Introduction
The Global Burden of Diseases study 2015 reported a
total of 42.43 million cases of cerebrovascular disease worldwide
and a total of 6.33 million moralities due to stroke (1). Individuals >40 years of age had an
increased prevalence of stroke, with the highest rate for those
aged 74-79 years. The age-standardized prevalence of ischemic
stroke was greater than that of hemorrhagic stroke in the majority
of regions and resulted in 57% of all stroke mortalities in 2015.
The incidence rate was 54/100,000 in males and 44/100,000 in
females (1). There was a markedly
higher stroke mortality rate in non-Hispanic African-American
individuals compared with all other ethnic groups and >25% of
stroke mortalities in individuals aged ≥45 occurred to those in the
45-64 years age group (28.6%) (2).
In China age-standardized stroke mortality was 127/100,000
individuals in 2010 (3). In the last
decade China has observed significant growth in its elderly
population and individuals ≥75 years old accounted for 3.5% of the
total population in 2013. Residents ≥65 years old reached 200
million in 2014 and there was a 4.3% increase in annual first-ever
stroke incidence from 1992 to 2012 (4). Stroke is the primary cause of mortality
and a prominent factor associated with disability-adjusted
life-years in China, with high social and economic costs (5). Therefore urgent investigation into
novel therapies for the effective treatment of stroke is
required.
Patients with acute stroke suffer from a sudden
decrease in cerebral blood flow in partial or global brain tissues,
which leads to a reduction in oxygen and glucose supply and
ultimately results in permanently infarcted tissue (ischemic core)
surrounded by a reversible and salvageable zone (penumbra)
(6). The degree and duration of
tissue hypoperfusion determines the outcome of the ischemic tissue
(7,8). An assessment of penumbral presence may
be used to identify patients who are ineligible for acute stroke
treatment but may still benefit from reperfusion therapy (9). Various imaging methods may be used to
assess the presence of penumbral tissue (10), including perfusion weighted magnetic
resonance imaging (PWI), diffusion weighted magnetic resonance
imaging (DWI) and PWI-DWI mismatch, which is useful for the
evaluation of cerebral hemodynamics, particularly in indicating
hypoperfusion (11). When abnormal
PWI lesions are larger than DWI lesions (PWI>DWI) it represents
the existence of penumbra following stroke, as previously described
(12). Galinovic et al
(13) and Mishra et al
(14) demonstrated an association
between reperfusion and a positive outcome in patients with a
substantial PWI-DWI mismatch. However this was not observed in
patients without a PWI-DWI mismatch in cohort studies. At present,
the only approved drug for acute ischemic stroke is recombinant
tissue plasminogen activator (rtPA), which is administered within a
time window of 3-4.5 h (14).
However, the majority of patients do not benefit from this therapy
as they are typically admitted to hospitals ~4.5 h after symptom
onset or there are contraindications to systemic thrombolysis,
including recent major surgery or active bleeding (15). Beyond this time window, a delayed
intervention is required. Delayed intervention for acute stroke is
defined as therapies beyond the 4.5 h time window, and are based on
the principle of arterial recanalization and rapid reperfusion of
the ischemic penumbra (16).
Improving microcirculation surrounding the ischemic penumbra has
been suggested to be an effective way to prevent ischemic injury to
neurons (16).
Salvianolic acid (SA) is an extract of Salvia
miltiorrhiza Bunge (Danshen), which is a traditional Chinese
medicine that has been used within clinical settings for >2,000
years in China (17). Danshen is a
perennial herb and within traditional Chinese medicine it is used
to improve blood circulation (18).
SA is a compound containing multiple salvianolic acids including,
salvianolic acid A and B (19). In
previous studies it has been demonstrated that salvianolic acid A
and B may have neuroprotective effects in an animal model of
ischemia (20). The aim of the
present study was to investigate whether SA was able to improve
penumbral microcirculation in patients who had suffered from acute
stroke.
Patients and methods
Patients
Diagnosis of acute ischemic stroke was confirmed
using diffusion-weighted magnetic resonance imaging (DWI) in 159
patients (117 male and 42 female; age, 60.10±13.02 years), who were
prospectively recruited between June 2015 and March 2016 at the
People's Liberation Army 153 Central Hospital (Zhengzhou, China).
Inclusion criteria included <72 h from symptom onset, a Glasgow
coma scale (GCS) score >5 (21)
and patients without contraindications for magnetic resonance
imaging (MRI), including those who were not allergic to
paramagnetic contrast agents. Patients with cerebral hemorrhage,
resuscitated encephalopathy, or a GCS score ≤4 were excluded from
the present study. Written informed consent was obtained from all
patients and the present study was approved by the Ethics Committee
of PLA 153 Central Hospital (Zhengzhou, China).
Patients were randomly divided into the SA group
(n=85) and the control group (n=74). In the control group, patients
were treated with standard therapy, which was aspirin
enteric-coated tablets 100 mg/day (Bayer AG, Leverkusen, Germany)
and atorvastain tablets 20 mg/day (Pfizer, Inc., New York, NY,
USA). Patients continued to be treated with drugs for pre-existing
conditions, including hypertension and diabetes mellitus. The
control group was treated with a total of 250 ml normal saline
administered intravenously everyday for 14 days. In the SA group,
SA (0.13 g/day; Tasly Pharmaceutical Group Co., Ltd., Tianjin,
China) was administered intravenously dissolved in 250 ml normal
saline for 14 days in addition to the standard therapy as performed
in the control group. If the patient was eligible for rtPA
thrombolysis (symptom onset to treatment ≤3 h and no bleeding at
admission), intravenous rtPA treatment (0.9 mg/kg; Boehringer
Ingelheim International GmbH, Ingelheim am Rhein, Germany) was
received whether in the control or SA group. One patient received
rtPA treatment in each group.
Magnetic resonance imaging
All images were acquired on a Siemens 3 T whole body
Trio scanner (Siemens Healthineers, Erlangen, Germany). The MRI
protocol included DWI, fluid-attenuated inversion recovery (FLAIR)
and dynamic susceptibility contrast perfusion perfusion-weighted
MRI (PWI). DWI, FLAIR and PWI were performed on day 1 or 2 of
admission, and DWI and PWI were repeated post-admission on day
15.
DWI was obtained with the use of a multislice,
single-shot, spin-echo echo-planar imaging sequence with the
following parameters: Time of repetition (TR), 4,200 msec; time of
echo (TE), 96 msec; field of view (FOV), 24×24 cm; and matrix size,
256×256. A total of 21 6-mm thick slices with a 10% gap were
obtained, and these images were collected with b=1,000
s/mm2, from which the apparent diffusion coefficient was
determined.
The following FLAIR sequence parameters were used:
TR, 6,500 msec; TE, 110 msec; inversion time, 2,200 msec; FOV,
24×24 cm; and matrix 256×256, for 21 6-mm thick slices with a 10%
gap.
For PWI, a gradient echo, echo planar imaging
sequence was employed. Gadolinium diethylenetriaminepentaacetic
acid (Gd-DTPA; Guangzhou Consun Pharmaceutical, Co., Ltd.,
Guangzhou, China) at 0.2 mmol/kg was administered using a
large-bore cannula (20 G; 1.1×30 mm) in the antecubital fossa
(speed, 5 ml/sec) through a power injector (Medrad Inc.; Bayer,
Newbury, UK), followed by 20 ml normal saline with the same speed.
A total of 19 slices were obtained with a slice thickness of 5 mm
and a 30% gap (FOV, 23×23 cm; matrix, 128×128; TR, 1,550 msec; and
TE, 32 msec). At the end of the third scanning procedure of 50
continuous scans with a total scanning time 84 sec, a bolus
injection of Gd-DTPA was administered. Subsequently, images were
obtained at 50 time points per slice, with a total of 950 images.
The raw PWI data were processed using an MRI workstation (Siemens
Healthineers) to produce PWI maps, including cerebral blood volume
(CBV), cerebral blood flow (CBF), mean transit time (MTT) and time
to peak (TTP) maps. On these maps the abnormal perfusion was
expressed as red, making it easily identified by the naked eye,
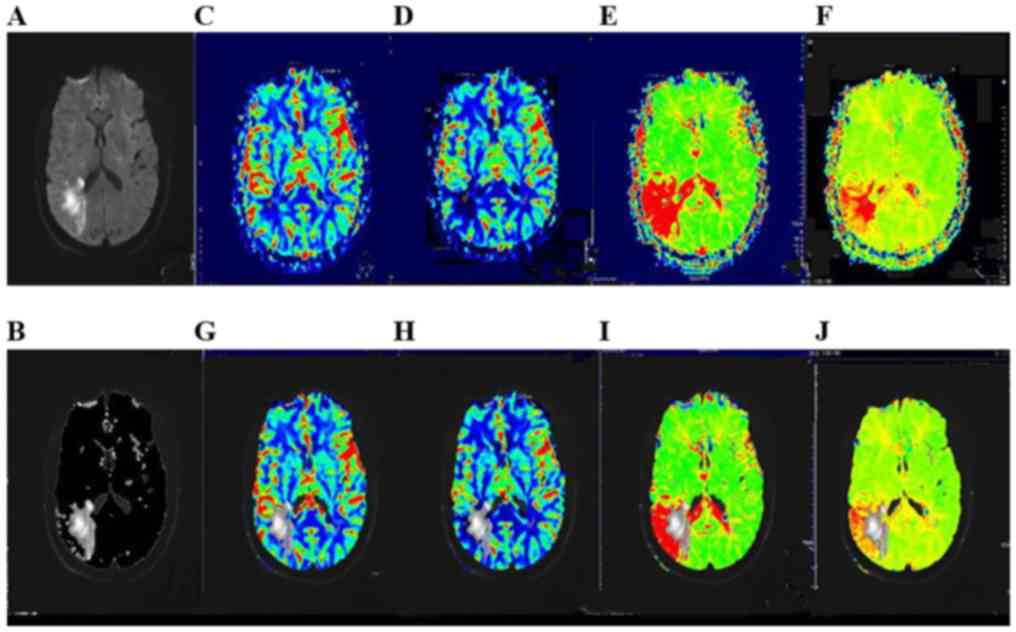
which is known as the visual assessment method.
