Introduction
Elevated intraocular pressure, intermittent or
persistent, is often a clinical symptom of glaucoma. Gradual
apoptosis of retinal ganglion cells (RGCs) due to elevated
intraocular pressure, as well as retinal neovascularization, lead
to thinning of retinal nerve fiber layer and visual field damage
(1,2). Clinical studies in recent years
provided increasing number of evidences that suggest aberrant
immune activity may play an important role in occurrence of
glaucoma. The response of the immune system to an immune stimulus,
such as pathological intraocular pressure elevation, can cause
optic nerve damage directly or indirectly, suggesting that the
immune system plays an important role in regulation of RGCs
(3,4). In the pathogenesis of glaucoma,
vascular endothelial growth factor (VEGF) plays an important role
in induction of neovascularization (5). In an animal study, it was reported that
intravitreal injection of VEGF induced formation of new blood
vessels, which disappeared following injection of anti-VEGF drug
(6). Ranibizumab is an anti-VEGF
drug that shows good efficacy profile. Cytokines also play an
important role in the pathogenesis of glaucoma. The cytokine
interleukin-6 (IL-6) binds to the signal transducing transmembrane
subunit gp130, thus activating the STAT3 pathway which regulates
the expression of Bcl-xL and Bcl-2. Upregulation of anti-apoptotic
Bcl-xL and Bcl-2 prevent apoptosis of RGCs (7,8). The
purpose of this study was to investigate the effects of
intravitreal injection of ranibizumab on the expression of IL-6 and
VEGF in peripheral blood and aqueous humor of glaucoma rat model to
evaluate the efficacy for treating optic nerve injury.
Materials and methods
Animal subjects
Specific Pathogen Free (SPF) SD rats were purchased
from Wuhan Hualianke Biotechnology Co., Ltd. (Wuhan, China). The
rats, aged 7–11 days and weighing 16–25 g, were fed with SPF
ShooBree, rat food obtained from Jiangsu Province Collaborative
Pharmaceutical Bioengineering Co., Ltd. (Jiangsu, China). All rats
were maintained in a controlled environment with a light/dark cycle
of 12 h, a temperature of 21±2°C and a humidity of 30–70%, and were
allowed free access to food and drinking water. The food tray and
water bottle were replaced 1–2 times a week with fresh food and
water. The study was approved by the Ethics Committee of People's
Hospital of Dongying (Dongying, China).
SD rat glaucoma model
establishment
One hundred and twenty-five SD rats were given
intraperitoneal injection of pentobarbital sodium (50 mg/kg). After
satisfactory induction of general anesthesia, topical ocular
anesthesia was given by instilling oxybuprocaine hydrochloride eye
drops (4 g/l) into the conjunctival sac. Then, the rat intraocular
pressure was measured three times and the average was recorded.
After the rat head was positioned under a microscope (Lufeifan
Biotecs, Jiaozuo, China), laser photocoagulation was performed on
the scleral vein by using a 532-nm diode laser at 0.075-watt laser
power and 0.3-sec duration. Approximately 150 laser burns were
delivered in each eye. After the procedure, lincomycin
hydrochloride and erythromycin were applied to reduce inflammatory
reactions in the eye. On the second day (day 2), a slit-lamp
microscopic examination was performed to evaluate corneal edema,
conjunctival hyperemia and anterior chamber reaction. Intraocular
pressure was measured three times on the day after operation (day
1), day 7, 14 and 21, and the average values were recorded. The rat
glaucoma model establishment was successful if the increase of
intraocular pressure was no <15 mmHg on day 7.
After model establishment, the rats were randomly
divided into two groups, the control group (n=40) and the
observation group (n=40). Rats in the observation group were
treated by intravitreal injections of ranibizumab (Shanghai
TheraMabs Biotechnology Co., Ltd., Shanghai, China) at a dose of
0.05 ml/day of 10 mg/ml solution. Drug treatment in this group
continued until the end of the experiment. Rats in the control
group received no treatment.
Indicators observed
Rats in the two groups were sacrificed by
decapitation on day 7 (n=13), day 14 (n=13) and day 21 (n=14) after
start of treatment. Seven days before sacrifice, retrograde
labeling of all RGCs was performed by bilateral injection of
fluorogold (0.5 µl, 50 g/l) into superior colliculi. After rats
were sacrificed at the time-points, the retina was harvested.
