Introduction
Cerebral concussion is a kind of mild traumatic
brain injury, as well as the most common and mildest disease in
primary brain injury, which has attracted widespread attention in
recent years due to its high incidence and disability rates
(1,2). The clinical diagnostic criteria for
cerebral concussion are as follows: transient disturbance of
consciousness after head injury without focal neurological signs,
and no abnormal primary brain injury in imaging examination
(3). General cerebral concussion can
be restored after one week, but injuries on numerous occasions
leads to more serious learning, memory and neurological disorders
(4). Cerebral concussion often
occurs in intense physical activity, accounting for approximately
90% of exercise-induced brain injuries, causing headache, blurred
vision, tinnitus and amnesia in patients. Recent epidemiological
studies have shown that the vulnerable population of cerebral
concussion has been constantly expanded (5,6).
Research on cerebral concussion has mainly focused
on epidemiology. However, few studies have focused on animal
experiments and the injury mechanism. Hehar et al (7) effectively copied the cerebral
concussion rat model using a metal simple-pendulum striking device
to effectively conduct the research on the damage and related
mechanism of cerebral concussion. Webster et al (8) proposed that the balance beam (BB) test
and beam walking (BW) test can be used to effectively evaluate the
balance motion behavior of animals, and the Morris water maze (MWM)
test can be used to effectively evaluate the learning and memory
abilities of animals.
In this study, the multiple cerebral concussion
(MCC) rat model was established to study the changes in balance
motion behavior and learning and memory abilities of cerebral
concussion rats, and its possible mechanisms was investigated via a
number of detection methods.
Materials and methods
Animal feeding, treatment and
grouping
A total of 30 male Sprague-Dawley (SD) rats weighing
(280±20) g were purchased from the Shanghai Bioray Laboratory Inc.,
(production license number of experimental animal: SCXK2014-0022)
and fed in a specific-pathogen-free (SPF) animal room at 25°C under
the humidity of 45-50%; the sunshine/darkness time was set as 12 h
light/dark. All animals had free access to food and water. After
adaptation to the environment for one week, the rats were divided
into two groups: 4MCC group (n=15) and control group (C group,
n=15). All the animal experiments in the present study were
approved by the Animal Ethics Committee of The First Affiliated
Hospital of Kunming Medical University (Kunming, China), and all
operations strictly followed the regulations of the National
Institute Guide for the Care and Use of Laboratory Animals.
Rats in 4MCC group were fixed on the iron bench with
the head tilted up to 45°, following the angle of pendulum. The
pendulum was placed above the head of rats, freely fell down and
hit the middle forehead. The procedure was repeated 4 times with
12-h interval, and whether the establishment of the rat model was
successful was evaluated according to the criteria of cerebral
concussion animal model: transient apnea for 5-15 sec during
striking; transient disappearance of pain reflex, external auditory
canal reflex and corneal reflex during striking; no intracranial
hemorrhage; no visible brain tissue injury; no dyskinesia and
abnormal reflex after consciousness recovery.
BB test
Before modeling, the rats in both groups were placed
on the BB at 90 cm above the ground (the device was revised from
Dixon laboratory) for training, and the standing time of rats on BB
was recorded with 60 sec as the limit; the operation was repeated 3
times for each rat, making rats in both groups stay on the BB for
60 sec before modeling. After modeling, BB test was performed for
rats in both groups, the balance latencies (BL) were recorded in
detail, and the changes in balance motion behavior after cerebral
concussion were evaluated.
BW test
Rats were placed on a BB device (the device was
revised from Dixon laboratory) made of a 90 cm-long wooden runway
at 120 cm above the ground, and the runway was equipped with four
obstacles with the same interval. The experiments were performed
under quiet conditions, and a 90-dB noise generator was installed
on the beginning end of runway to stimulate the experimental rats.
Before modeling, the rats received the BW test training, enabling
them to pass through the four obstacles within 5 sec. After the
establishment of cerebral concussion model, BW test was performed
for the two groups of rats, followed by scoring: 1 point: rats ran
from the starting point along the runway and passed through the
first obstacle; 2 points: rats ran from the starting point along
the runway and passed through the second obstacle; 3 points: rats
ran from the starting point along the runway and passed through the
third obstacle; 4 points: rats ran from the starting point along
the runway and passed through the fourth obstacle; 5 points: rats
ran from the starting point along the runway to the stopping
point.
MWM test
MWM test device purchased from RWD Biotech was used
for MWM test. The water maze was divided into 4 quadrants. The
quadrant in which the target platform was located was quadrant I,
followed by quadrants II, III and IV in a clockwise direction.