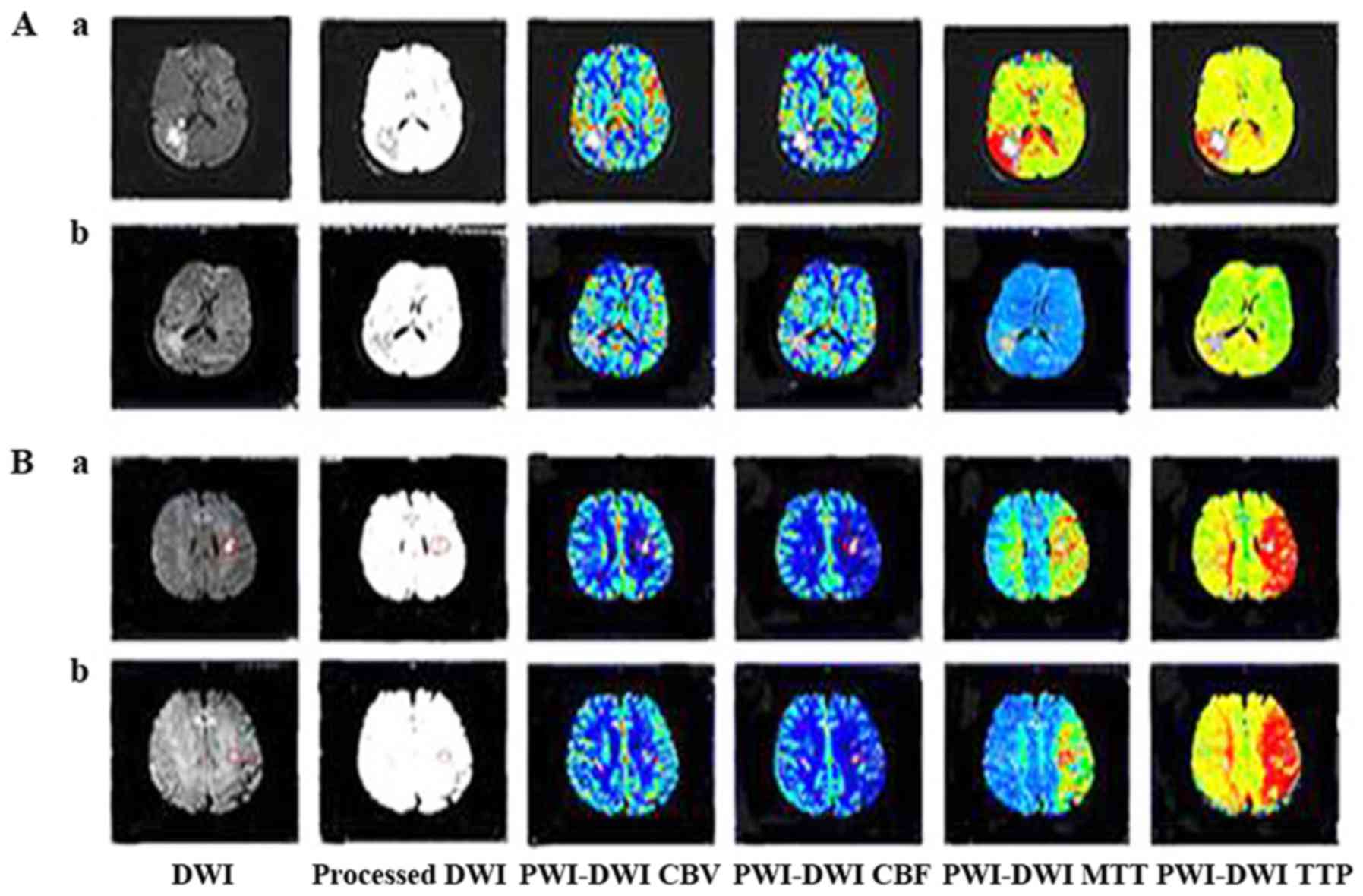
Manual imaging coregistration
Slices with the most notable changes in DWI were
selected, and non-transparent and reverse conversions were
performed on the DWI lesions that were identified as diaphanous via
photo processing (Photoshop CS5; Adobe Systems, Inc., San Jose, CA,
USA). Subsequently, the processed images were superimposed on the
same or the nearest slice of the PWI map with the same
magnification; and imaging coregistration was completed (Fig. 1).
Definitions
The 0.16-cm2 area selected on the
ipsilateral hemisphere of the DWI lesion on the PWI maps was
defined as the region of interest (ROI). ROIs were located either
in the lesion or in its surrounding tissue using coregister
imaging. The signal intensity of ROI was calculated automatically
(Syngo® MR B17; Siemens AG, Munich, Germany) on CBV,
CBF, MTT and TTP maps, respectively. A mirror of the ROI with the
same area was placed on the contralateral hemisphere symmetrically
with avoidance of vessel and cortical sulci, and its signal
intensity was also calculated automatically (Syngo® MR
B17).
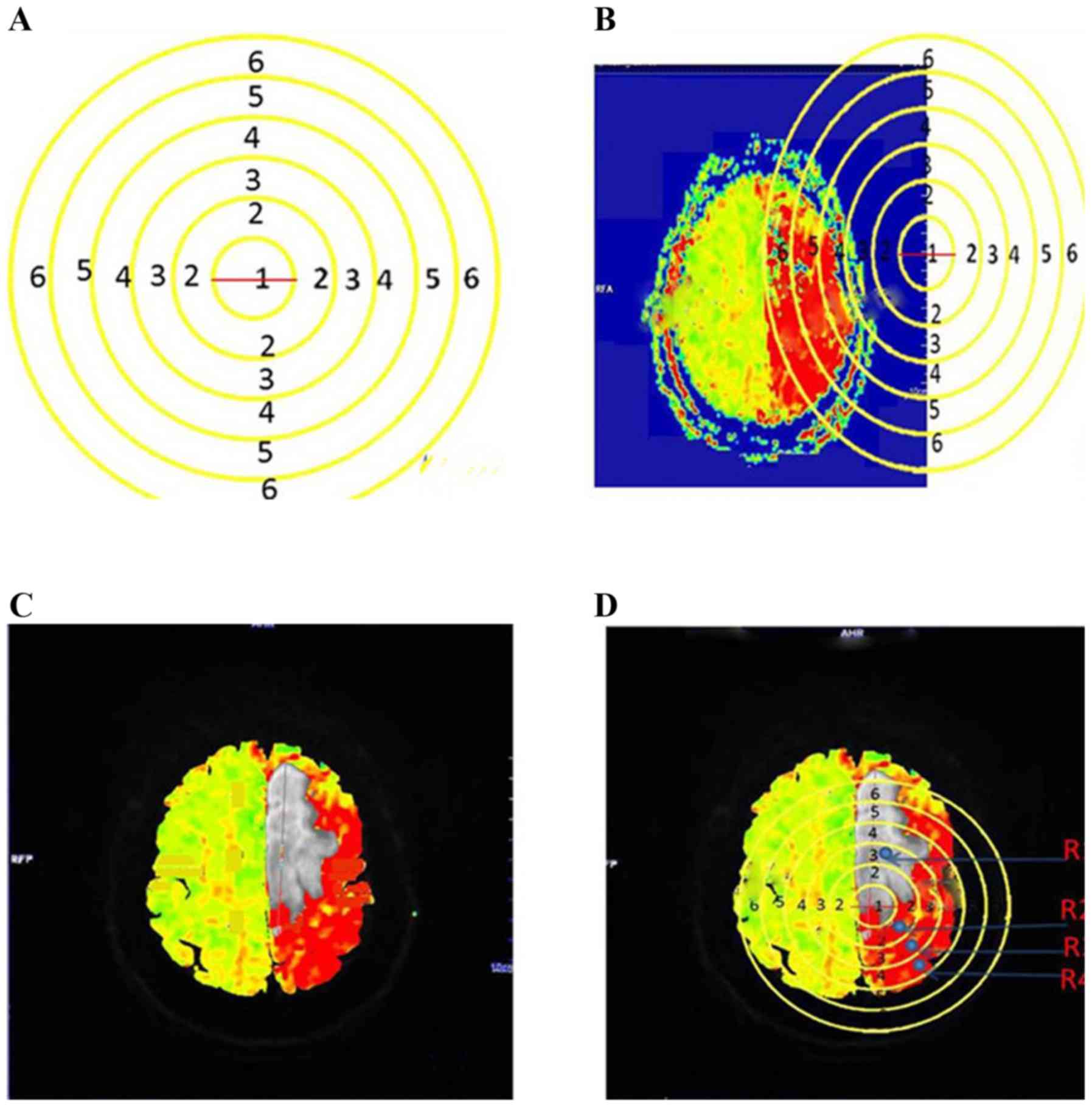
To perform circle calibrations, six circles with
diameters of 1, 2, 3, 4, 5 and 6 cm, were drawn to form a
concentric circle. Subsequently, the concentric circle was placed
on the ruler of the PWI maps to identify the magnification factor.
The minimum circle of the concentric circle thereafter was placed
on the border of the DWI lesion on the coregistered PWI map. A 2-3
cm distance from the border of the DWI lesion was defined as the
lesion surroundings, where ROIs represented the ischemic penumbra
(Fig. 2).
Furthermore, the ratio of the signal value of ROI in
the injured hemisphere to that of its mirror was considered to
indicate the relative PWI parameter, that is, relative (r)CBV,
rCBF, rMTT and rTTP.
According to the territory of arterial supplies, the
internal cranial artery (ICA) was defined as the responsibility
vessel when the DWI lesion was located in ICA territory. Similarly,
the vertebral basilar artery (VBA) was defined as the
responsibility vessel when there was a DWI lesion in its
territory.
Due to the reversed association of the signal value
with the real CBV, CBV may be reasonably speculated from rCBV as
follows: If rCBV was <1, meaning that the blood perfusion in ROI
was greater than that in its mirror, the speculated CBV=[1 +
(1-rCBV)] ×100%; whereas, if rCBV was >1, meaning that the blood
perfusion in ROI was less than that in it's mirror, the speculated
CBV=[1-(rCBV-1)] ×100%.
The modified Rankin Scale (mRS) (22) was used to measure the extent of
disability in patients after stroke with ordinal scores from 0-6 as
follows: 0, no symptoms or disability; 1, symptoms but no
disability; 2, slight disability but no assistance was required; 3,
moderate disability and some assistance was required with
activities of daily living, but patients were able to walk
independently; 4, moderately severe disability and inability to
walk or care for bodily requirements without assistance; 5, severe
disability where patients were bedridden and required constant
care; and 6, patients succumbed to fatality. An mRS score of 0-2
was considered a favorable outcome and scores ≥3 were considered as
unfavorable. mRS scores were recorded in all patients at the
3-month follow-up.
The National Institutes of Health Stroke Scale
(NIHSS) (23) was applied to measure
the extent of neurological dysfunction or deficit after stroke
across multiple domains, including motor, sensory, visual and
language functions. Scores ranged from 0-42, with a higher score
indicating a more severe neurological deficit. NIHSS was recorded
at admission and the 3-month follow-up.
Statistical analysis
All data were presented as mean ± standard
deviation, and IBM SPSS software (version 19.0; IBM Corp., Armonk,
NY, USA) was applied for statistical analysis of the data.
Independent or unpaired sample t-test was used to compare the
differences between the groups mean values. The constitutive ratio
was analyzed using Fisher's exact test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinical characteristics
Among the total 168 patients that were screened, 4
patients were excluded from the study due to allergy to
sulfanilamide (n=2) and claustrophobia (n=2). A total of 5 patients
in the control group dropped out from the trial due to the
requirement of the patients to transfer to another hospital during
treatment.
Baseline characteristics of the patients are
indicated in Table I. Among the 159
patients, 85 patients were allocated to the SA group and 74
patients were allocated to the control group. No statistically
significant differences were identified regarding the sex, age, and
time from symptom onset to hospital admission between the control
and SA groups. The median time from symptom onset to hospital
admission was 6 h in the SA group and 9 h in the control group.
Furthermore, the median GCS at admission was ~12 in the SA and
control groups.
 | Table I.Clinical characteristics of
patients. |
Table I.
Clinical characteristics of
patients.
| Characteristic | SA | Control | P-value |
|---|
| Patients (n) | 85 | 74 |
|
| Sex (n;
male/female) | 65/20 | 52/22 | 0.47 |
| Age (years) | 60.07±12.96 | 61.36±12.98 | 0.52 |
| Symptom onset
(h) | 17.33±20.05 | 20.10±24.10 | 0.77 |
| GCS at
admission | 12.27±1.91 | 12.13±1.89 | 0.85 |
| Responsibility
vesselsa (ICA/VBA) | 69/14 | 61/13 | 0.91 |
| Ipsilateral
hypoperfusion (positive/negative) | 62/23 | 51/23 | 0.60 |
| Contralateral
hypoperfusion (positive/negative) | 15/70 | 5/69 | 0.05 |
| Contralateral DWI
lesion (positive/negative) | 8/77 | 3/71 | 0.22 |
Through visual assessment (24) 62 patients in the SA group and 51
patients in the control group were recorded as exhibiting
hypoperfusion at admission. In addition, 23 patients exhibited
normal perfusion in both the control and SA groups; however,
contralateral hypoperfusion occurred in 15 patients in the SA group
and 5 patients in the control group. No significant difference was
indicated in ipsilateral hypoperfusion between the control or SA
groups in the constitutive ratio (Table
I). Additionally, the results indicated that there was no
significant difference in contralateral DWI lesion between the
control and SA groups. The present findings suggested that the ICA
was associated with 69 cases in the SA group and 61 cases in the
control group. Furthermore, 14 cases in the SA group and 13 cases
in the control group were verified VBA as the responsible artery.