Numbers of RGCs were counted under a fluorescence microscope
(Lufeifan Biotecs). In the meantime, whole blood and aqueous humor
were collected. The levels of IL-6 and VEGF in whole blood and
aqueous humor were determined by using an enzyme-linked
immunosorbent assay (ELISA) kit for IL-6 and an ELISA kit for VEGF,
respectively (Shanghai Jingkang Biological Engineering Co., Ltd.,
Shanghai, China).
Statistical analysis
Statistical software SPSS 19.0 [AsiaAnalytics
(formerly SPSS China), Shanghai, China] was used in data
processing. Measurement data were expressed as mean ± SD.
Chi-square test, non-parametric K-S test and t-test were used,
respectively, for comparison of rate, comparison between two groups
and comparison between different time-points within a group.
Comparison between multiple groups was done using one-way ANOVA
test followed by post hoc test (Least Significant Difference).
Correlations of IL-6 and VEGF with RGCs were examined by using
logistic regression analysis. A difference was statistically
significant if P<0.05.
Results
General information of animal
subjects
Of 125 SD rats that were used for establishment of
rat glaucoma model, 80 were successful, 15 died (accidental death
caused by improper operation) and 30 failed (with the increase of
intraocular pressure <15 mmHg). The success rate was 64%. Rats
in the observation group (24 males and 16 females) were aged
8.2±1.3 days and weighed 22.4±3.8 g, and rats in the control group
(19 males and 21 females) were aged 9.8±1.6 days and weighed
26.7±4.2 g. The rats in both groups were allowed free access to
food and water. There were no statistically significant differences
in sex, age and weight between the two groups (P>0.05). Compared
with the intraocular pressure on day 1, the decline started to be
statistically significant when the ranibizumab treatment reached
day 14 (P<0.05). The detailed results are shown in Table I.
 | Table I.General information of rat subjects in
both groups. |
Table I.
General information of rat subjects in
both groups.
| Items | Observation group
(n=40) | Control group
(n=40) | t | P-value |
|---|
| Age (days) | 8.2±1.3 | 9.8±1.6 | 0.226 | 0.899 |
| Sex
(male/female) | 24/16 | 19/21 | 0.967 | 0.645 |
| Weight (g) | 22.4±3.8 | 26.7±4.2 | 0.742 | 0.785 |
| Intraocular pressure
(mm/Hg) after anesthesia | 17.1±2.1 | 16.7±1.8 | 0.623 | 0.812 |
| Intraocular pressure
(mm/Hg) on day 1 | 27.6±2.1 | 28.4±1.5 | 0.569 | 0.865 |
| Intraocular pressure
(mm/Hg) on day 7 | 26.4±1.7 | 28.1±2.3 | 0.857 | 0.742 |
| Intraocular pressure
(mm/Hg) on day 14 | 20.4±2.1 | 31.1±2.8 | 2.973 | 0.037 |
| Intraocular pressure
(mm/Hg) on day 21 | 17.8±1.9 | 31.4±3.1 | 4.569 | 0.019 |
IL-6 levels in peripheral blood and
aqueous humor of rats in both groups
Levels of IL-6 in peripheral blood and aqueous humor
were quantitatively analyzed by ELISA and the results are shown in
Table II. In the observation group,
the levels of IL-6 in peripheral blood and aqueous humor decreased
gradually over the treatment time, and the level of IL-6 on day 21
was significantly different from that on day 7 (P<0.05). In the
control group, the levels of IL-6 in peripheral blood and aqueous
humor increased gradually over time, and the level of IL-6 on day
21 was significantly different from that on day 7 (P<0.05). At
the same time-point, the levels of IL-6 in serum and aqueous humor
were higher in the control group than those in the observation
group (P<0.05).
 | Table II.Levels of IL-6 (pg/ml) in rat
peripheral blood and aqueous humor. |
Table II.