After modeling, the rats in both groups were placed into the water
maze for 7-day training: The target platform was placed at 1 cm
below the surface of water, and the ink was added into the pool,
making rats unable to see the platform. Then rats were randomly
placed into the pool with 120 sec as the limit, and the time
climbing onto the platform (escape latency) was recorded. If rats
could not find the platform after 120 sec, they were taken onto the
platform for 30 sec, and the escape latency of rats in each group
was recorded during training. On the 8th day, the test was formally
carried out. The rats were placed into the pool with the back to
the platform, and the motion track and escape latency of rats in
the pool were recorded.
Magnetic resonance spectroscopy
(MRS)
After the behavior detection of rats in each group,
the brain tissues of rats in each group were scanned via
superconducting magnetic resonance imaging instrument (GE
Healthcare, Chicago, IL, USA). After anesthesia, the heads of rats
were fixed on the track with the body covered with a towel to keep
warm. Under a prone position, the rats received MRS on the magnetic
resonance coil (scanning site: septal coronal section; scanning
parameters: pulse repetition time = 2,000 ms, echo time = 144 ms,
pixels = 10 mm × 10 mm × 10 mm), and the procedure was repeated for
each rat 5 times. The metabolic profiling graphs were constructed
using spectrum software to detect choline (Cho), creatinine (Cr),
naphthalene acetic acid (NAA) and myo-inositol (MI).
Glial fibrillary acidic protein (GFAP)
immunohistochemical staining
The rats were sacrificed immediately after MRI scan.
After the brain tissues were removed, they were cut using the
freezing microtome to obtain the 25 µm-thick brain slices in the
septal coronal and hippocampal sections. The brain sections were
placed onto the glass slide, followed by serial section and
immunohistochemical staining using GFAP antibody (Milipore). After
hydration, the water on the section was sucked dry using the
absorbent paper, and the sections were placed on the staining rack,
washed with distilled water 3 times (3 min/time), and then washed
again with phosphate-buffered saline (PBS) + 0.1% Triton 3 times (2
min/time). The oxidase blocking solution was added for reaction for
15 min with the lid covered, followed by washing with PBS + 0.1%
Triton 3 times (3 min/time), membrane perforation using 3% Triton
X-100 for 10 min, washing again with PBS + 0.1% Triton twice. The
cleaning solution was sucked dry, non-immune serum was added for 90
min, the serum was sucked dry and GFAP antibody (diluted at 1:500)
(Sigma, St. Louis, MO, USA) was added for incubation at 4°C
overnight. The section was then washed with PBS + 0.1% Triton 3
times (10 min/time), added with biotin-labeled secondary antibody
for incubation for 90 min, washed 3 times (2 min/time), added with
biotin-peroxidase for reaction for 12 min, and washed with PBS +
0.1% Triton 3 times (3 min/time). Freshly-prepared diaminobenzidine
(DAB) developing solution was added for color development (~10
min). After dehydration, the sections were sealed with neutral gum
and observed under a microscope (Nikon ECLiPSE 90i; Nikon
Corporation, Tokyo, Japan), followed by analysis via Image. The
number of GFAP-positive cells per unit visual field was
calculated.
Terminal deoxynucleotidyl transferase
dUTP nick end-labeling (TUNEL) apoptosis assay
The apoptosis of brain slices in septal coronal and
hippocampal sections of rats in each group was determined using the
TUNEL apoptosis kit (Roche Applied Science, Penzberg, Germany). For
TUNEL staining, after hydration, the sections were washed with PBS.
After protease K working solution was prepared, the sections were
placed in the blocking solution for reaction for 30 min. The
paraffin sections were permeabilized with Triton X-100/sodium
citrate (0.1%) and sealed with Vectashield Hard Set (Vector
Laboratories, Inc., Burlingame, CA, USA). Fluorescein
isothiocyanate (FITC) was used as the fluorescent developing
reagent. The number of TUNEL-positive cells was calculated in 10
visual fields.
