Introduction
With the changes in people's diet structure, such as
the popularization of Western-style diet, incidence of type 2
diabetes mellitus (T2DM) shows an increasing trend (1). Zinman et al (2) reported that T2DM affected 3 million
patients in 2015. Green et al (3) predicted that the number of patients
with T2DM will exceed 5 million in 2030. Presently, T2DM is the
most common type of chronic disease worldwide (4). T2DM is a chronic metabolic disorder
characterized by insufficient insulin secretion or resistance
(5). Complications of T2DM mainly
include glucose and lipid metabolism disorder, of which fatty liver
is the most common complication (6).
At present, there is no radical treatment of T2DM in clinical
practice, and conventional treatment can only delay the development
of T2DM (7). Hypoxia-inducible
factor-1α (HIF-1α) is a protein with critical roles in
post-transcriptional and post-transcriptional regulation of gene
expression. Studies in recent years (8–10) have
shown that HIF-1α is closely correlated with the onset and
development of T2DM, while its involvement in T2DM-induced fatty
liver is unclear. Saxagliptin, as a potent inhibitor of dipeptidyl
peptidase-4 (DPP-4), can increase the level of endogenous
glucagon-like peptide-1 (GLP-1) to regulate blood glucose. In this
study, a rat model of T2DM complicated with fatty liver was
established and the expression of HIF-1α in liver tissue during the
treatment with saxagliptin was detected. Our study provided new
insights for the treatment of T2DM complicated with fatty
liver.
Materials and methods
Animals
Eighty male Wistar rats weighted 170-250 gr were
provided by the Experimental Animal Center of Hunan Normal
University. Feeding conditions: Room temperature 26°C, humidity
75%, five rats in one cage, normal light and free access to water.
The study was approved by the Ethics Committee of Dahua Hospital of
Xuhui District (Shanghai, China).
Model construction
All rats were randomly divided into two groups. One
group was subjected to T2DM modeling (n=40) and the other group was
control (n=40). Rats in model group were fed with high fat diet
(0.5% cholic acid, 1% cholesterol, 15% lard, 20% sucrose, 63.5%
basal diet) after 1 week adaptive feeding. After continuous feeding
for 4 weeks, intraperitoneal injection of streptozotocin (30 mg/kg,
once a day) was performed. After 8 weeks of feeding, fasting blood
glucose, insulin sensitivity index and indexes of liver function
were detected. Based on the diagnostic criteria for fatty liver
proposed in 2015 (11), fasting
blood glucose (>16 mmol/l) and the decrease of insulin
sensitivity index were used as the indicators of the successfully
established models.
Experimental methods
Successfully established rat T2DM models were
randomly divided into two groups: One group was treatment group
that received intraperitoneal injection of saxagliptin solution (10
mg/kg, once per day), the other was a model group with normal
breeding. Six mice in each of the three groups were sacrificed at
12 h after the model construction, 1 and 3 weeks after treatment.
Blood (2 ml) was extracted, followed by centrifugation at 3,500 × g
for 5 min. Beckman Coulter AU5800 automatic biochemical analyzer
(Beckman Coulter, Inc., Brea, CA, USA) was used to detect blood
glucose, blood lipids and liver function indexes. Rat liver was
removed and fixed in 10% neutral formalin. Expression of HIF-1α
protein in liver tissue was detected by western blot analysis.
Western blot analysis
After total protein extraction, 10% PAGE gel
electrophoresis was performed, followed by transmembrane under 100
V for 2 h. After blocking for 1 h at room temperature, membranes
were incubated with rabbit monoclonal HIF-α antibody (cat. no.
ab51608; dilution 1:250) overnight, followed by incubation with
secondary goat anti-rabbit (HRP) IgG antibody (cat. no. ab6721;
dilution 1:1000) for 1-2 h at room temperature. All the antibodies
were all purchased from Abcam (Cambridge, MA, USA). Test was in
accordance with BD's test kit instructions (BD Biosciences,
Franklin Lakes, NJ, USA).