The remaining 2 patients in the SA group had DWI lesions in both
ICA and VBA territories.
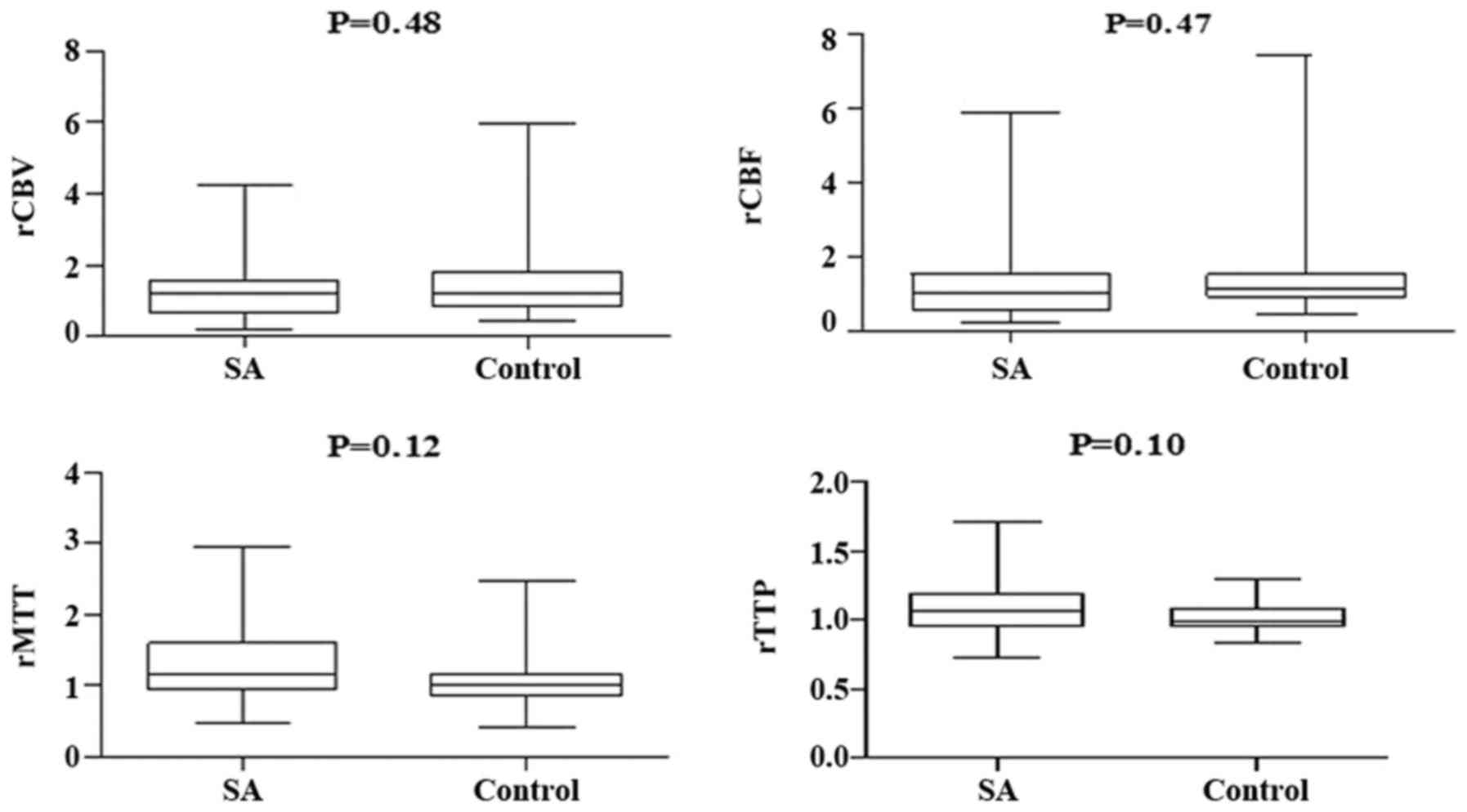
Changes of relative PWI parameters at
admission
The relative PWI parameters at admission within DWI
lesions, which were located using the coregister imaging method,
were indicated to be <1 for rCBF (0.68±0.45 in the SA group vs.
0.85±0.51 in the control group), and >1 for rMTT (1.31±0.56 in
the SA group vs. 1.34±0.56 in the control group) and rTTP
(1.17±0.24 in the SA group vs. 1.15±0.36 in the control group)
(Table II). In addition, the mean
rCBV was 1.27±0.59 in the SA group and 1.21±0.68 in the control
group. Due to the reversed association of the signal value with the
real CBV (25), the value of rCBV
>1 implied hypoperfusion compared with its contralateral
hemisphere. Changes in the relative PWI parameters indicated that
hypoperfusion and longer blood perfusion times were similar in the
DWI lesions of SA and control groups (Table II). Table III demonstrated similar
observations of the relative PWI parameters regarding the
environment surrounding the DWI lesion, which was limited in 1-3 cm
around the lesion using the concentric circle calibration method;
and similar hypoperfusion and longer blood perfusion times were
also indicated. Results suggested there was no significant
difference identified in rCBV, rCBF, rMTT and rTTP between the SA
and control groups.
 | Table II.Relative perfusion-weighted magnetic
resonance imaging parameters of the diffusion-weighted magnetic
resonance imaging lesion at admission. |
Table II.
Relative perfusion-weighted magnetic
resonance imaging parameters of the diffusion-weighted magnetic
resonance imaging lesion at admission.
| Group | rCBV | rCBF | rMTT | rTTP |
|---|
| SA | 1.27±0.59 | 0.68±0.45 | 1.31±0.56 | 1.17±0.24 |
| Control | 1.21±0.68 | 0.85±0.51 | 1.34±0.56 | 1.15±0.36 |
| P-value | 0.62 | 0.08 | 0.73 | 0.73 |
 | Table III.Relative perfusion-weighted magnetic
resonance imaging parameters of the surrounding region of the
diffusion-weighted magnetic resonance imaging lesion at
admission. |
Table III.
Relative perfusion-weighted magnetic
resonance imaging parameters of the surrounding region of the
diffusion-weighted magnetic resonance imaging lesion at
admission.
| Group | rCBV | rCBF | rMTT | rTTP |
|---|
| SA | 1.02±0.56 | 0.80±0.35 | 1.32±0.57 | 1.05±0.17 |
| Control | 1.01±0.55 | 0.84±0.51 | 1.44±0.71 | 1.14±0.29 |
| P-value | 0.82 | 0.61 | 0.31 | 0.09 |
In either ICA or VBA, there were no significant
differences in rCBV, rCBF, rMTT, or rTTP between the SA and control
groups in the DWI lesion or in its surroundings (Table IV); which indicated a similar effect
was observed regarding the relative PWI parameters between SA and
control groups. In normal perfusion or hypoperfusion, the relative
PWI parameters were not significantly different in the DWI lesion
or in its surroundings in the SA and control groups at admission
(Table V).
 | Table IV.Relative PWI parameters with
different responsibility vessels at admission. |
Table IV.
Relative PWI parameters with
different responsibility vessels at admission.
| A, ICA |
|---|
| Vessels | rCBV | rCBF | rMTT | rTTP |
|---|
| DWI lesion |
| SA | 1.27±0.58 | 0.72±0.49 | 1.40±0.59 | 1.19±0.23 |
|
Control | 1.24±0.73 | 0.82±0.47 | 1.39±0.60 | 1.15±0.39 |
|
P-value | 0.82 | 0.35 | 0.96 | 0.57 |
| Surrounding of DWI
lesion |
| SA | 1.02±0.58 | 0.78±0.37 | 1.37±0.57 | 1.08±0.14 |
|
Control | 1.02±0.60 | 0.80±0.54 | 1.56±0.76 | 1.16±0.32 |
|
P-value | 0.96 | 0.89 | 0.20 | 0.20 |
|
| B, VBA |
|
| DWI lesion |
| SA | 1.26±0.68 | 0.56±0.24 | 1.00±0.33 | 1.08±0.25 |
|
Control | 1.07±0.40 | 0.84±0.40 | 1.15±0.26 | 1.12±0.27 |
|
P-value | 0.47 | 0.07 | 0.26 | 0.71 |
| Surrounding of DWI
lesion |
| SA | 1.06±0.54 | 0.83±0.29 | 1.18±0.56 | 0.95±0.25 |
|
Control | 0.90±0.29 | 1.00±0.38 | 1.01±0.15 | 1.06±0.15 |
|
P-value | 0.39 | 0.24 | 0.32 | 0.20 |
 | Table V.Relative PWI parameters with
different perfusion state at admission. |
Table V.
Relative PWI parameters with
different perfusion state at admission.