Western blotting
The hippocampal and septal areas were separated from
the brain tissues of rats in each group, added with the lysis
buffer in a ratio of 1 g: 1 ml, and smashed on an ultrasonic
pulverizer till no visible tissues could be seen by naked eyes,
followed by centrifugation using a centrifugal machine at 9,600 × g
and 4°C for 10 min. The total protein in brain tissues of the above
rats was quantified using bicinchoninic acid (BCA) protein assay
kit (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA), and
added with the loading buffer to prepare the loading system in
equal concentration. The sample was added into the sample sink for
sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE). After electrophoresis, the protein was transferred onto
a polyvinylidene fluoride (PVDF) membrane (IPVH00010; EMD
Millipore, Billerica, MA, USA), and sealed with 5% skim milk powder
for 1 h. Then the target band was cut, and rabbit anti-rat p-tau,
Aβ1-40 and glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) monoclonal antibodies were incubated at 4°C overnight
(1:1,000; cat. nos. ab151559, ab62658 and ab181602, respectively;
all purchased from Abcam, Cambridge, MA, USA). The band was
removed, washed with Tris-buffered saline Tween (TBST) 3 times (5
min/time), incubated by horseradish peroxidase-conjugated secondary
goat anti-rabbit polyclonal antibody (1:600; cat. no. AGPS002-91C;
Shanghai Yihyson Biological Technology Co., Ltd., Shanghai, China)
at room temperature for 2 h and then washed with TBST 3 times (5
min/time). Then electrochemiluminescence (ECL) luminescent liquid
was added for development in the dark and the corresponding protein
expression levels were analyzed via p-tau/GAPDH and
Aβ1-40/GAPDH.
Statistical analysis
Data in this study were presented as mean ± standard
deviation, and processed using Statistical Product and Service
Solutions (SPSS) 19.0 software (SPSS Inc., Chicago, IL, USA).
Student's t-test was used for the intergroup comparison. Chi-square
test was used for the enumeration data. Analysis of variance and
SNK post hoc test were used for the comparison among groups.
P<0.05 indicated that the difference was statistically
significant.
Results
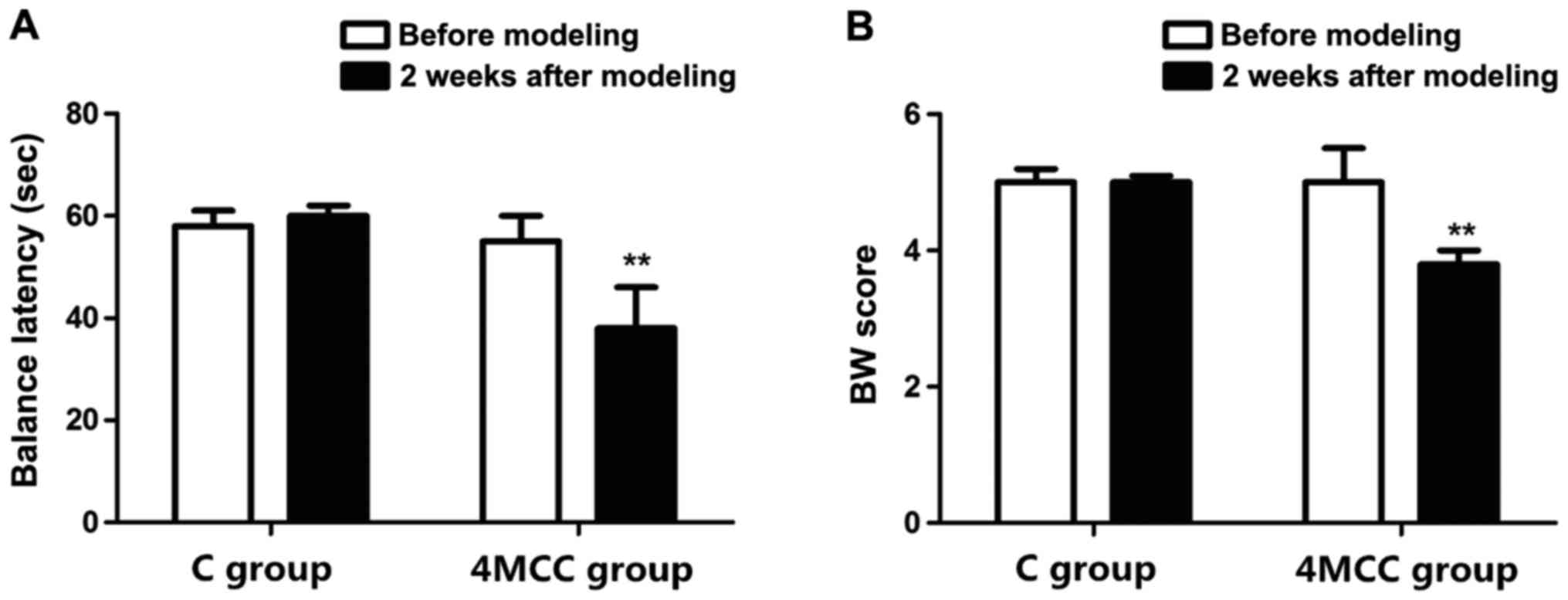
Evaluation of balance motion behavior
via BB test and BW test
Changes in balance motion behavior of rats after MCC
were evaluated via BB test and BW test. The results showed that the
BL of rats in 4MCC group was similar to that in C group before
modeling, and they could stay on the BB for 60 sec (p>0.05). At
2 weeks after modeling, the BL of rats in 4MCC group was
significantly shorter than that in C group (p<0.01). The BW test
revealed that the score of rats in 4MCC group was similar to that
in C group before modeling (p>0.05), but the score of rats in
4MCC group was obviously lower than that in C group (p<0.01)
(Fig. 1).