Levels of IL-6 (pg/ml) in rat
peripheral blood and aqueous humor.
|
| Peripheral blood | Aqueous humor |
|---|
|
|
|
|
|---|
| Groups | Day 7 (n=13) | Day 14 (n=13) | Day 21 (n=14) | Day 7 (n=13) | Day 14 (n=13) | Day 21 (n=14) |
|---|
| Observation
group | 10.13±2.21 | 9.03±1.54 |
7.63±1.24a | 9.45±1.38 | 8.67±1.52 |
5.78±1.11b |
| Control group | 13.31±1.94 | 14.96±2.68 |
15.44±2.49c | 11.44±2.01 | 12.19±3.11 |
13.97±2.94d |
| t-test | 3.136 | 3.445 | 4.237 | 3.001 | 3.711 | 3.952 |
| P-value | 0.031 | 0.028 | 0.021 | 0.034 | 0.027 | 0.023 |
VEGF levels in peripheral blood and
aqueous humor of rats in both groups
Levels of VEGF in peripheral blood and aqueous humor
were quantitatively analyzed by ELISA and the results are shown in
Table III. In the observation
group, the levels of VEGF in peripheral blood and aqueous humor
decreased gradually over the treatment time, and the level of VEGF
on day 21 was significantly different from that on day 7
(P<0.05). In the control group, the levels of VEGF in peripheral
blood and aqueous humor did not show significant differences
between different time-points (P>0.05). At the same time-point,
the levels of VEGF in serum and aqueous humor were higher in the
control group than those in the observation group (P<0.05).
 | Table III.Levels of VEGF (pg/ml) in rat
peripheral blood and aqueous humor. |
Table III.
Levels of VEGF (pg/ml) in rat
peripheral blood and aqueous humor.
|
| Peripheral blood | Aqueous humor |
|---|
|
|
|
|
|---|
| Groups | Day 7 (n=13) | Day 14 (n=13) | Day 21 (n=14) | Day 7 (n=13) | Day 14 (n=13) | Day 21 (n=14) |
|---|
| Observation
group | 43.57±6.47 | 36.14±9.44 |
31.32±5.33a | 40.14±4.24 | 34.59±8.33 |
27.17±3.62b |
| Control group | 68.44±11.39 | 67.45±3.44 | 66.34±9.54 | 57.11±5.64 | 54.64±7.58 | 55.18±4.49 |
| t-test | 3.115 | 4.237 | 4.913 | 3.445 | 3.816 | 4.569 |
| P-value | 0.032 | 0.021 | 0.013 | 0.028 | 0.024 | 0.019 |
Rat RGC counts in both groups
Rat RGCs were counted after fluorogold labeling. The
count results were shown in Table