Observation indicators
Blood glucose, triglyceride (TG), total cholesterol
(TC), high-density lipoprotein cholesterol (HDL-C), low-density
lipoprotein cholesterol (LDL-C); liver function indexes: Alanine
aminotransferase (ALT), aspartate aminotransferase (AST),
γ-glutamyl transferase (GGT); HIF-1α protein expression.
Statistical analysis
SPSS 22.0 statistical software (IBM Corp., Armonk,
NY, USA) was used for all statistical analyses. Enumeration data
are expressed as rate and processed using Chi-square test.
Measurement data are expressed as mean ± standard deviation, and
comparisons among multiple groups and between two groups were
performed by analysis of variance followed by Least Significant
Difference as its post hoc test and t-test, respectively. P<0.05
indicates a difference with statistical significance.
Results
Results of model construction
Thirty-seven model rats were successfully
established, and success rate was 92.5% (37/40). Those rat models
were randomly divided into treatment group (n=19) and model group
(n=18).
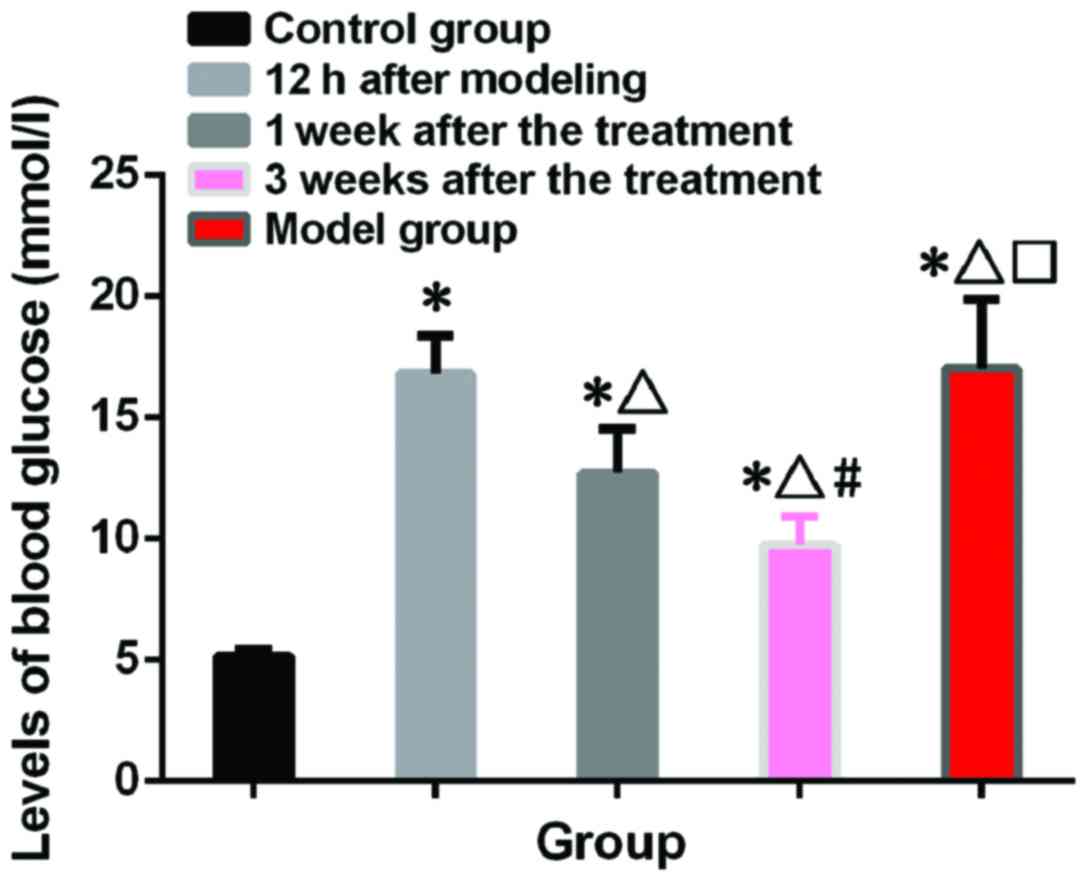
Blood glucose test results
Blood glucose in control group was 5.16±0.24 mmol/l,
and blood glucose in model group was 17.04±2.82 mmol/l. Blood
glucose levels in treatment group at 12 h after model construction,
1 and 3 weeks after treatment were 16.82±2.56, 12.72±1.84 and
9.73±1.18 mmol/l, respectively. Levels of blood glucose in model
and treatment group were significantly higher than those in control
group (p<0.05). There was no significant difference between
model and treatment group at 12 h after model construction
(p>0.05). Blood glucose level 3 weeks after treatment were lower
than those 1 week after treatment (p<0.05) (Fig. 1).