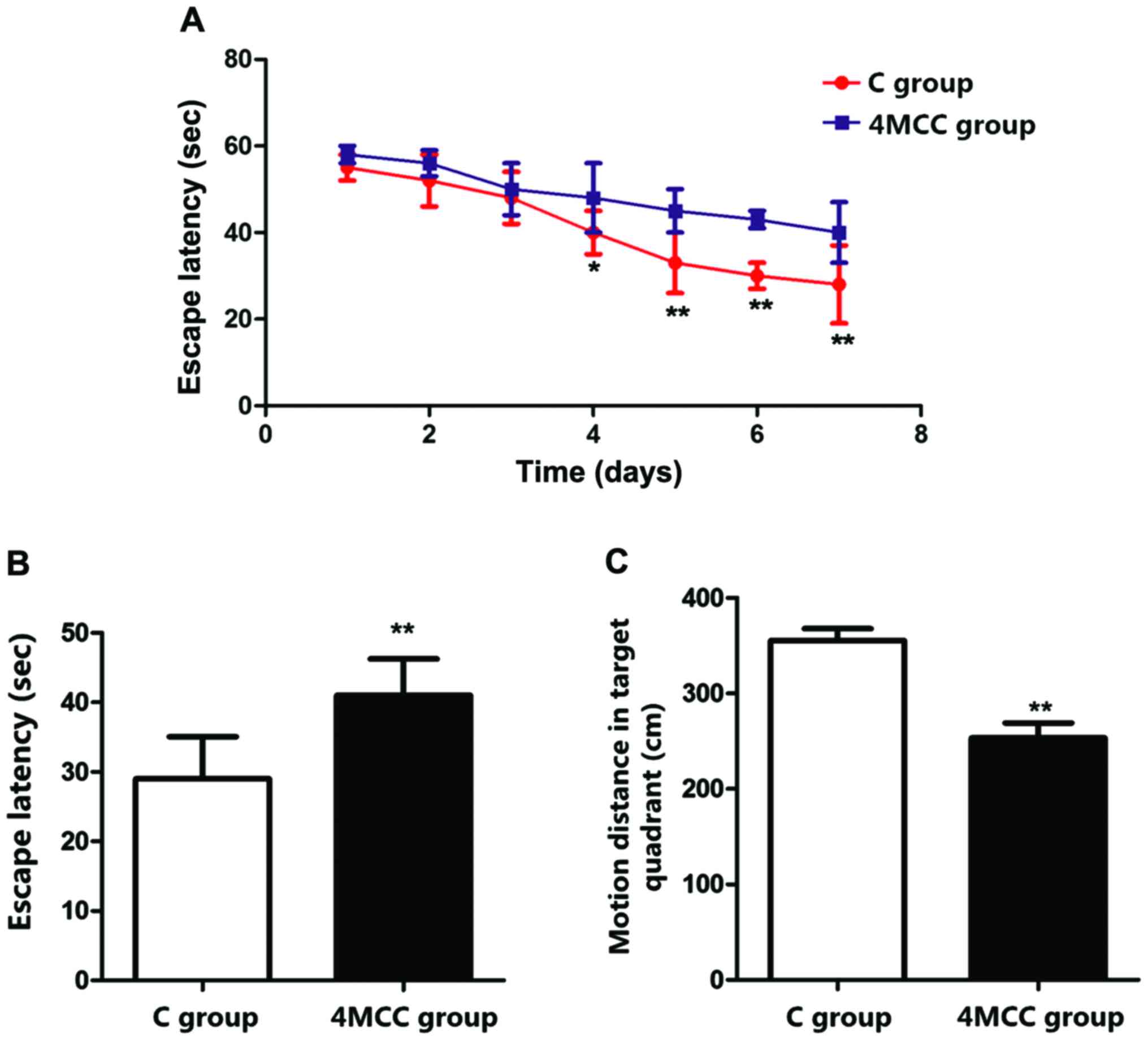
Evaluation of learning and memory
abilities via MWM test
Changes in learning and memory abilities of rats
after MCC were evaluated via MWM test. Rats received the training
for 7 days at 2 weeks after modeling. The results showed that the
escape latencies of rats in 4MCC group on the 4th-7th days were
significant longer than those in C group (p<0.01), and the
escape latency of rats in 4MCC group on the 8th day was obviously
longer than that in C group (p<0.01). The motion distance of
rats in 4MCC group in target quadrant was significantly shorter
than that in C group (p<0.01) (Fig.