IV. In the observation group, the number of RGCs increased
gradually over the treatment time. The number difference between
day 7 and 14, as well as between day 7 and 21, was statistically
significant (P<0.05). In the control group, the number of RGCs
decreased gradually over time. The differences between every two
time-points were significant (P<0.05). At the same time-point,
the number of RGCs was lower in the control group than that in the
observation group (P<0.05).
 | Table IV.RGC counts in both groups. |
Table IV.
RGC counts in both groups.
|
| RGC counts |
|---|
|
|
|
|---|
| Groups | Day 7 (n=13) | Day 14 (n=13) | Day 21 (n=14) |
|---|
| Observation
group | 185.41±28.44 |
191.12±31.32a |
197.24±16.51b |
| Control group | 145.25±15.86 |
128.94±18.54c |
104.39±13.24d |
| t-test | 3.012 | 3.255 | 4.673 |
| P-value | 0.033 | 0.027 | 0.016 |
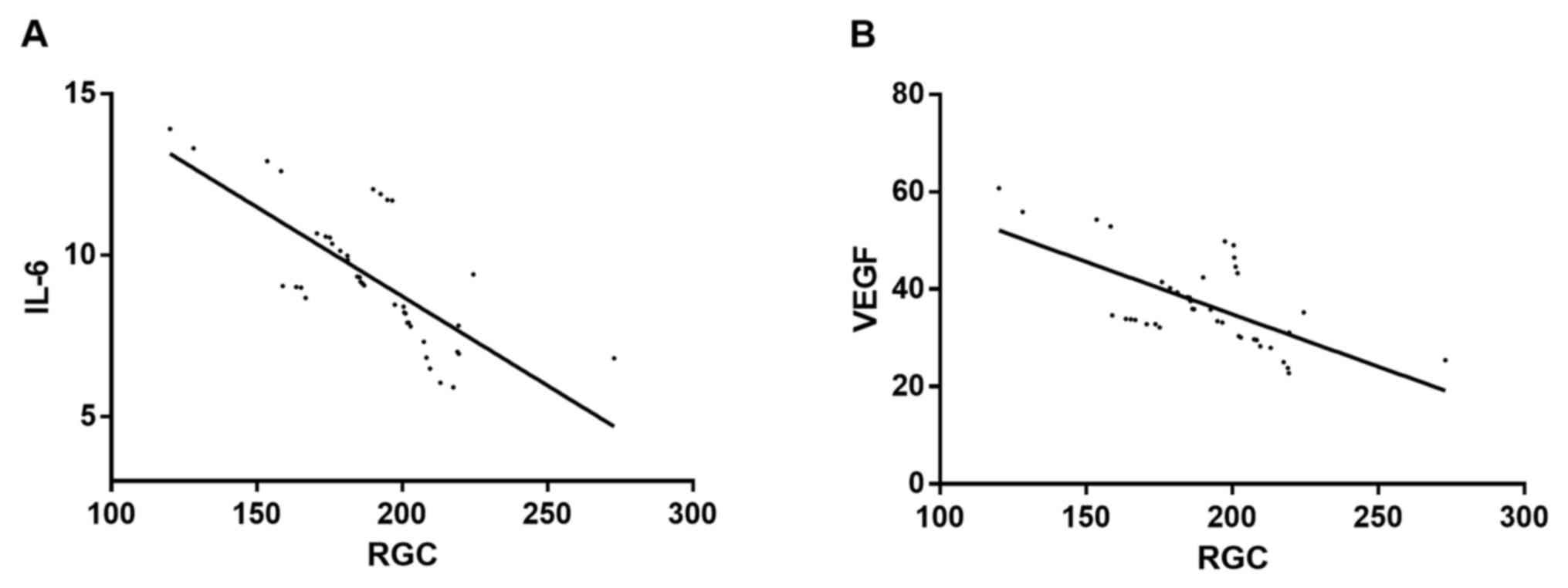
Correlations of IL-6 and VEGF with RGC
counts
Correlations of IL-6 and VEGF with RGC counts were
evaluated by logistic regression analysis. The analysis revealed
that IL-6 and RGC counts, as well as VEGF and RGC counts, were
negatively correlated. As the number of RGCs increased, the levels
of IL-6 and VEGF gradually decreased (r=−0.743 and P=0.012 for
IL-6; r=−0.675 and P=0.022 for VEGF) (Fig. 1).
Discussion
Glaucoma leading to optic nerve injury and visual
loss is a non-negligible public health issue globally. According to
reports, the number of patients with glaucoma could reach up to 80
million in the world, and 6 million in China alone by 2020
(9,10). Glaucoma has a significant negative
impact on vision function, however, it does not respond well to
treatment of drugs and surgery. The primary goal of current
treatments for glaucoma is focused on reduction of symptoms
(11,12). In this study, therapeutic efficacy of
ranibizumab in treatment of visual impairment due to glaucoma was
evaluated by monitoring levels of IL-6 and VEGF, and the
correlations of IL-6 and VEGF with visual impairment were
examined.