Blood lipid test results
Levels of TC, TG, LDL-C and HDL-C in treatment and
model group were significantly higher than those in control group
(p<0.05). After treatment, levels of TC, TG, LDL-C and HDL-C in
treatment group gradually decreased (p<0.05) (Table I).
 | Table I.Blood lipid test results of two groups
of rats (mmol/l). |
Table I.
Blood lipid test results of two groups
of rats (mmol/l).
|
|
| Treatment group |
|
|---|
|
|
|
|
|
|---|
| Items | Control group | 12 h after model
construction | 1 week after
treatment | 3 weeks after
treatment | Model group |
|---|
| TC | 0.32±0.24 |
4.48±1.27a |
3.54±0.94a,b |
1.92±0.76a–c |
4.52±1.36a–d |
| TG | 0.61±0.25 |
1.52±0.92a |
1.14±0.46a,b |
0.89±0.24a–c |
1.61±0.87a–d |
| LDL-C | 0.24±0.10 |
2.08±1.09a |
1.52±0.86a,b |
0.96±0.31a–c |
2.04±1.02a–d |
| HDL-C | 1.04±0.24 |
2.28±0.53a |
1.83±0.47a,b |
1.37±0.56a–c |
2.20±0.41a–d |
Liver function test results
Levels of AST, ALT and GGT in treatment and model
group were significantly higher than those in control group
(p<0.05). After treatment, levels of AST, ALT and GGT in rats of
treatment group gradually decreased (p<0.05) (Table II).
 | Table II.Liver function test results of two
groups of rats (IU/l). |
Table II.
Liver function test results of two
groups of rats (IU/l).
|
|
| Treatment group |
|
|---|
|
|
|
|
|
|---|
| Items | Control group | 12 h after model
construction | 1 week after
treatment | 3 weeks after
treatment | Model group |
|---|
| AST | 18.25±4.51 |
36.34±6.27a |
30.69±4.01a,b |
24.36±5.83a–c |
35.84±6.04a–d |
| ALT | 24.43±4.92 |
37.42±8.22a |
33.14±7.16a,b |
28.77±6.04a–c |
36.62±8.04a–d |
| GGT | 27.15±6.27 |
42.52±9.81a |
38.05±4.37a,b |
34.53±6.98a–c |
41.60±9.70a–d |
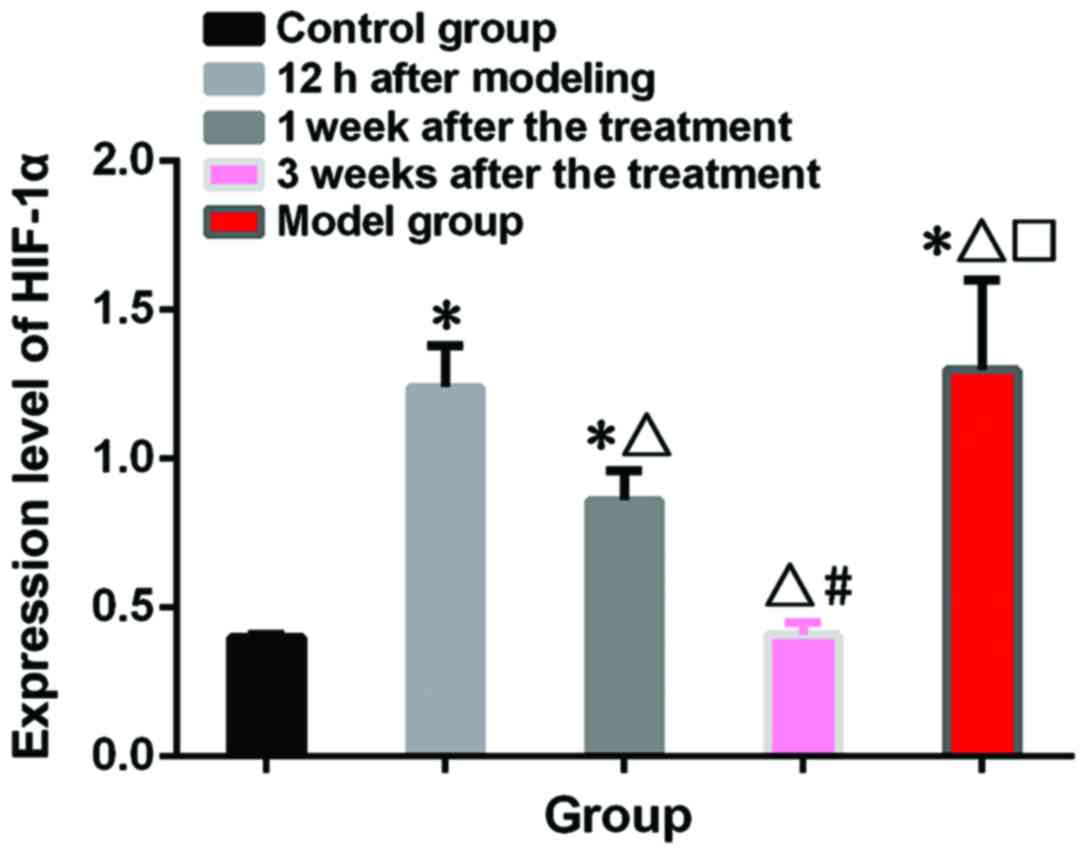
HIF-1α test results
Relative HIF-1α expression level was 0.40±0.01 in
control group and 1.30±0.30 in the model group. Relative HIF-1α
expression level was 1.24±0.14 in treatment group at 12 h after
model construction, 0.86±0.10 at 1 week after treatment and
0.41±0.03 at 3 weeks after treatment. Expression level of HIF-1α
was significantly higher in model and treatment group than in
control group at 12 h after model construction and 1 week after