2).
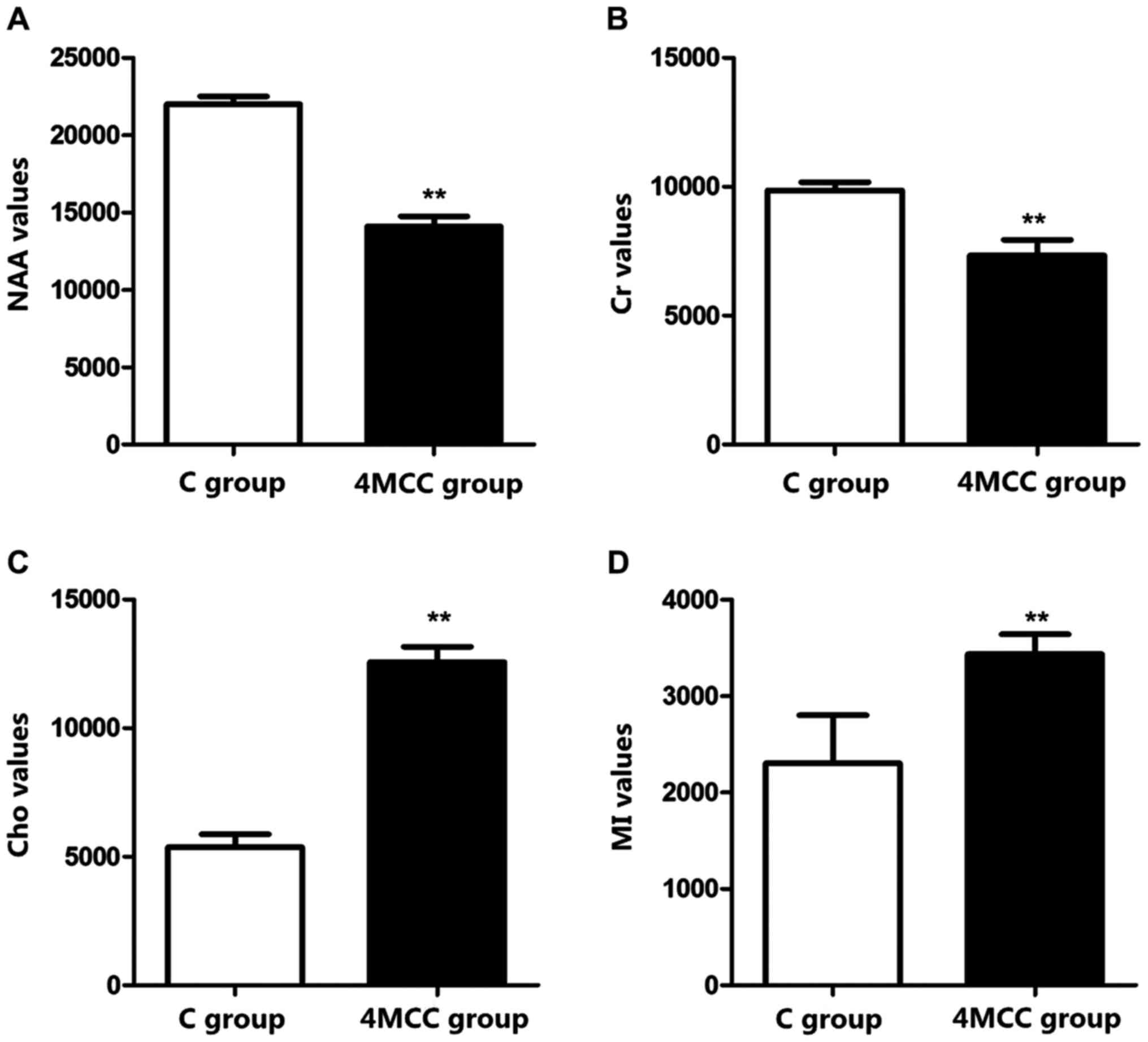
MRS
The septal coronal section of rats in each group was
scanned via MRS to measure the values of Cho, Cr, NAA and MI
metabolites. The results revealed that NAA and Cr values of rats in
4MCC group at 2 weeks after modeling were significantly decreased
compared with those in C group, but Cho and MI values were
obviously increased (p<0.01) (Fig.
3).