Successful establishment of glaucoma rat model was
indicated by a difference of no less than 15 mmHg in intraocular
pressures before and after the model establishment. All rats in
this study, as well as the rat food, were SPF grade. Experimental
results showed that the number of RGCs labeled with fluorogold was
higher in the observation group treated with ranibizumab than that
in the control group (P<0.05). Ranibizumab is a recombinant
humanized monoclonal antibody fragment. Clinically it was
demonstrated to alleviate macular edema, and reduce
neovascularization and vascular leakage in patients by effectively
antagonizing VEGF (13,14). In this study, the levels of VEGF in
rat serum and aqueous humor were determined, and it was found that
the VEGF levels of rats treated with ranibizumab were significantly
lower (P<0.05) than those of the control group, and decreased
further over time in the treatment. Improvement of VEGF levels by
ranibizumab was also reported by Froger et al and Molokhia
et al (15,16). The levels of IL-6 were also measured
by ELISA, which showed that the IL-6 levels of rats treated with
ranibizumab were significantly lower (P<0.05) than those of the
control group. Similar to VEGF, the IL-6 levels decreased further
over time in the treatment as well. Therefore, ranibizumab improved
not only the VEGF levels but also the IL-6 levels, which was very
important in alleviating the inflammatory response. Currently more
studies of ranibizumab were focused on its effect on improving the
VEGF levels (17,18) and there has been no report in
literature about its effect on improving the IL-6 levels. Thus,
this study represents the first study of ranibizumab on its effect
on improving the IL-6 levels. Due to using experimental animal
model in this study, the findings need further support from studies
by larger sample sets and clinical studies. Correlations of IL-6
and VEGF levels with optic nerve injury were also examined in this
study. Using RGC count as an indicator of the extent of optic nerve
injury, it was found that the RGC count was higher when levels of
IL-6 and VEGF were lower, suggesting the levels of IL-6 and VEGF
were negatively correlated with optic nerve damage. Thus, lower
levels of IL-6 and VEGF indicate lower extent of optic nerve
injury. Johnson et al reported that elevated IL-6 levels
caused damage to the optic nerve head, compromising the axonal
integrity (19). In Fisher J's
study, microglia cell survival was increased in IL-6 gene knockout
mice (20). Microglia cells play an
important role in the sequelae of neurological injury. These
reports confirmed that increased IL-6 levels resulted in optic
nerve injury, which were in accordance with the findings in this
study. Bennett et al reported that decreased VEGF levels
improved papilledema, as well as visual impairment (21). This was consistent with our findings.
The findings in this study were obtained by establishing and using
animal models. Further studies using larger sample set and clinical
studies are needed to support these findings.
In conclusion, ranibizumab alleviated optic nerve
injury by reducing levels of IL-6 and VEGF in peripheral blood and
aqueous humor of glaucoma rat model.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors contributions
YS, QS and LL conceived and designed the study. YS,
JX and XL were responsible for the collection and analysis of the
experimental data. QS and LL interpreted the data and drafted the
manuscript. YS, QS and JX revised the manuscript critically for
important intellectual content. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
People's Hospital of Dongying (Dongying, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tham YC, Li X, Wong TY, Quigley HA, Aung T
and Cheng CY: Global prevalence of glaucoma and projections of
glaucoma burden through 2040: A systematic review and
meta-analysis. Ophthalmology. 121:2081–2090. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weinreb RN, Aung T and Medeiros FA: The
pathophysiology and treatment of glaucoma: A review. JAMA.
311:1901–1911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jia Y, Wei E, Wang X, Zhang X, Morrison
JC, Parikh M, Lombardi LH, Gattey DM, Armour RL, Edmunds B, et al:
Optical coherence tomography angiography of optic disc perfusion in
glaucoma. Ophthalmology. 121:1322–1332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gramlich OW, Ding QJ, Zhu W, Cook A,
Anderson MG and Kuehn MH: Adoptive transfer of immune cells from
glaucomatous mice provokes retinal ganglion cell loss in
recipients. Acta Neuropathol Commun. 3:562015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun Y, Liang Y, Zhou P, Wu H, Hou X, Ren
Z, Li X and Zhao M: Anti-VEGF treatment is the key strategy for
neovascular glaucoma management in the short term. BMC Ophthalmol.
16:1502016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gu X, Yu X and Dai H: Intravitreal
injection of ranibizumab for treatment of age-related macular
degeneration: Effects on serum VEGF concentration. Curr Eye Res.
39:518–521. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berg K, Hadzalic E, Gjertsen I, Forsaa V,
Berger LH, Kinge B, Henschien H, Fossen K, Markovic S, Pedersen TR,
et al: Ranibizumab or bevacizumab for neovascular age-related
macular degeneration according to the lucentis compared to avastin
study treat-and-extend protocol: Two-year results. Ophthalmology.
123:51–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Andreoli MT, Pinnolis M, Kieser T, Sun J
and Andreoli CM: Feasibility and efficacy of a mass switch from
ranibizumab (Lucentis) to bevacizumab (Avastin) for treatment of
neovascular age-related macular degeneration. Digit J Ophthalmol.