treatment (p<0.05). No significant differences in the expression
level of HIF-1α were found between model and treatment group at 12
h after model construction (p>0.05). In treatment group, the
expression level of HIF-1α decreased with the prolonged treatment,
and no significant differences in expression level of HIF-1α were
found between treatment and control group 3 weeks after treatment
(p>0.05) (Fig. 2).
Discussion
T2DM can easily lead to damage of important organs
in human body and has been proved to be a major cause of a variety
of heart, brain, liver and eye diseases (12). At present, there is no clear
conclusion on the pathogenesis of T2DM-induced fatty liver disease.
Insulin resistance and oxidative stress lipid peroxidation is a
major cause of inflammatory necrosis and fibrosis in the liver
(13,14). HIF-1, a DNA-binding protein
discovered by Semenza and Wang (15)
in 1992, is composed of two subunits, α and β, and has been shown
to have various immune responses in the human body through
hypoxic-ischemic conditions (16,17). The
role of HIF-1 in patients with T2DM complicated with fatty liver is
unclear. In this study, rats T2DM and fatty liver models were
induced by high-fat diet and treated with saxagliptin. The
expression of HIF-1α was detected to examine the role of HIF-1α in
T2DM and fatty liver.
In this study, compared with control group, the
expression level of HIF-1α in model group was significantly higher
than that in control group, and the expression levels of HIF-1α in
rat models were decreased gradually with the prolonged saxagliptin
treatment and reached the level of control group 3 weeks after
treatment, indicating the involvement of HIF-1α in the onset and
development of T2DM, which is consistent with the findings of Nayak
et al (18). The possible
explanation is that HIF-1α promotes the transcription of VEGF under
the condition of hypoxia, so as to induce the regeneration of liver
cells in rats with fatty liver (19). Over-proliferation of cells
accelerates the consumption of oxygen in rats, and the internal
circulatory system cannot provide enough oxygen, which in turn
induces the expression of HIF-1α, resulting in a vicious circle.