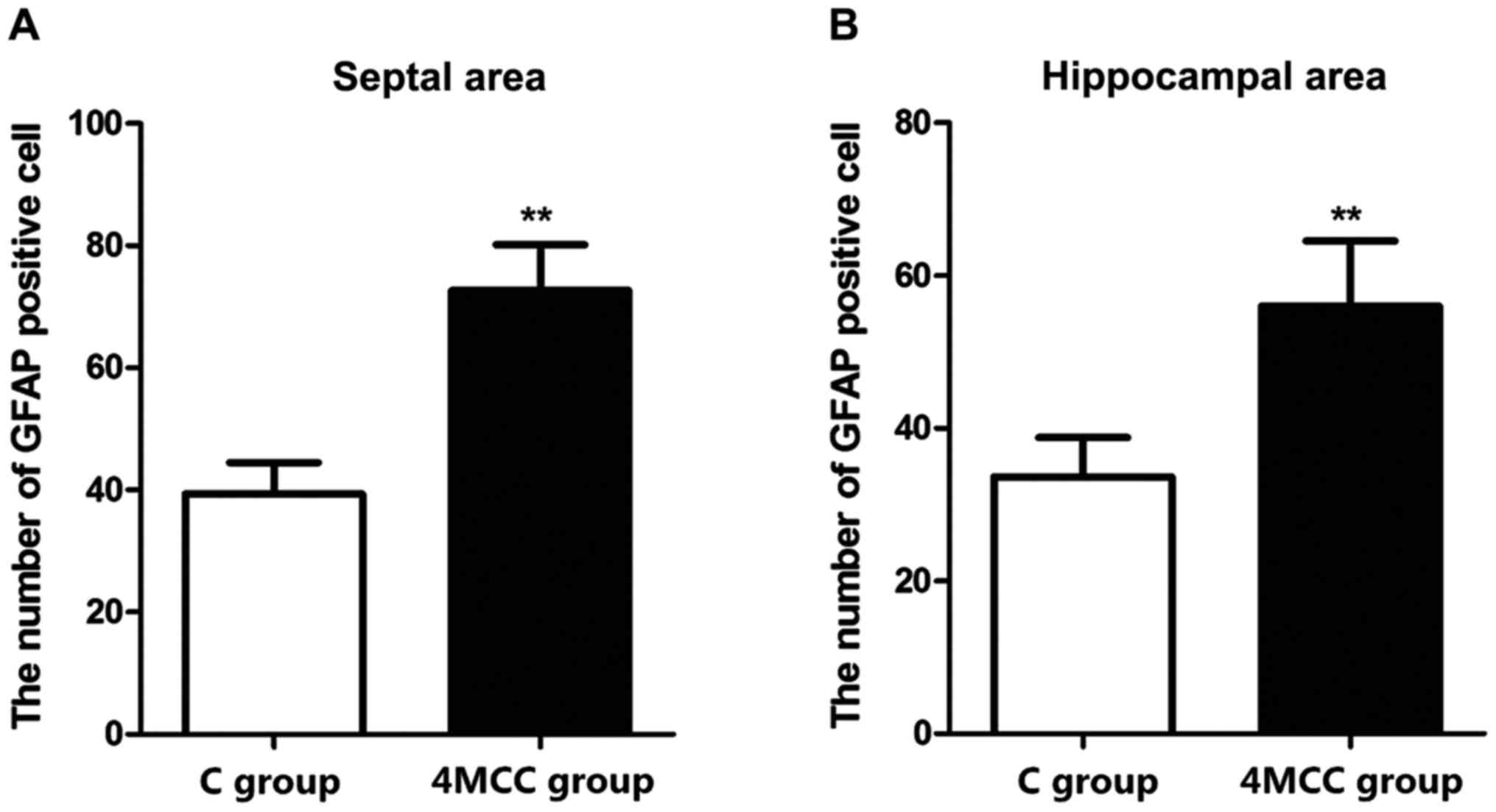
GFAP immumohistochemical staining
The numbers of GFAP-positive cells in hippocampal
area and septal area of rats in each group were detected via
immumohistochemical staining. The results revealed that the number
of GFAP-positive cells in hippocampal area and septal area of rats
in 4MCC group at 2 weeks after modeling was significantly larger
than those in C group (p<0.01) (Fig.
4).