21:1–17. 2015.PubMed/NCBI
|
|
9
|
Kapetanakis VV, Chan MPY, Foster PJ, Cook
DG, Owen CG and Rudnicka AR: Global variations and time trends in
the prevalence of primary open angle glaucoma (POAG): A systematic
review and meta-analysis. Br J Ophthalmol. 100:86–93. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu L, Jia Y, Takusagawa HL, Pechauer AD,
Edmunds B, Lombardi L, Davis E, Morrison JC and Huang D: Optical
coherence tomography angiography of the peripapillary retina in
glaucoma. JAMA Ophthalmol. 133:1045–1052. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kyari F, Wormald R, Murthy GV, Evans JR
and Gilbert CE: Nigeria National Blindness and Visual Impairment
Study Group: Ethnicity and deprivation are associated with
blindness among adults with primary glaucoma in nigeria: Results
from the nigeria national blindness and visual impairment survey. J
Glaucoma. 25:e861–e872. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gharahkhani P, Burdon KP, Fogarty R,
Sharma S, Hewitt AW, Martin S, Law MH, Cremin K, Bailey JNC, Loomis
SJ, et al: Wellcome Trust Case Control Consortium 2, NEIGHBORHOOD
consortium: Common variants near ABCA1, AFAP1 and GMDS confer risk
of primary open-angle glaucoma. Nat Genet. 46:1120–1125. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Narayanan R, Panchal B, Das T, Chhablani
J, Jalali S and Ali MH: MARVEL study group: A randomised,
double-masked, controlled study of the efficacy and safety of
intravitreal bevacizumab versus ranibizumab in the treatment of
macular oedema due to branch retinal vein occlusion: MARVEL Report
No. 1. Br J Ophthalmol. 99:954–959. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Türkcü FM, Cinar Y, Türkcü G, Sahin A,
Cingü AK, Yüksel H, Sahin M, Yıldırım A and Caça I: Topical and
subconjunctival ranibizumab (lucentis) for corneal
neovascularization in experimental rat model. Cutan Ocul Toxicol.
33:138–144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Froger NG, Forster V, Ivkovic I, Matonti
F, Sahel JA and Picaud SA: Ranibizumab (Lucentis®)
suppresses the autocrine VEGF-elicited survival of purified retinal
ganglion cells. Invest Ophthalmol Vis Sci. 55:2391. 2014.
|
|
16
|
Molokhia S, Burr RM, Flood M, Vallrath M,
Winter G and Ambati BK: Lens capsule biodegradable lipid implant
for sustained-release anti-VEGF therapy of neovascular AMD. Invest
Ophthalmol Vis Sci. 57:4004. 2016.
|
|
17
|
Korhonen T: Developing Medical Record for
Follow-Up of Wet Age-Related Macular DegenerationLeadership,
Innovation and Entrepreneurship as Driving Forces of the Global
Economy. Springer International Publishing; pp. 77–83. 2017,
View Article : Google Scholar
|
|
18
|
Niwa Y, Kakinoki M, Sawada T, Wang X and
Ohji M: Ranibizumab and aflibercept: Intraocular pharmacokinetics
and their effects on aqueous VEGF level in vitrectomized and
nonvitrectomized macaque eyes. Invest Ophthalmol Vis Sci.
56:6501–6505. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Johnson EC, Doser TA, Cepurna WO, Dyck JA,
Jia L, Guo Y, Lambert WS and Morrison JC: Cell proliferation and
interleukin-6-type cytokine signaling are implicated by gene
expression responses in early optic nerve head injury in rat
glaucoma. Invest Ophthalmol Vis Sci. 52:504–518. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fisher J, Mizrahi T, Schori H, Yoles E,
Levkovitch-Verbin H, Haggiag S, Revel M and Schwartz M: Increased
post-traumatic survival of neurons in IL-6-knockout mice on a
background of EAE susceptibility. J Neuroimmunol. 119:1–9. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bennett JL, Thomas S, Olson JL and Mandava
N: Treatment of nonarteritic anterior ischemic optic neuropathy
with intravitreal bevacizumab. J Neuroophthalmol. 27:238–240. 2007.
View Article : Google Scholar : PubMed/NCBI
|