HIF-1α stimulates the secretion of a large number of cellular
stimulating factors, so as to exacerbate insulin secretion and
reduce insulin sensitivity, thus contributing to the development of
fatty liver (20). Saxagliptin can
also effectively improve blood glucose and lipids and liver
function by improving insulin resistance metabolic disorders
(21). With the prolonged treatment,
those indexes gradually decreased, indicating that saxagliptin can
effectively improve the blood glucose, blood lipid and liver
function. Insulin resistance induces the synthesis of free fatty
acids in adipose tissue, which can result in elevation of GLP-1 in
rats, leading to further deterioration of fatty liver (22). Overload of GLP-1 directly affects the
normal metabolic function of the digestive system of rats and
greatly reduces the absorption of nutrients. Saxagliptin increases
the synthesis and metabolism of cyclic adenosine monophosphate and
inhibits the secretion of glucagon, which can not only promote the
proliferation and differentiation of β cells, but also activate the
protective effect of GLP-1 receptor on liver of rats (23). Therefore, re-deterioration of liver
cirrhosis in rats was suppressed. With saxagliptin, insulin in rats
is effectively maintained and the activation of VEGF is inhibited,
resulting in gradually decreased expression level of HIF-1α.
In this study, upregulated expression of HIF-1α was
observed in diabetic rats combined with fatty liver and saxagliptin
effectively inhibited the expression of HIF-1α. However, animal
system may be different from human body. Therefore, clinical
studies are needed to further confirm our conclusions.
In conclusion, HIF-1α is highly expressed in rats
with T2DM and fatty liver. Saxagliptin can effectively improve the
blood glucose, blood lipid and liver function, and reduce the
expression level of HIF-1α protein.
Acknowledgements
Not applicable.
Funding
This study was funded by the Medical Science Program
of Xuhui District (no. SHXH201427; Shanghai, China).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ was responsible for writing the manuscript and
model construction. QX and XM analyzed and interpreted blood lipid
results and blood glucose tests. JC performed liver function test.
WY helped with HIF-1α test. YZ performed western blot analysis. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Dahua Hospital of Xuhui District (Shanghai, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Diabetes Association, : Standards
of medical care in diabetes-2017 abridged for primary care
providers. Clin Diabetes. 35:5–26. 2017.PubMed/NCBI
|
|
2
|
Zinman B, Wanner C, Lachin JM, Fitchett D,
Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ,
et al: EMPA-REG OUTCOME Investigators: Empagliflozin,
cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J
Med. 373:2117–2128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Green JB, Bethel MA, Armstrong PW, Buse
JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, et al
TECOS Study Group, : Effect of sitagliptin on cardiovascular
outcomes in type 2 diabetes. N Engl J Med. 373:232–242. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
American Diabetes Association, : 2.
Classification and diagnosis of diabetes. Diabetes Care. 40 Suppl
1:S11–S24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marso SP, Daniels GH, Brown-Frandsen K,
Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR,
Ravn LS, et al LEADER Steering Committee, ; LEADER Trial
Investigators, : Liraglutide and cardiovascular outcomes in type 2
Diabetes. N Engl J Med. 375:311–322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Byrne CD and Targher G: EASL-EASD-EASO
Clinical Practice Guidelines for the management of non-alcoholic
fatty liver disease: Is universal screening appropriate?
Diabetologia. 59:1141–1144. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheung O, Kapoor A, Puri P, Sistrun S,
Luketic VA, Sargeant CC, Contos MJ, Shiffman ML, Stravitz RT,
Sterling RK, et al: The impact of fat distribution on the severity
of nonalcoholic fatty liver disease and metabolic syndrome.