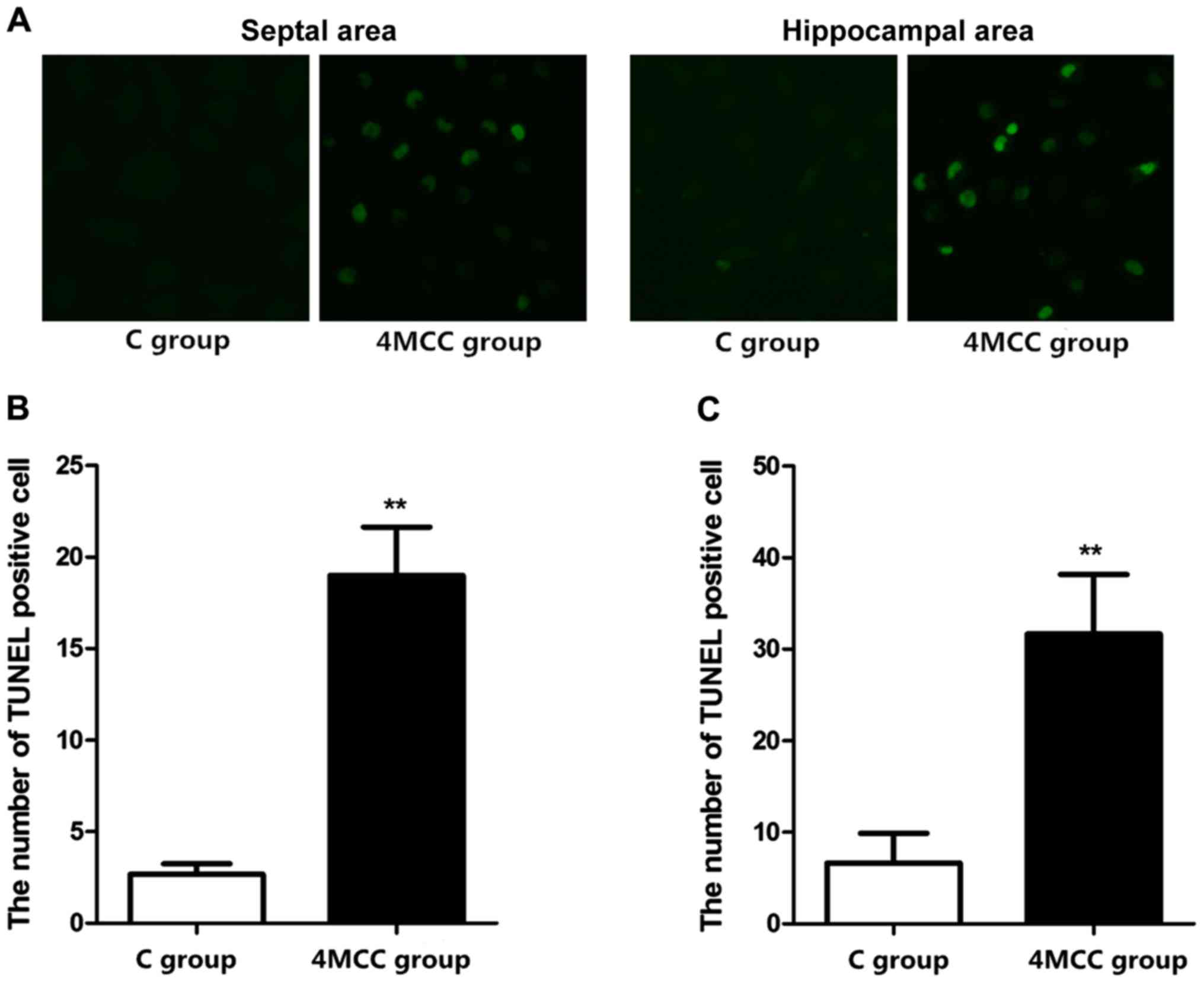
Detection of the number of apoptotic
cells via TUNEL staining
The number of apoptotic cells in hippocampal area
and septal area of rats in each group were detected via TUNEL
staining. The results showed that the numbers of apoptotic cells in
hippocampal area and septal area of rats in 4MCC group at 2 weeks
after modeling were obviously larger than those in C group
(p<0.01) (Fig. 5).