| A, Normal
perfusion |
|---|
| Perfusion
state | rCBV | rCBF | rMTT | rTTP |
|---|
| DWI lesion |
| SA | 0.88±0.39 | 0.92±0.41 | 1.09±0.37 | 1.01±0.08 |
|
Control | 0.74±0.28 | 1.20±0.64 | 1.02±0.14 | 0.98±0.05 |
|
P-value | 0.30 | 0.20 | 0.53 | 0.35 |
| Surrounding of DWI
lesion |
| SA | 0.77±0.26 | 1.01±0.42 | 1.00±0.18 | 0.99±0.09 |
|
Control | 0.79±0.35 | 1.01±0.56 | 1.03±0.29 | 1.04±0.15 |
|
P-value | 0.90 | 0.10 | 0.77 | 0.32 |
|
| B,
Hypo-Perfusion |
|
| DWI lesion |
| SA | 1.35±0.40 | 0.60±0.41 | 1.37±0.59 | 1.21±0.25 |
|
Control | 1.38±0.70 | 0.72±0.39 | 1.47±0.61 | 1.15±0.22 |
|
P-value | 0.82 | 0.22 | 0.47 | 0.26 |
| Surrounding of DWI
lesion |
| SA | 1.11±0.60 | 0.73±0.30 | 1.42±0.61 | 1.18±0.21 |
|
Control | 1.110±0.61 | 0.72±0.39 | 1.63±0.69 | 1.26±0.26 |
|
P-value | 0.96 | 0.92 | 0.16 | 0.17 |
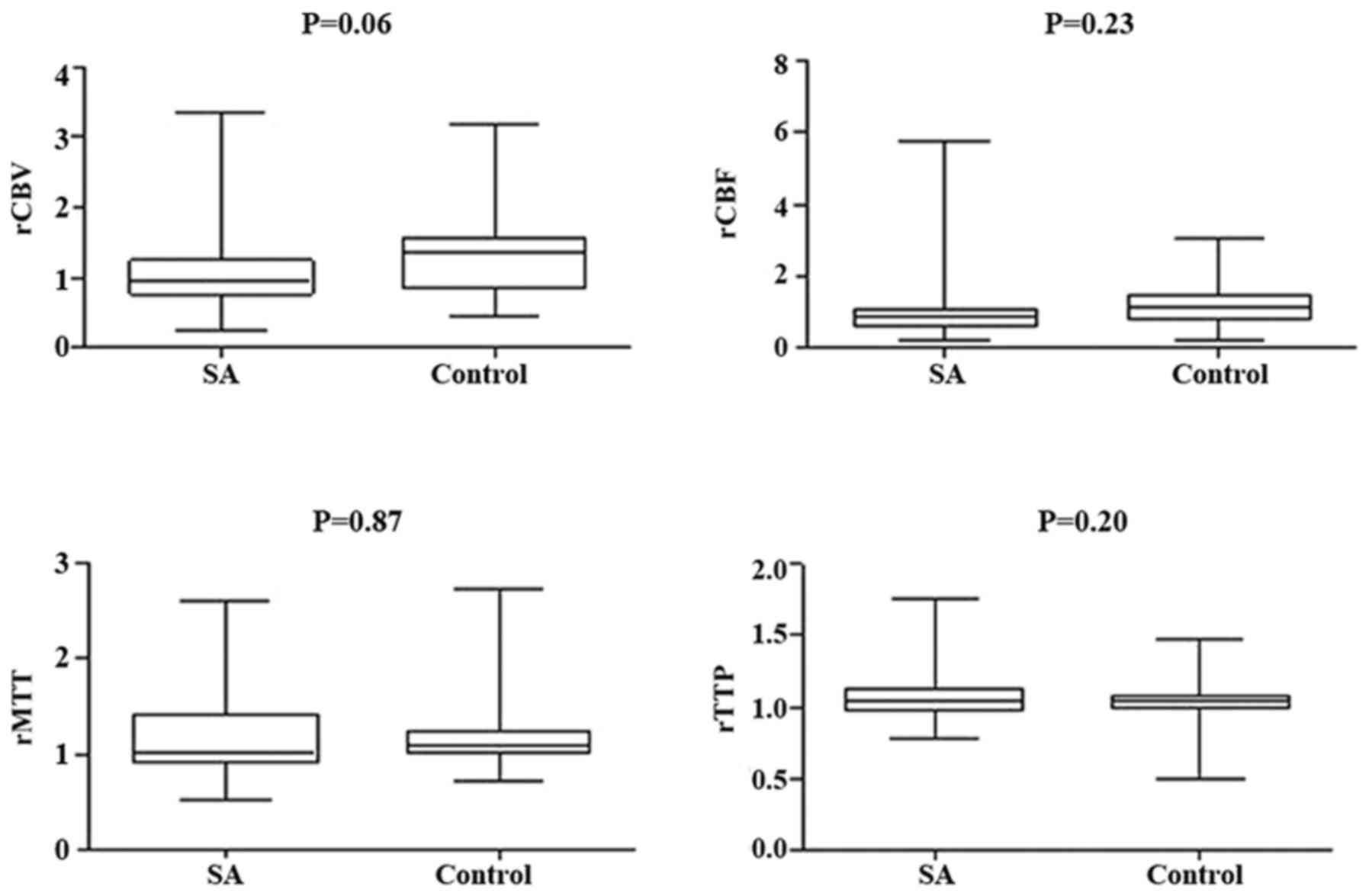
Changes in relative PWI parameters
following treatment
There was no significant differences identified
between the SA and control groups post-treatment in terms of rCBV,
rCBF, rMTT, and rTTP in the DWI lesion or its surroundings
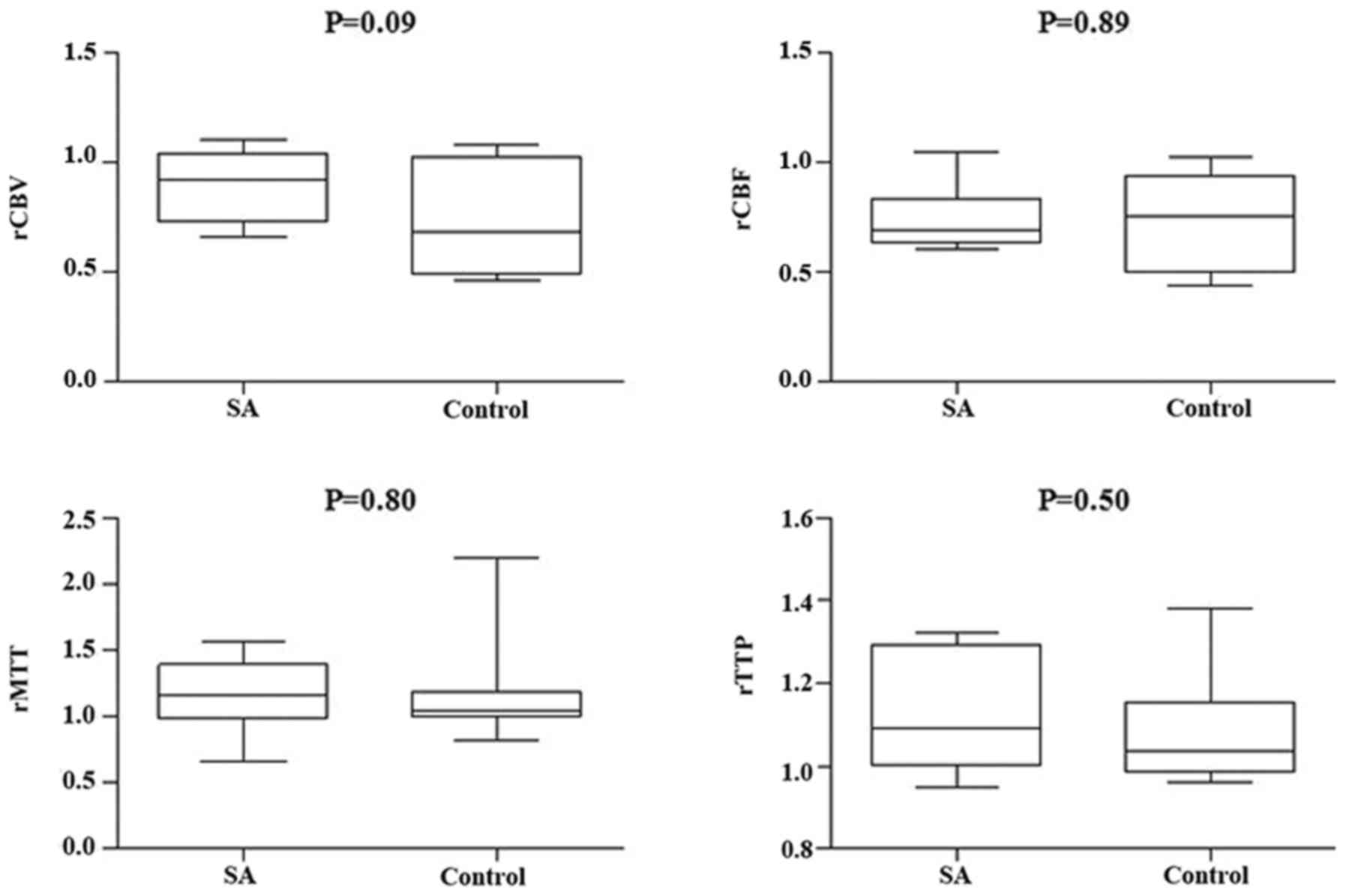
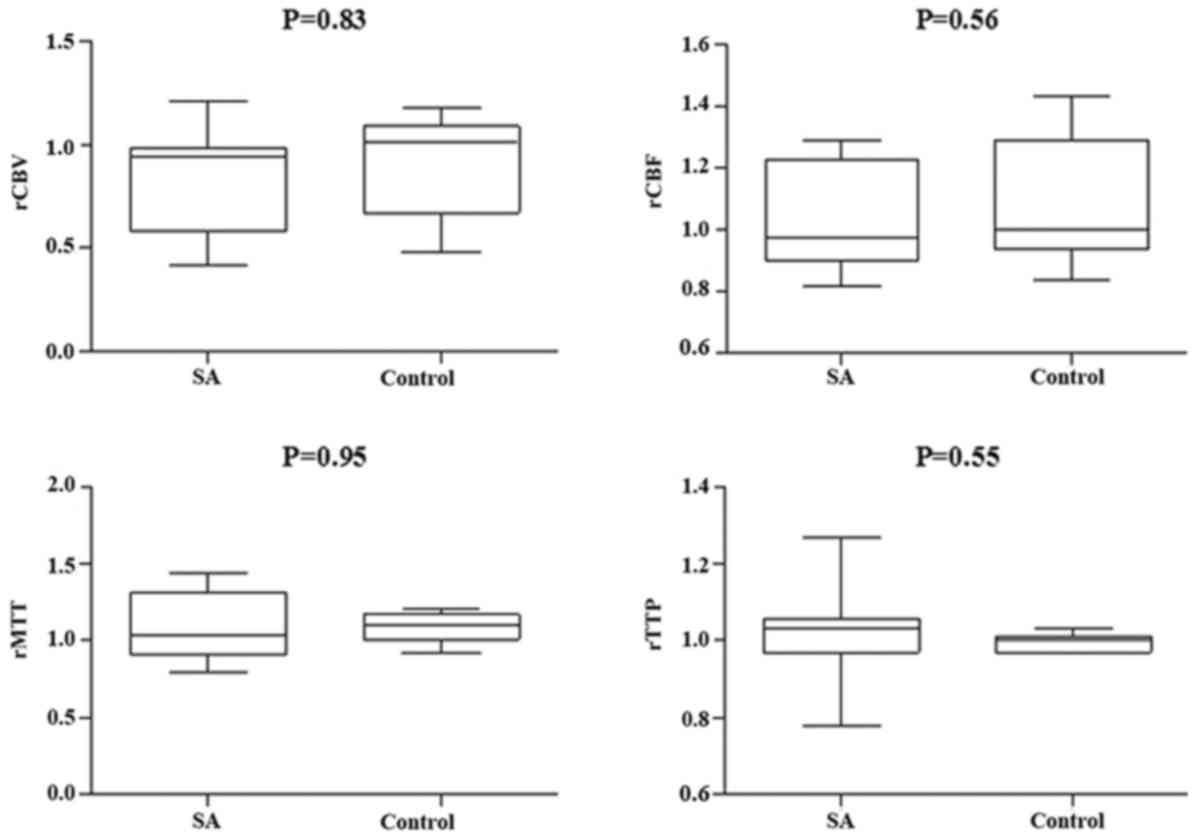
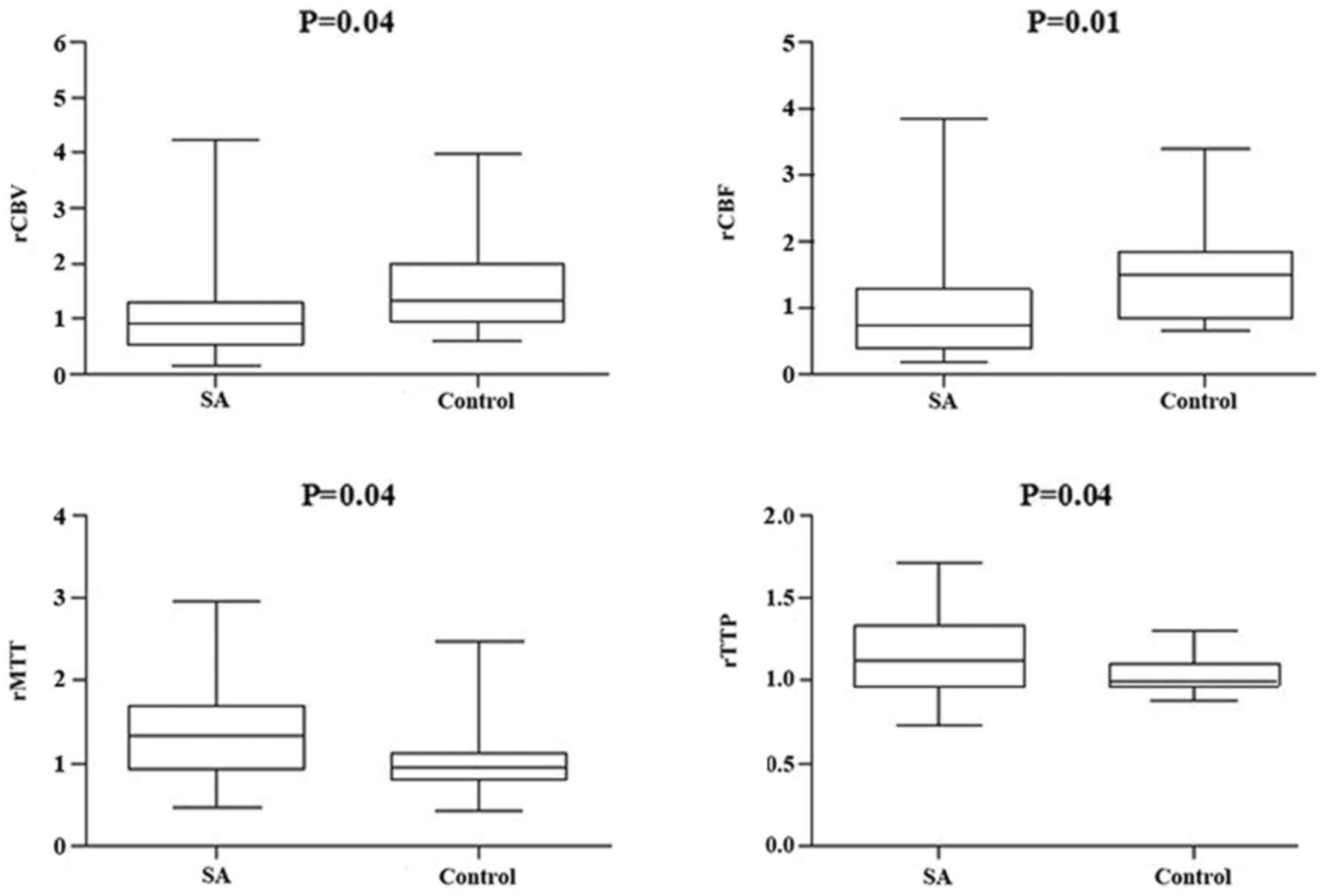
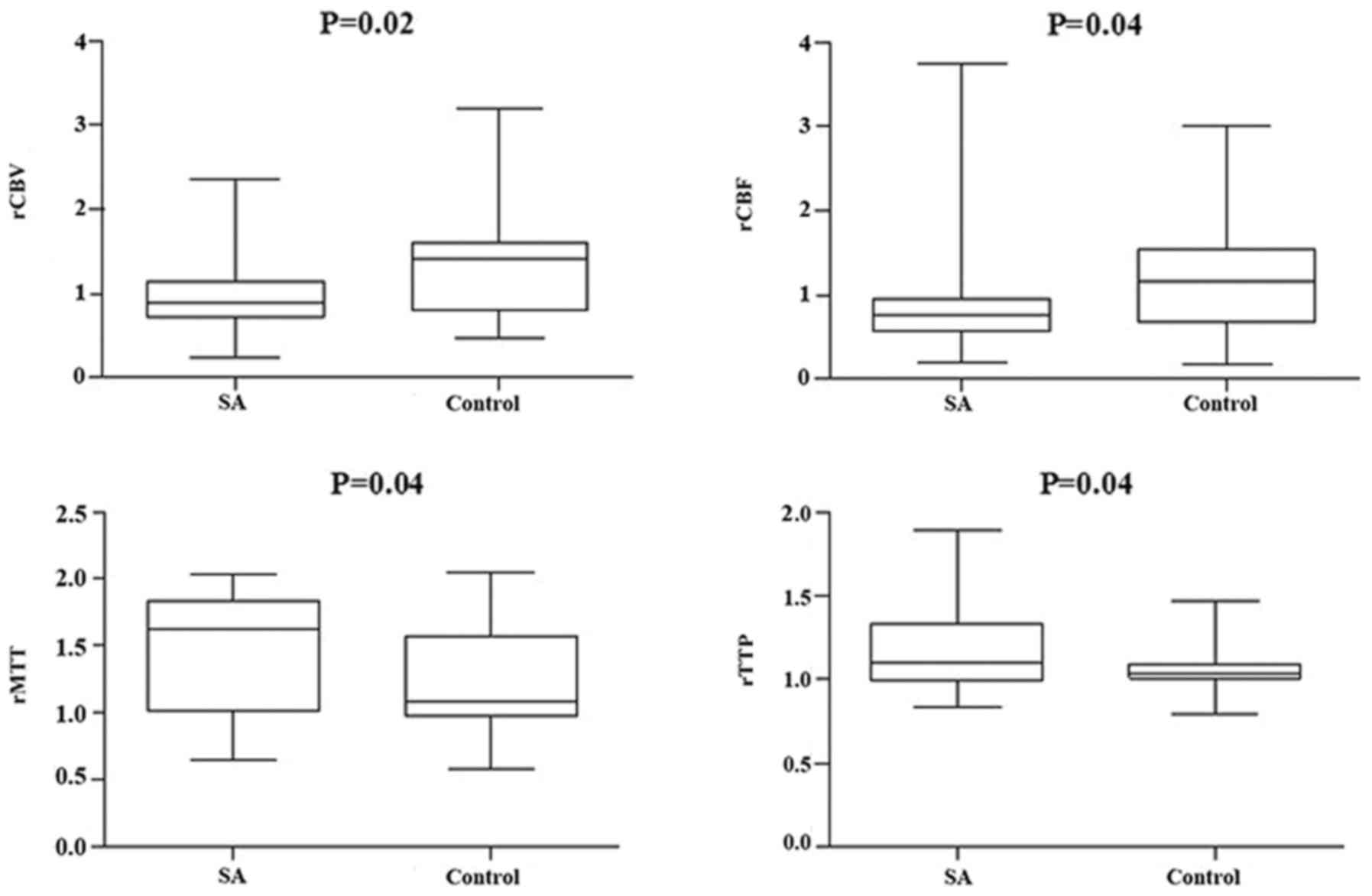
(Figs. 3 and 4, respectively). However, compared with the
different responsible vessel, there was a significant difference