Hepatology. 46:1091–1100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mehrabani M, Najafi M, Kamarul T, Mansouri
K, Iranpour M, Nematollahi MH, Ghazi-Khansari M and Sharifi AM:
Deferoxamine preconditioning to restore impaired HIF-1α-mediated
angiogenic mechanisms in adipose-derived stem cells from
STZ-induced type 1 diabetic rats. Cell Prolif. 48:532–549. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen H, Jia P, Kang H, Zhang H, Liu Y,
Yang P, Yan Y, Zuo G, Guo L, Jiang M, et al: Upregulating HIF-1α by
hydrogel nanofibrous scaffolds for rapidly recruiting angiogenesis
relative cells in diabetic wound. Adv Healthc Mater. 5:907–918.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sunkari VG, Lind F, Botusan IR, Kashif A,
Liu ZJ, Ylä-Herttuala S, Brismar K, Velazquez O and Catrina SB:
Hyperbaric oxygen therapy activates hypoxia-inducible factor 1
(HIF-1), which contributes to improved wound healing in diabetic
mice. Wound Repair Regen. 23:98–103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lonardo A, Ballestri S, Marchesini G,
Angulo P and Loria P: Nonalcoholic fatty liver disease: A precursor
of the metabolic syndrome. Dig Liver Dis. 47:181–190. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oei L, Rivadeneira F, Zillikens MC and Oei
EH: Diabetes, diabetic complications, and fracture risk. Curr
Osteoporos Rep. 13:106–115. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Asrih M and Jornayvaz FR: Metabolic
syndrome and nonalcoholic fatty liver disease: Is insulin
resistance the link? Mol Cell Endocrinol. 418:55–65. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen S, Zhao X, Ran L, Wan J, Wang X, Qin
Y, Shu F, Gao Y, Yuan L, Zhang Q, et al: Resveratrol improves
insulin resistance, glucose and lipid metabolism in patients with
non-alcoholic fatty liver disease: A randomized controlled trial.
Dig Liver Dis. 47:226–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Semenza GL and Wang GL: A nuclear factor
induced by hypoxia via de novo protein synthesis binds to the human
erythropoietin gene enhancer at a site required for transcriptional
activation. Mol Cell Biol. 12:5447–5454. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Balamurugan K: HIF-1 at the crossroads of
hypoxia, inflammation, and cancer. Int J Cancer. 138:1058–1066.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang H, Lu H, Xiang L, Bullen JW, Zhang
C, Samanta D, Gilkes DM, He J and Semenza GL: HIF-1 regulates CD47
expression in breast cancer cells to promote evasion of
phagocytosis and maintenance of cancer stem cells. Proc Natl Acad
Sci USA. 112:E6215–E6223. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nayak BK, Shanmugasundaram K, Friedrichs
WE, Cavaglierii RC, Patel M, Barnes J and Block K: HIF-1 mediates
renal fibrosis in OVE26 type 1 diabetic mice. Diabetes.
65:1387–1397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dodd KM, Yang J, Shen MH, Sampson JR and
Tee AR: mTORC1 drives HIF-1α and VEGF-A signalling via multiple
mechanisms involving 4E-BP1, S6K1 and STAT3. Oncogene.
34:2239–2250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rigiracciolo DC, Scarpelli A, Lappano R,
Pisano A, Santolla MF, De Marco P, Cirillo F, Cappello AR, Dolce V,
Belfiore A, et al: Copper activates HIF-1α/GPER/VEGF signalling in
cancer cells. Oncotarget. 6:34158–34177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Toh S, Hampp C, Reichman ME, Graham DJ,
Balakrishnan S, Pucino F, Hamilton J, Lendle S, Iyer A, Rucker M,
et al: Risk for hospitalized heart failure among new users of
saxagliptin, sitagliptin, and other antihyperglycemic drugs: A
retrospective cohort study. Ann Intern Med. 164:705–714. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Armstrong MJ, Gaunt P, Aithal GP, Barton
D, Hull D, Parker R, Hazlehurst JM, Guo K, Abouda G, Aldersley MA,
et al LEAN trial team, : Liraglutide safety and efficacy in
patients with non-alcoholic steatohepatitis (LEAN): A multicentre,
double-blind, randomised, placebo-controlled phase 2 study. Lancet.
387:679–690. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dyson JK, Anstee QM and McPherson S:
Non-alcoholic fatty liver disease: A practical approach to
treatment. Frontline Gastroenterol. 5:277–286. 2014. View Article : Google Scholar : PubMed/NCBI
|