanisms of action. Immunopharmacology.
47:85–118. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee SH, Kim CD, Huh KH, Cho BH, Ju MK, Lee
DR, Cho HR, Park JW, Lee JJ, Lee S, et al: Low-dose mycophenolate
mofetil in tablet form or capsule form combined with tacrolimus in
the early period after kidney transplantation: A prospective
randomized trial. Clin Nephrol. 86:319–327. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Glander P, Hambach P, Braun KP, Fritsche
L, Waiser J, Mai I, Neumayer HH and Budde K: Effect of
mycophenolate mofetil on IMP dehydrogenase after the first dose and
after long-term treatment in renal transplant recipients. Int J
Clin Pharmacol Ther. 41:470–476. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hou JH, Le WB, Chen N, Wang WM, Liu ZS,
Liu D, Chen JH, Tian J, Fu P, Hu ZX, et al: Mycophenolate mofetil
combined with prednisone versus full-dose prednisone in IgA
nephropathy with active proliferative lesions: A randomized
controlled trial. Am J Kidney Dis. 69:788–795. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hogg RJ, Bay RC, Jennette JC, Sibley R,
Kumar S, Fervenza FC, Appel G, Cattran D, Fischer D, Hurley RM, et
al: Randomized controlled trial of mycophenolate mofetil in
children, adolescents, and adults with IgA nephropathy. Am J Kidney
Dis. 66:783–791. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu X, Dewei D, Sun S, Xu G, Liu H, He L
and Zhang P: Treatment of severe IgA nephropathy: Mycophenolate
mofetil/prednisone compared to cyclophosphamide/prednisone. Int J
Clin Pharmacol Ther. 52:95–102. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu XW, Li DM, Xu GS and Sun SR:
Comparison of the therapeutic effects of leflunomide and
mycophenolate mofetil in the treatment of immunoglobulin A
nephropathy manifesting with nephrotic syndrome. Int J Clin
Pharmacol Ther. 48:509–513. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang SC, Tang AW, Wong SS, Leung JC, Ho YW
and Lai KN: Long-term study of mycophenolate mofetil treatment in
IgA nephropathy. Kidney Int. 77:543–549. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang S, Leung JC, Chan LY, Lui YH, Tang
CS, Kan CH, Ho YW and Lai KN: Mycophenolate mofetil alleviates
persistent proteinuria in IgA nephropathy. Kidney Int. 68:802–812.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Frisch G, Lin J, Rosenstock J, Markowitz
G, D'Agati V, Radhakrishnan J, Preddie D, Crew J, Valeri A and
Appel G: Mycophenolate mofetil (MMF) vs placebo in patients with
moderately advanced IgA nephropathy: A double-blind randomized
controlled trial. Nephrol Dial Transplant. 20:2139–2145. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maes BD, Oyen R, Claes K, Evenepoel P,
Kuypers D, Vanwalleghem J, Van Damme B and Vanrenterghem YF:
Mycophenolate mofetil in IgA nephropathy: Results of a 3-year
prospective placebo-controlled randomized study. Kidney Int.
65:1842–1849. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen X, Chen P, Cai G, Wu J, Cui Y, Zhang
Y, Liu S and Tang L: A randomized control trial of mycophenolate
mofeil treatment in severe IgA nephropathy. Zhonghua Yi Xue Za Zhi.
82:796–801. 2002.(In Chinese). PubMed/NCBI
|
|
18
|
Du B, Jia Y, Zhou W, Min X, Miao L and Cui
W: Efficacy and safety of mycophenolate mofetil in patients with
IgA nephropathy: An update meta-analysis. BMC Nephrol. 18:2452017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vecchio M, Bonerba B, Palmer SC, Craig JC,
Ruospo M, Samuels JA, Molony DA, Schena FP and Strippoli GF:
Immunosuppressive agents for treating IgA nephropathy. Cochrane
Database Syst Rev. 2015:CD0039652015.
|
|
20
|
Chen Y, Li Y, Yang S, Li Y and Liang M:
Efficacy and safety of mycophenolate mofetil treatment in IgA
nephropathy: A systematic review. BMC Nephrol. 15:1932014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu G, Tu W, Jiang D and Xu C:
Mycophenolate mofetil treatment for IgA nephropathy: A
meta-analysis. Am J Nephrol. 29:362–367. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Higgins JP and Green S: Cochrane handbook
for systematic reviews of interventions. version 5.1.0. The
Cochrane Collaboration. 2011.http://handbook.cochrane.orgUpdated March
2011.
|
|
23
|
Knobloch K, Yoon U and Vogt PM: Preferred
reporting items for systematic reviews and meta-analyses (PRISMA)
statement and publication bias. J Craniomaxillofac Surg. 39:91–92.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deierhoi MH, Kauffman RS, Hudson SL,
Barber WH, Curtis JJ, Julian BA, Gaston RS, Laskow DA and Diethelm
AG: Experience with mycophenolate mofetil (RS61443) in renal
transplantation at a single center. Ann Surg. 217:476–484. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rovin BH: Lupus nephritis: Guidelines for
lupus nephritis-more recommendations than data? Nat Rev Nephrol.
8:620–621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Conti F, Ceccarelli F, Perricone C,
Massaro L, Cipriano E, Pacucci VA, Truglia S, Miranda F, Morello F,
Alessandri C, et al: Mycophenolate mofetil in systemic lupus
erythematosus: Results from a retrospective study in a large
monocentric cohort and review of the literature. Immunol Res.
60:270–276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Langman LJ, LeGatt DF, Halloran PF and
Yatscoff RW: Pharmacodynamic assessment of mycophenolic
acid-induced immunosuppression in renaltransplant recipients.
Transplantation. 62:666–672. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park JS, Kim GH, Jo CH, Kim S, Lee CH, Kim
YS and Kang CM: Effect of mycophenolic acid on cyclosporin
A-induced fibronectin expression in rat mesangial cells.
Pharmacology. 91:20–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dubus I, Vendrely B, Christophe I,
Labouyrie JP, Delmas Y, Bonnet J and Combe C: Mycophenolic acid
antagonizes the activation of cultured human mesangial cells.
Kidney Int. 62:857–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hauser IA, Renders L, Radeke HH, Sterzel
RB and Goppelt-Struebe M: Mycophenolate mofetil inhibits rat and
human mesangial cell proliferation by guanosine depletion. Nephrol
Dial Transplant. 14:58–63. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Al Hussain T, Hussein MH, Al Mana H and
Akhtar M: Pathophysiology of IgA Nephropathy. Adv Anat Pathol.
24:56–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lai KN: Pathogenesis of IgA nephropathy.
Nat Rev Nephrol. 8:275–283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Beckwith H, Medjeral-Thomas N, Galliford
J, Griffith M, Levy J, Lightstone L, Palmer A, Roufosse C, Pusey C,
Cook HT and Cairns T: Nephrol Dial Transplant. 32 Suppl
1:i123–i128. 2017. View Article : Google Scholar : PubMed/NCBI
|