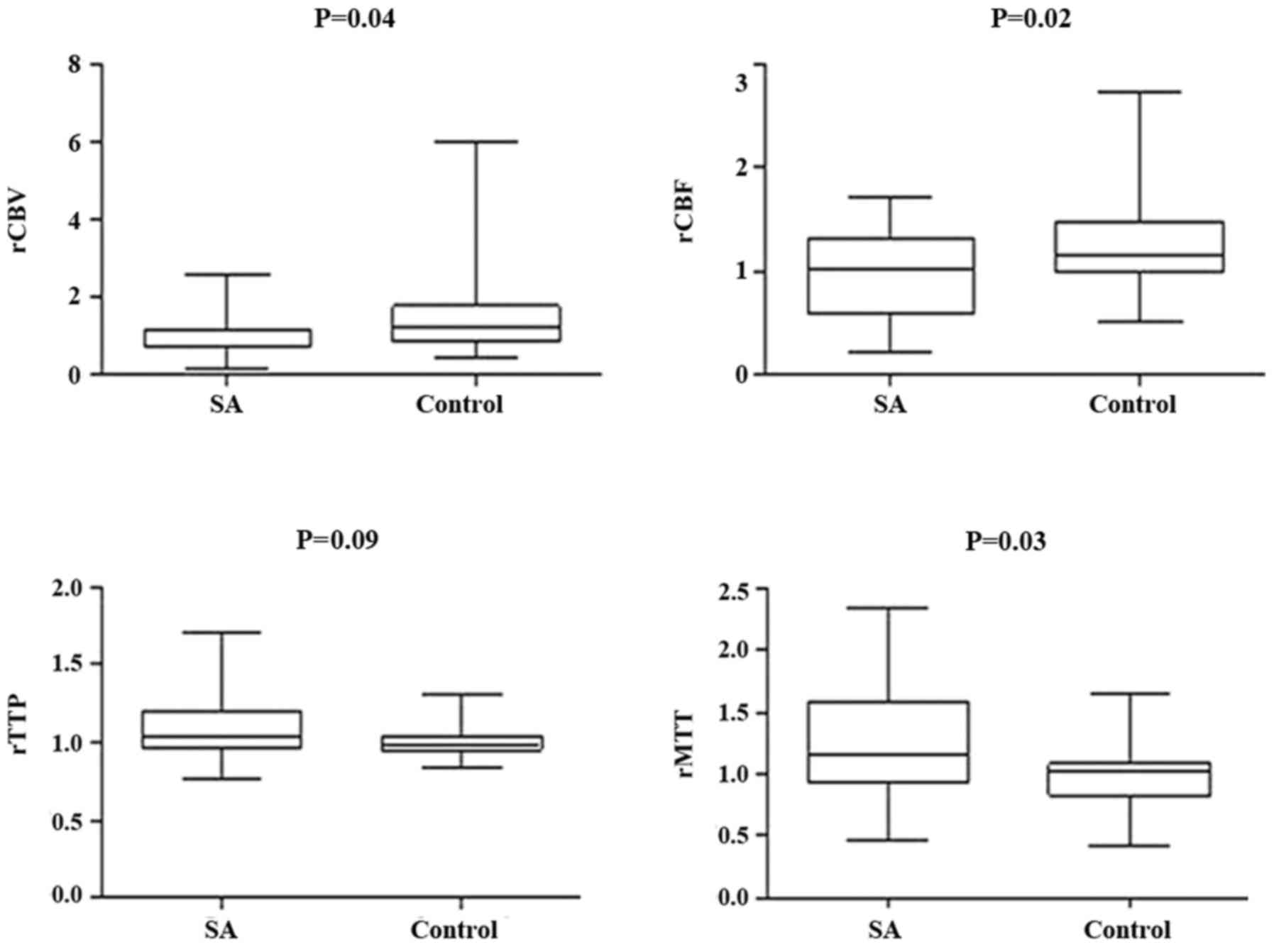
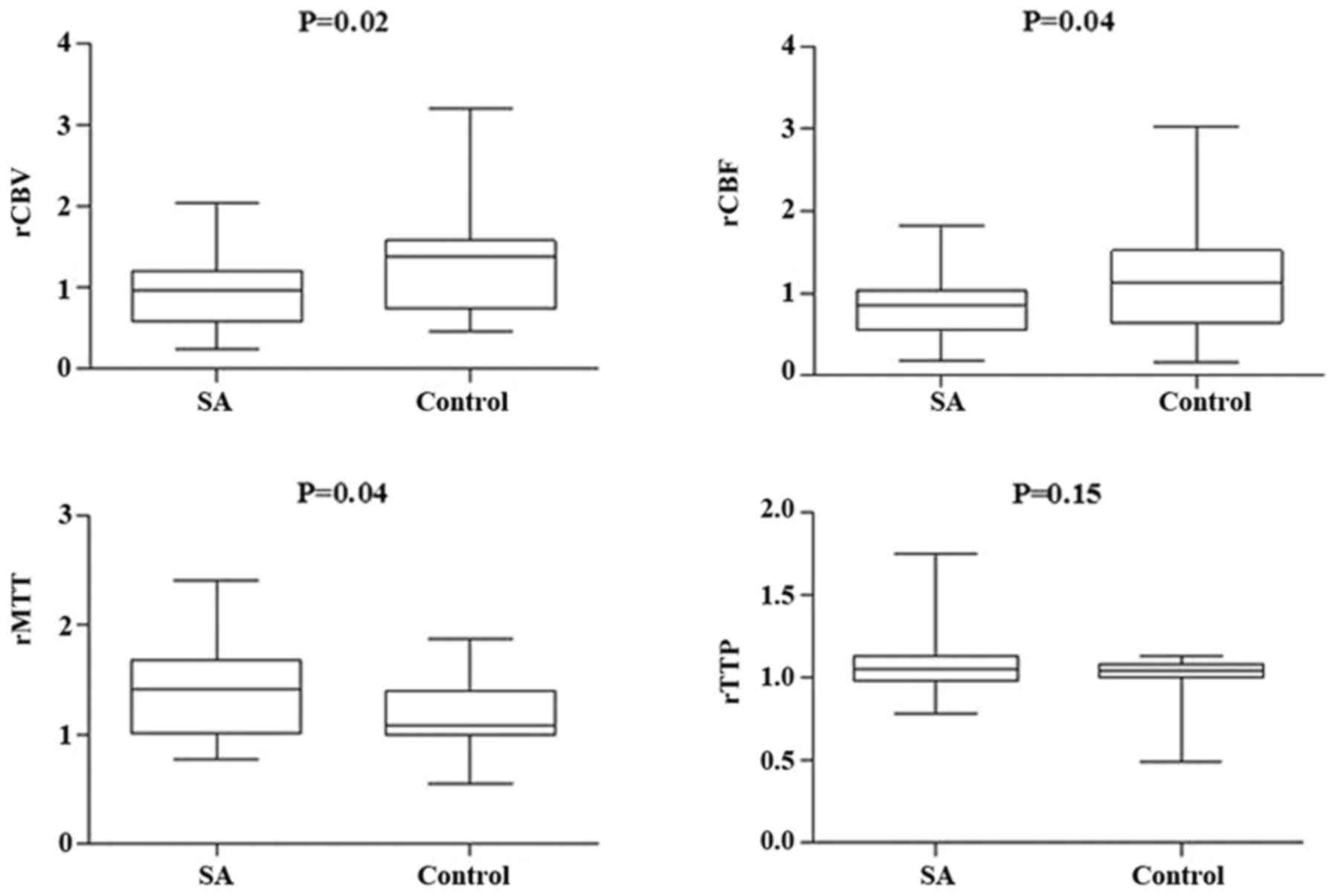
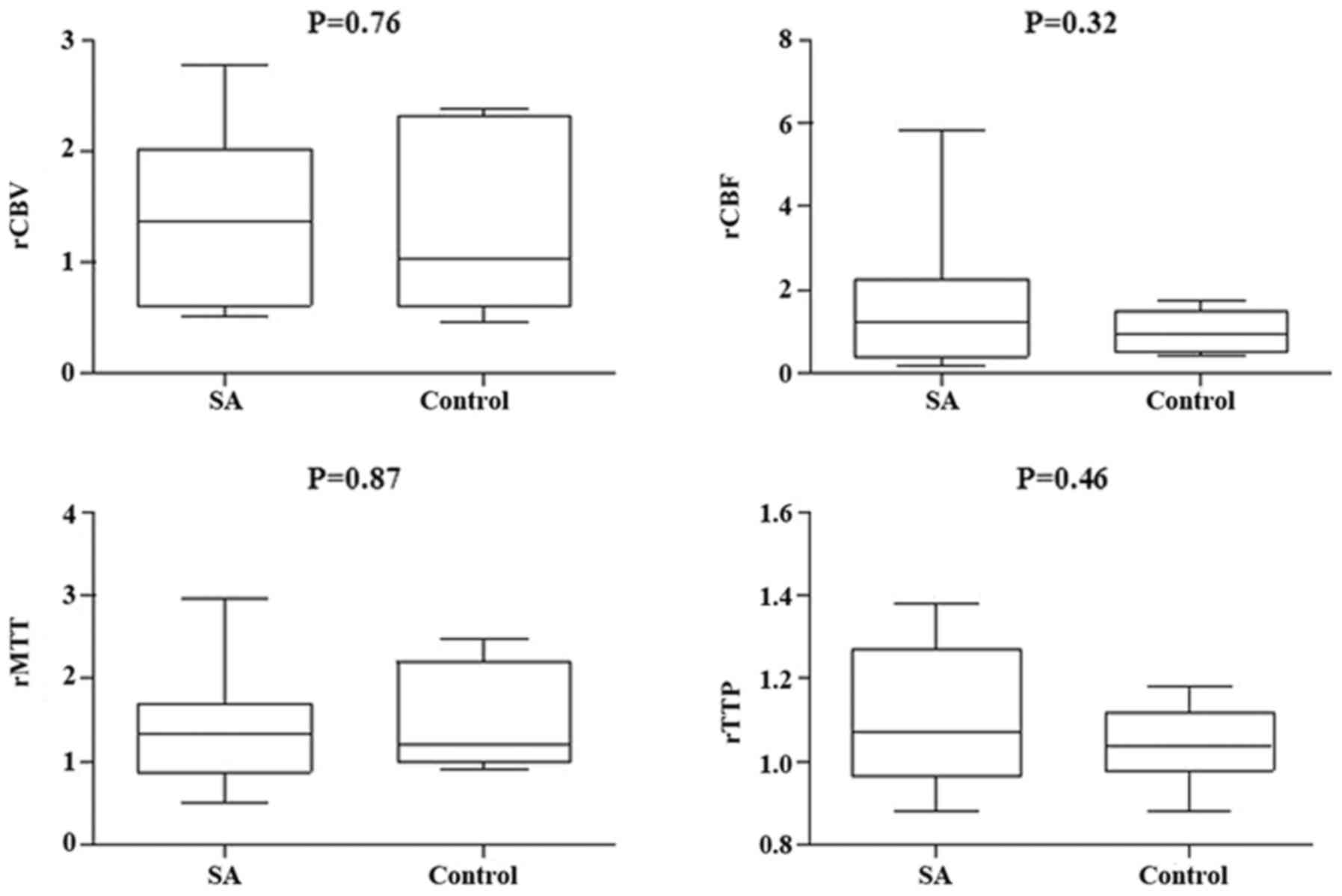
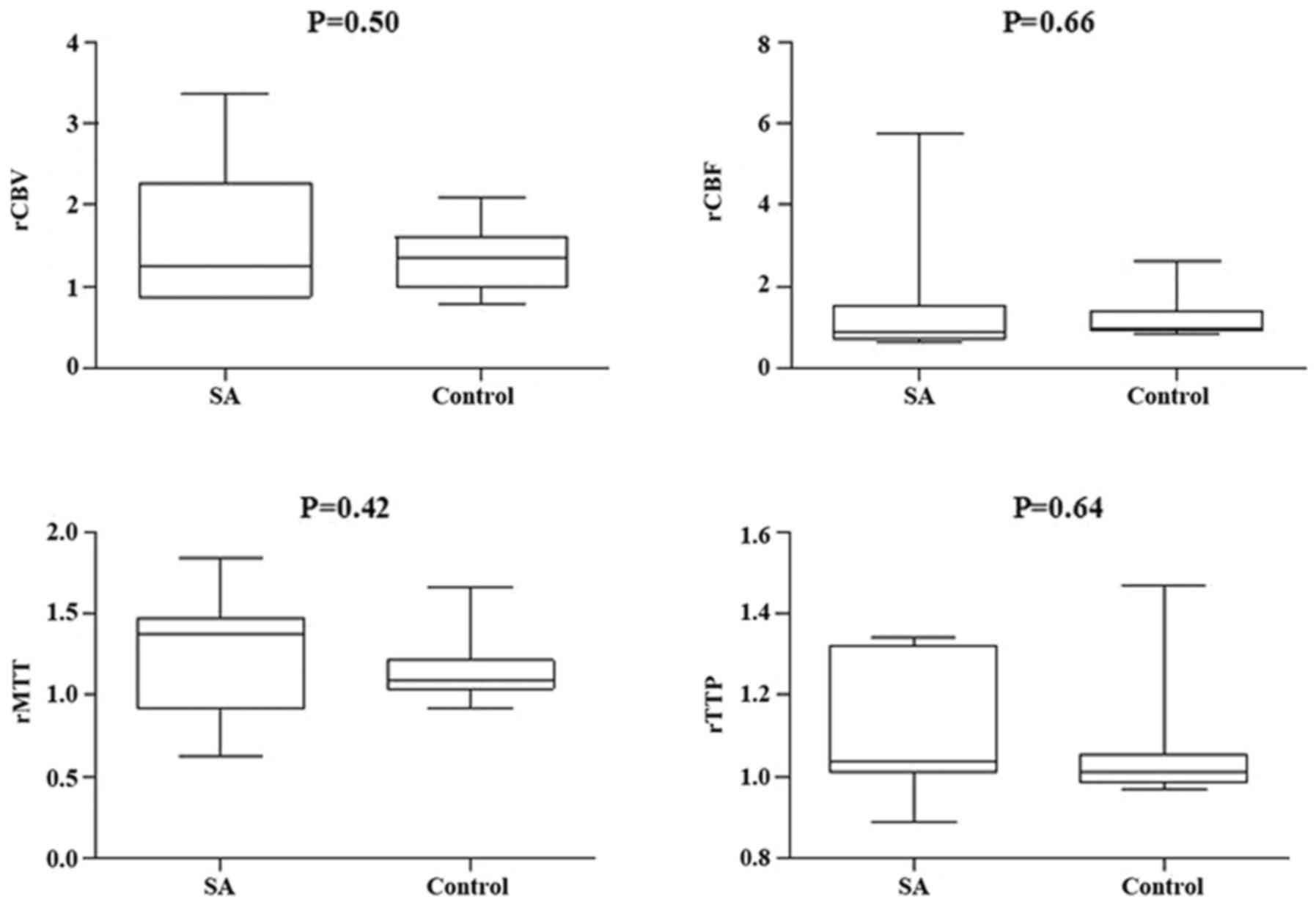
between the two groups in ICA in rCBV, rCBF, and rMTT (Figs. 5 and 6); however, there was no significant
difference in VBA (Figs. 7 and
8), which implied a superior
treatment effect on ICA to VBA. Comparisons of the perfusion states
indicated there was no significant difference in normal perfusion
(Figs. 9 and 10). However, a significant difference
appeared in the hypoperfusion between the two groups in rCBV, rCBF,
rMTT, and rTTP, either in the DWI lesion or in its surrounding
(Figs. 11 and 12). These findings indicate that patients
with hypoperfusion at admission may benefit from this therapy to a
greater extent compared with patients with normal perfusion.
In certain patients the abnormal PWI disappeared
following treatment with SA, whereas in other patients treated with
SA abnormal PWI persisted (Fig.
13). This may be due to the different reperfusion of CBV.
Speculated CBV using the speculation
method
The CBV at admission in the DWI lesion was 73 and
79% of the contralateral in the SA and control groups, respectively
(Table VI). In addition,
surrounding the DWI lesion the CBV was 98 and 99% of the
contralateral in the SA and control groups, respectively (Table VII). However, in patients with
hypoperfusion at admission the CBV was 93 and 46% of the
contralateral in the DWI lesions and 102 and 61% in the
surroundings in the SA and control groups, respectively following
treatment (Table VIII). In
patients with ICA responsible artery the CBV was 98 and 50% in the
DWI lesions and 104 and 67% in the surroundings following treatment
in the SA and control groups, respectively (Table IX).
 | Table VI.Speculated CBV at admission. |
Table VI.
Speculated CBV at admission.
| Group | ROI | rCBV | CBV (%) |
|---|
| SA | DWI | 1.27±0.59 | 73 |
| Control | DWI | 1.21±0.68 | 79 |
| SA | Surrounding | 1.02±0.56 | 98 |
| Control | Surrounding | 1.01±0.55 | 99 |
 | Table VII.Speculated CBV following
treatment. |
Table VII.
Speculated CBV following
treatment.
| Group | ROI | rCBV | CBV (%) |
|---|
| SA | DWI | 1.29±0.80 | 71 |
| Control | DWI | 1.45±1.08 | 55 |
| SA | Surrounding | 1.05±0.57 | 95 |
| Control | Surrounding | 1.33±0.60 | 67 |
 | Table VIII.Speculated CBV after treatment in
patients with hypoperfusion at admission. |
Table VIII.
Speculated CBV after treatment in
patients with hypoperfusion at admission.
| Group | ROI | rCBV | CBV (%) |
|---|
| SA | DWI | 1.07±0.78 | 93 |
| Control | DWI | 1.54±0.81 | 46 |
| SA | Surrounding | 0.98±0.51 | 102 |
| Control | Surrounding | 1.39±0.68 | 61 |
 | Table IX.Speculated CBV following treatment in
patients with ICA responsible artery. |
Table IX.
Speculated CBV following treatment in
patients with ICA responsible artery.
| Group | ROI | rCBV | CBV (%) |
|---|
| SA | DWI | 1.02±0.46 | 98 |
| Control | DWI | 1.50±1.16 | 50 |
| SA | Surrounding | 0.96±0.43 | 104 |
| Control | Surrounding | 1.33±0.69 | 67 |
Neurological function
The neurological function in patients was evaluated
using NIHSS and mRS. There was no significant difference in NIHSS
between the two groups at admission (8.43±6.05 in the SA group vs.
9.12±5.98 in the control group; P=0.47; Table X). However, at the 3-month follow-up,
NIHSS in the SA group was significantly decreased compared with
that in the control group (3.25±4.67 vs. 5.76±3.82; P=0.001), and
mRS was also significantly reduced in the SA group (1.26±1.58 vs.
2.01±1.58, P=0.005 (Table X), which
indicated that there was a minor deficit of neurological function
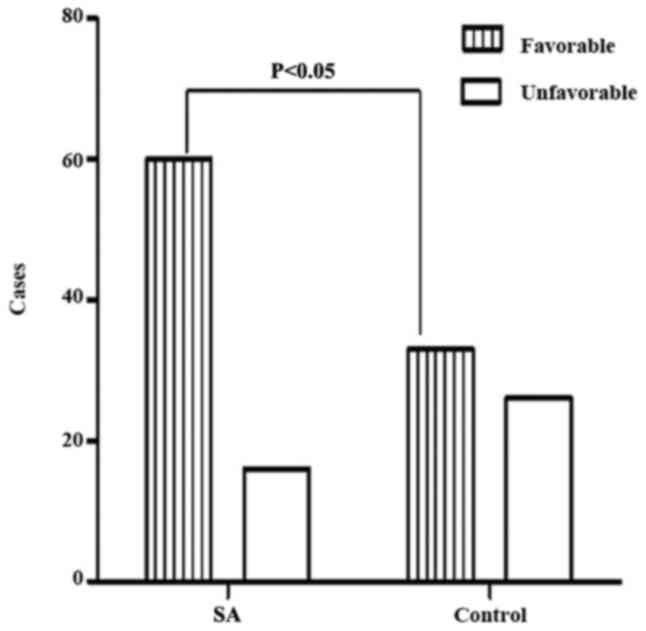
in the SA group compared with that in the control group. When mRS
was dichotomized, the proportion of patients with a favorable
outcome was significantly increased in the SA group compared with
the control group (Fig. 14).
 | Table X.NIHSS and mRS score. |
Table X.
NIHSS and mRS score.
|
| NIHSS |
|
|---|
|
|
|
|
|---|
| Group | Admission | 90-day | mRS 90-day |
|---|
| SA | 8.43±6.05 | 3.25±4.67 | 1.26±1.58 |
| Control | 9.12±5.98 | 5.76±3.82 | 2.01±1.58 |
| P-value | 0.47 | 0.001 | 0.005 |
Discussion
Stroke is one of the leading causes of morbidity and
the second most common cause of mortality worldwide (26). An acute ischemic stroke occurs due to
occlusion or a severe restriction in the blood supply to the brain,
resulting in hypoperfusion of the brain tissue, namely, ischemic
penumbra; which may cause permanent infarction (14). Therefore, the aim of acute stroke
treatment is to restore the blood supply of hypoperfused brain
tissues in a timely manner to prevent these tissues from undergoing
irreversible injury. This is referred to as reperfusion therapy
(13). Currently, effective
reperfusion therapy includes the intravenous and intraarterial
administration of rtPA, and the use of various thrombectomy devices
under X-ray guidance which is typically defined as dethrombosis
therapy or recanalization (27–32). The
focus of these therapies is on the restoration of the antegrade
flow of the supplying artery in the ischemic regions. However, the
time window limits its use in clinical practice (33,34).
With the progress of neuroimaging techniques, it is
possible to observe the existence of ischemic penumbra. Previous
studies have suggested that the mismatch between PWI and DWI
represents ischemic penumbra (35,36). If
increased CBV in the penumbra is achieved following treatment, it
may be concluded that there was a restoration of blood perfusion in
the penumbra, whether antegrade or retrograde flow.
In the present study, among the 159 cases of acute
ischemic stroke, 62 cases in the SA group and 51 cases in the
control group exhibited hypoperfusion in the ipsilateral hemisphere
of the DWI lesion, with another 23 cases in each group who
presented with normal perfusion. However, the hypoperfusion/normal
perfusion in the ipsilateral hemisphere between these two groups
was not significantly different at admission. In addition, relative
PWI parameter comparisons between the SA and control groups also
indicated no significant difference at admission, whether in DWI
lesion or in the surroundings of the DWI lesion. However, following
the 14-day treatment, a significant decrease in rCBV occurred in
the SA group compared with the control in the DWI lesion and its
surrounding region in patients with responsibility vessel ICA or
hypoperfusion at admission. Relative PWI parameters in the SA group
were compared with those in controls within the subgroups of ICA as
a responsible vessel or hypoperfused patients at admission. No
significant difference was indicated within the subgroup of VBA as
the responsible vessel or normal perfused patients at admission,
which implied that SA improved the perfusion of the hypoperfused
brain tissue in the DWI lesion and its surroundings, potentially by
a selective pattern to fit the metabolism demands of the
hypoperfused tissue, or by increasing blood perfusion in
hypoperfused tissues selectively and not influencing the
non-hypoperfused tissues, as SA did not significantly affect CBV in
normal perfused patients. Baron et al (37) found previously that oxygen extraction
fraction (OEF) increased from the normal value of approximately 40
to >80% in the area of penumbra in a hemodynamic cerebral
ischemia patient with positron emission tomography. Furthermore,
with the ischemia-reperfusion experiment model in cats, reversible
middle cerebral artery (MCA) occlusion occurred by reopening the
MCA after 60 min (37). Heiss
(38) previously reported that when
the OEF remained elevated throughout the ischemic episode,
reperfusion prevented large infarcts involving cortical areas, and
if the initial OEF increase disappeared during ischemia, extended
postischemic hyperperfusion-accompanied large infarcts developed.
These findings suggest that reperfusion may only fit the metabolic
demands of hypoperfused tissues, and consequently, a favorable
outcome was obtained. Therefore, the different energy requirements
for the maintenance of membrane function and for the propagation of
information may result in different blood flow volumes supplied for
the preservation of neuronal function and morphological integrity.
The present study indicated that SA treatment may be an effective
reperfusion treatment for the hypoperfused tissues when
administered <72 h following the onset of symptoms as it
responds appropriately to metabolic demands and consequently
provides an improved outcome compared with the control.
When compared with the different responsible
arteries, in the ICA territory, SA also appeared to have greater
benefits in improving the perfusion of the ischemic core and
ischemic penumbra compared with that in the VBA territory. These
findings suggest that microcirculation compensation may be a
potential mechanism associated with acute stroke treatment with SA.
However, further studies are required to fully elucidate this.
The present study indicated that blood perfusion in
ischemic core and it's surrounding was improved in hypoperfused
tissues following SA treatment. Similar changes were identified in
the neurological deficits using NIHSS and mRS. No significant
difference was indicated in the NIHSS between the SA and control
group at admission; however, a significant decrease in NIHSS was
observed in the SA group at the 3-month follow-up compared with
that in the control group, which indicated that the SA elicited a
neurological protective effect on ischemic brain tissue, and this
may be associated with microcirculation compensation. Additionally,
the rate of patients with a favorable outcome in the SA group at
the 3-month follow-up was significantly increased compared with
that in the control group, which suggested that SA improved the
neurological defect, potentially due to an improvement in blood
supply in the ischemic brain tissue caused by an increase in CBV.
The restoration of the blood flow, either antegrade or retrograde,
may save the ischemic tissue (16).
Mokin et al (39)
retrospectively reviewed cases of acute ischemic stroke due to MCA
M1 segment occlusion, and associated the favorable outcome with
preintervention CBV values. The findings concluded that
preintervention CBV values may be used as a predictor of the
outcome in patients undergoing intra-arterial stroke therapies
(39). Therefore, CBV in brain
tissue expressed by rCBV in the present study may be a useful
indicator of reperfusion in the ischemic region and may be used as
criteria to assess the therapeutic effect of drugs in acute stroke
therapy.
In addition to increased CBV in the ischemic region
following treatment with SA, decreased rCBF and increased rMTT were
also observed in the present study when compared with that in
controls in ICA or in hypoperfusion patients, which indicates
longer blood perfusion time. In the control group, significantly
increased rCBV and rCBF and significantly decreased rMTT were
observed compared with the SA group following treatment either in
ICA as the responsible vessel or in hypoperfusion patients,
implying a shorter blood perfusion time and blood traveling a
shorter distance by other vessels as opposed to the capillaries
(such as the arteries and veins).
SA, consists of the water-soluble components
isolated from the roots of Salvia miltiorrhiza Bunge, which
contains 1% SA A, 57% SA B, 37% rosmarinic acid and 5% other acids,
and is a Chinese herb widely used for the treatment of stroke
(40). The use of SA was approved in
2011 by the Chinese State food and Drug administration (Z20110011)
for the treatment of ischemic stroke (41). Previous findings have indicated that
SA inhibits lipid peroxidation, scavenges free radicals and
protects neural cells against injuries caused by anoxia (42,43). In
the present study, SA improved the perfusion of ischemic brain
tissues selectively, and induced a favorable outcome compared with
the controls at 3-month follow-up. Based on the present findings,
microcirculation compensation may be a potential mechanism
associated with the effect of SA on acute stroke. Further studies
are required, particularly in vivo experiments, to fully
explore the effects of microcirculation compensation in acute
stroke.
The present study did have some limitations.
Firstly, the study was semi-qualitative. However, relative PWI
parameters are simply obtained and it has been observed that
speculated CBV from rCBV is similar to that from volumetric
assessment (44). In a previous
study by the present authors, using the speculation method, it was
revealed that 118% of the contralateral CBV appeared in normal
perfused patients and 77% in hypoperfused patients within DWI
lesions; and surrounding the DWI lesion 121% of the contralateral
CBV in normal perfused patients and 90% in hypoperfused patients,
respectively; which suggests that >90% of the contralateral CBV
may fit the metabolic demands of the hypoperfused tissues, and
>120% of the contralateral CBVs may maintain a normal perfusion
map (45). In the present study,
increasing CBV appeared in the SA group after treatment whether in
DWI lesions or it's surrounding with patients of hypoperfusion or
ICA as the responsible artery but does not meet the 120% criteria
to reverse the hypoperfusion completely in the PWI map in all
patients Secondly, visual assessment is not as accurate as
volumetric assessment; however, hypoperfusion was easily detected
using visual assessment. Furthermore, in the present study, the ROI
was selected from a single time point of the PWI map and therefore
not all time points were considered, which increased the extent of
selection error. Additionally, the authors suggest that the
increased CBV in ischemic brain tissue following treatment with SA
was due to surrounding circulation compensation as opposed to the
normal microcirculation perfusion, however animal experiments are
required to confirm.
In conclusion, the present results indicate that SA
treatment may improve the perfusion of ischemic brain tissues,
including the ischemic core and penumbra in ICA or hypoperfused
patients with acute stroke. Furthermore, these findings suggest
that SA may be used to improve neurological function and obtain
favorable outcomes for patients that have suffered with acute
stroke.
Competing interests
All authors declare that there are no competing
interests.
References
|
1
|
Roth GA, Johnson C, Abajobir A, Abd-Allah
F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, et al:
Global, regional, and national burden of cardiovascular diseases
for 10 causes, 1990-2015. J Am Coll Cardio. 4:1–25. 2017.
View Article : Google Scholar
|
|
2
|
Vagal A, Sanelli P, Sucharew H, Alwell KA,
Khoury JC, Khatri P, Woo D, Flaherty M, Kissela BM, Adeoye O, et
al: Age, sex, and racial differences in neuroimaging use in acute
stroke: A population-based study. AJNR Am J Neuroradiol.
38:1905–1910. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim AS, Cahill E and Cheng NT: Global
stroke belt: Geographic variation in stroke burden worldwide.
Stroke. 46:3564–3570. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Long X, Lou Y, Gu H, Guo X, Wang T, Zhu Y,
Zhao W, Ning X, Li B, Wang J and An Z: Mortality, recurrence, and
dependency rates are higher after acute ischemic stroke in elderly
patients with diabetes compared to younger patients. Front Aging
Neurosci. 8:1422016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gan Y, Wu J, Zhang S, Li L, Yin X, Gong Y,
Herath C, Mkandawire N, Zhou Y, Song X, et al: Prevalence and risk
factors associated with stroke in middle-aged and older Chinese: A
community-based cross-sectional study. Sci Rep. 7:95012017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Motyer R, Asadi H, Thornton J, Nicholson P
and Kok HK: Current evidence for endovascular therapy in stroke and
remaining uncertainties. J Intern Med. 283:2–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wey HY, Desai VR and Duong TQ: A review of
current imaging methods used in stroke research. Neurological Res.
35:1092–1102. 2013. View Article : Google Scholar
|
|
8
|
An H, Ford AL, Eldeniz C, Chen Y, Vo KD,
Zhu H, Powers WJ, Lin W and Lee JM: Reperfusion beyond 6 hours
reduces infarct probability in moderately ischemic brain tissue.
Stroke. 47:99–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mishra NK, Albers GW, Davis SM, Donnan GA,
Furlan AJ, Hacke W and Lees KR: Mismatch-based delayed
thrombolysis: A meta-analysis. Stroke. 41:e25–e33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Albers GW: Expanding the window for
thrombolytic therapy in acute stroke. The potential role of acute
MRI for patient selection. Stroke. 30:2230–2237. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim SJ, Seok JM, Bang OY, Kim GM, Kim KH,
Jeon P, Chung CS, Lee KH, Alger JR and Liebeskind DS: MR mismatch
profiles in patients with intracranial atherosclerotic stroke: A
comprehensive approach comparing stroke subtypes. J Cereb Blood
Flow Metab. 29:1138–1145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Neumann-Haefelin T, Wittsack HJ, Wenserski
F, Siebler M, Seitz RJ, Mödder U and Freund HJ: Diffusion- and
perfusion-weighted MRI. The DWI/PWI mismatch region in acute
stroke. Stroke. 30:1591–1597. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Galinovic I, Ostwaldt AC, Soemmer C, Bros
H, Hotter B, Brunecker P, Schmidt WU, Jungehülsing J and Fiebach
JB: Search for a Map and threshold in perfusion MRI to accurately
predict tissue fate: A protocol for assessing lesion growth in
patients with persistent vessel occlusion. Cerebrrovasc Dis.
32:186–193. 2011. View Article : Google Scholar
|
|
14
|
Mishra NK, Albers GW, Christensen S, Marks
M, Hamilton S, Straka M, Liggins JT, Kemp S, Mlynash M, Bammer R,
et al: Comparison of magnetic resonance imaging mismatch criteria
to select patients for endovascular stroke therapy. Stroke.
45:1369–1374. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prabhakaran S, Ruff I and Bernstein RA:
Acute stroke intervention: A systematic review. JAMA.
313:1451–1462. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Davis S, Campbell B, Christensen S, Ma H,
Desmond P, Parsons M, Levi C, Bladin C, Barber PA and Donnan G:
Perfusion/Diffusion mismatch is valid and should be used for
selecting delayed interventions. Transl Stroke Res. 3:188–197.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun K, Fan J and Han J: Ameliorating
effects of traditional Chinese medicine preparation, Chinese
materia medica and active compounds on ischemia/reperfusion induced
cerebral microcirculatory disturbances and neuron damage. Acta
Pharm Sin B. 5:8–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chien MY, Chuang CH, Chern CM, Liou KT,
Liu DZ, Hou YC and Shen YC: Salvianolic acid A alleviates ischemic
brain injury through the inhibition of inflammation and apoptosis
and the promotion of neurogenesis in mice. Free Radic Biol Med.
99:508–519. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhuang P, Wan Y, Geng S, He Y, Feng B, Ye
Z, Zhou D, Li D, Wei H, Li H, et al: Salvianolic acids for
injection (SAFI) suppresses inflammatory responses in activated
microglia to attenuate brain damage in focal cerebral ischemia. J
Ethnopharmacol. 198:194–204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng SQ, Aa N, Geng JL, Huang JQ, Sun RB,
Ge C, Yang ZJ, Wang LS, Aa JY and Wang GJ: Pharmacokinetic and
metabolomic analyses of the neuroprotective effects of salvianolic
acid A in a rat ischemic stroke model. Acta Pharmacol Sin.
38:1435–1444. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murphy S, Thomas NJ, Gertz SJ, Beca J,
Luther JF, Bell MJ, Wisniewski SR, Hartman AL and Tasker RC:
Tripartite stratification of the Glasgow coma scale in children
with severe traumatic brain injury and mortality: An analysis from
a multi-center comparative effectiveness study. J Neurotrauma. Feb
27–2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stefanovic Budimkic M, Pekmezovic T,
Beslac-Bumbasirevic L, Ercegovac M, Berisavac I, Stanarcevic P,
Padjen V and Jovanović DR: Long-term prognosis in ischemic stroke
patients treated with intravenous thrombolytic therapy. J Stroke
Cerebrovasc Dis. 26:196–203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suwanwela NC, Chutinet A, Mayotarn S,
Thanapiyachaikul R, Chaisinanunkul N, Asawavichienjinda T,
Muengtaweepongsa S, Nilanont Y, Samajarn J, Watcharasaksilp K, et
al: A randomized controlled study of intravenous fluid in acute
ischemic stroke. Clin Neurol Neurosurg. 161:98–103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Siemonsen S, Fitting T, Thomalla G,
Krutzelmann A and Fiehler J: Visual assessment of magnetic
resonance imaging perfusion lesions in a large patient group. Clin
Neuroradiol. 22:305–313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Østergaard L: Principles of cerebral
perfusion imaging by bolus tracking. J Magn Reson Imaging.
22:710–717. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Robertson CA, McCabe C, Gallagher L,
Lopez-Gonzalez Mdel R, Holmes WM, Condon B, Muir KW, Santosh C and
Macrae IM: Stroke penumbra defined by an MRI-based oxygen challenge
technique: 1. Validation using [14C]2-deoxyglucose autoradiography.
J Cere Blood Flow Metab. 31:1778–1787. 2011. View Article : Google Scholar
|
|
27
|
The National Institute of Neurological
Disorders and Stroke rt-PA Stroke Study Group, : Tissue plasminogen
activator for acute ischemic stroke. N Engl J Med. 333:1581–1587.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ciccone A, Valvassori L, Ponzio M,
Ballabio E, Gasparotti R, Sessa M, Scomazzoni F, Tiraboschi P and
Sterzi R; SYNTHESIS Investigators, : Intra-arterial or intravenous
thrombolysis for acute ischemic stroke? J Neurointerv Surg.
2:74–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Berkhemer OA, Fransen PS, Beumer D, van
den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn
PJ, Wermer MJ, et al: A randomized trial of intra-arterial
treatment for acute ischemic stroke. N Engl J Med. 372:11–20. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goyal M, Demchuk AM, Menon BK, Eesa M,
Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL,
et al: Randomized assessment of rapid endovascular treatment of
ischemic stroke. N Engl J Med. 372:1019–1030. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Campbell BC, Mitchell PJ, Kleinig TJ,
Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley
TJ, et al: Endovascular therapy for ischemic stroke with
perfusion-imaging selection. N Engl J Med. 372:1009–1018. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saver JL, Goyal M, Bonafe A, Diener HC,
Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, et
al: : Solitaire TM with the intention for thrombectomy
as primary endovascular treatment for acute ischemic stroke stroke
(SWIFT PRIME) trial: Protocol for a randomized, controlled,
multicenter study comparing the Solitaire revascularization device
with IV tPA with IV tPA alone in acute ischemic stroke. Int J
Stroke. 10:439–448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schwamm LH, Ali SF, Reeves MJ, Smith EE,
Saver JL, Messe S, Bhatt DL, Grau-Sepulveda MV, Peterson ED and
Fonarow GC: Temporal trends in patient characteristics and
treatment with intravenous thrombolysis among acute ischemic stroke
patients at Get with the Guidelines-Stroke hospitals. Circ
Cardiovasc Qual Outcomes. 6:543–549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Menon BK, Saver JL, Goyal M, Nogueira R,
Prabhakaran S, Liang L, Xian Y, Hernandez AF, Fonarow GC, Schwamm L
and Smith EE: Trends in endovascular therapy and clinical outcomes
within the nationwide get with the guidelines-stroke registry.
Stroke. 46:989–995. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fujioka M, Okuchi K, Iwamura A, Taoka T
and Siesjö BK: A mismatch between the abnormalities in diffusion-
and susceptibility-weighted magnetic resonance imaging may
represent an acute ischemic penumbra with misery perfusion. J
Stroke Cerebrovasc Dis. 22:1428–1431. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gersing AS, Ankenbrank M, Schwaiger BJ,
Toth V, Janssen I, Kooijman H, Wunderlich S, Bauer JS, Zimmer C and
Preibisch C: Mapping of cerebral metabolic rate of oxygen using
dynamic susceptibility contrast and blood oxygen level dependent MR
imaging in acute ischemic stroke. Neuroradio. 57:1253–1261. 2015.
View Article : Google Scholar
|
|
37
|
Baron JC, Bousser MG, Rey A, Guillard A,
Comar D and Castaigne P: Reversal of focal ‘misery-perfusion
syndrome’ by extra-intracranial arterial bypass in hemodynamic
cerebral ischemia. A caes study with 15O positron emission
tomography. Stroke. 12:454–459. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Heiss WD: The ischemic penumbra:
Correlates in imaging and implications for treatment of ischemic
stroke. The Johann Jacob Wepfer award 2011. Cerebrovasc Dis.
32:307–320. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mokin M, Morr S, Fanous AA, Shallwani H,
Natarajan SK, Levy EI, Snyder KV and Siddiqui AH: Correlation
between cerebral blood volume values and outcomes in endovascular
therapy for acute ischemic stroke. J NeuroInterv Surg. 7:705–708.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ren DC, Du GH and Zhang JT: Inhibitory
effect of salvianolic acids on endothelial cells damage induced by
hydrogen peroxide. Chin J Pharmacol Toxicol. 17:333–337. 2003.
|
|
41
|
Tang H, Pan CS, Mao XW, Liu YY, Yan L,
Zhou CM, Fan JY, Zhang SY and Han JY: Role of NADPH oxidase in
total salvianolic acid injection attenuating ischemia-reperfusion
impaired cerebral microcirculation and neurons: Implication of
AMPK/AKt/PKC. Microcirculation. 21:615–627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen YH, Du GH and Zhang JT: Salvianolic
acid B protects brain against injuries caused by
ischemia-reperfusion in rats. Acta Pharmacol Sin. 21:463–466.
2000.PubMed/NCBI
|
|
43
|
Hou S, Zhao MM, Shen PP, Liu XP, Sun Y and
Feng JC: Neuroprotective effect of salvianolic acids against
cerebral ischemia/reperfusion injury. Int J Mol Sci. 17(pii):
E11902016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Knash M, Tsang A, Hameed B, Saini M,
Jeerakathil T, Beaulieu C, Emery D and Butcher K: Low cerebral
blood volume is predictive of diffusion restriction only in
hypeeracute stroke. Stroke. 41:2795–2800. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu Y, Peng JW and Yu LF: Changes of
cerebral perfusion and potential targets for intervention in acute
ischemic stroke. Chin J Pract Nerv Dis. 20:11–16. 2017.(In
Chinese).
|