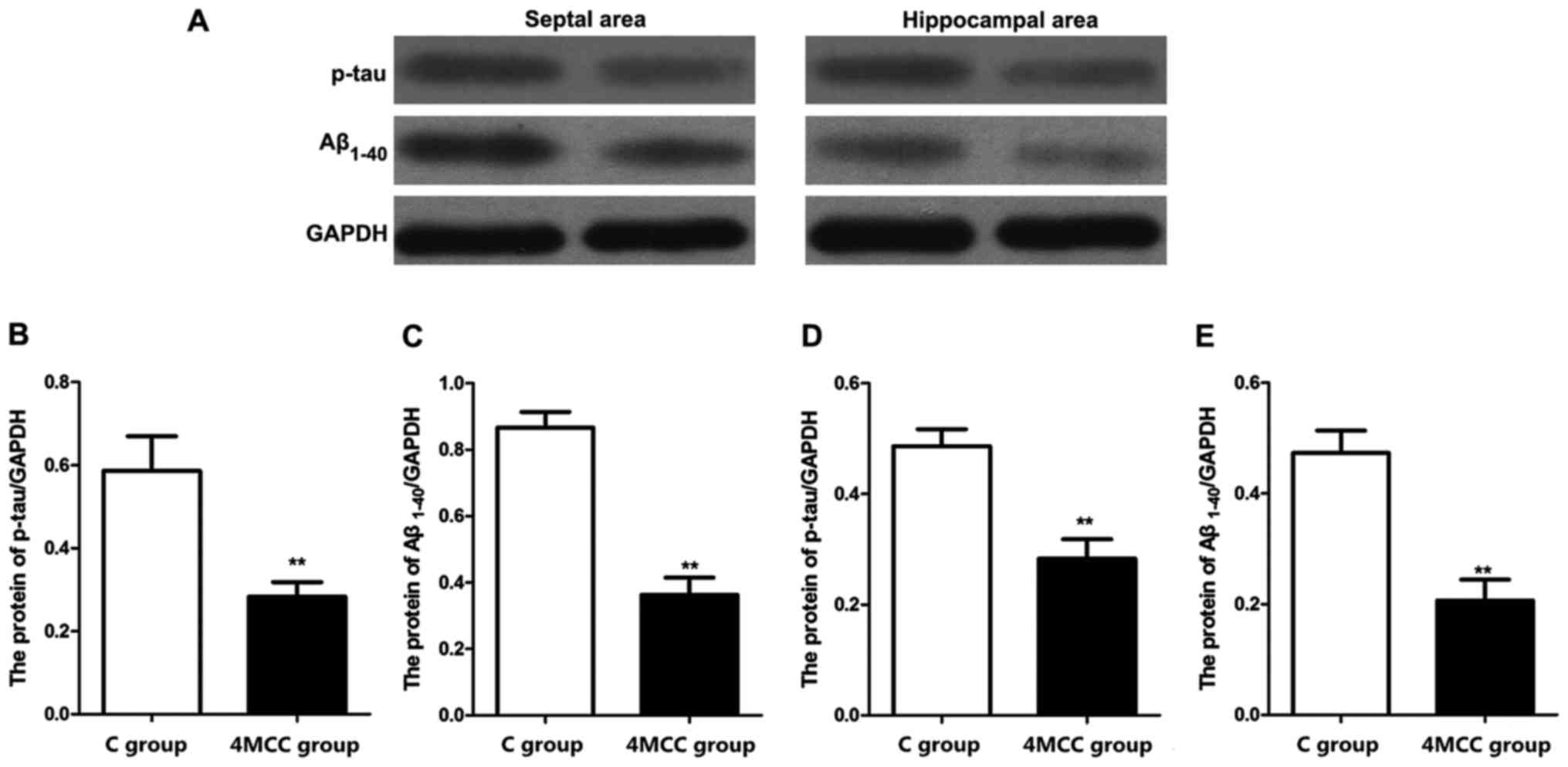
Detection of related protein
expression levels via western blot analysis
The expression levels of phosphorylated tau (p-tau)
and Aβ1-40 proteins in hippocampal area and septal area
in each group were detected via western blot analysis. The results
revealed that the expression levels of p-tau and Aβ1-40
proteins in hippocampal area and septal area of rats in 4MCC group
at 2 weeks after modeling were significantly higher than those in C
group (p<0.01) (Fig. 6).
Discussion
Cerebral concussion caused by traumatic biological
force can produce complex effects on the brain, causing a series of
pathophysiological reactions and leading to rapid and transient
neurological impairment. With the increasing number of trauma, it
will further lead to severe damage to learning and memory abilities
and balance motion behavior (9,10).
Benson et al (11) studied
and found that when cerebral concussion in patients has not
restored, the stimulation of cerebral concussion once again can
lead to learning and memory and neurological disorders in patients.
MCC often leads to serious neurological sequelae, seriously
affecting the patients' health and even life safety (12). Through establishing the MCC rat
model, Mychasiuk et al (13)
simulated the process of cerebral concussion, which effectively
clarified that MCC can seriously damage the learning and memory
abilities. MWM test, invented by Morris, a British psychologist, is
currently an internationally-accepted test for spatial recognition
learning model, in which the escape latency of rats searching for
the platform can effectively reflect the spatial learning ability
(14). BB test and BW test are used
to evaluate the static and dynamic balance motion behaviors of rats
after injury, respectively, so as to evaluate the effect of
cerebral concussion on balance motion behavior of rats (15).
In this study, MCC rat models were established via
multiple striking. BB test and BW test were used to verify the
effect of MCC on balance motion ability of rats. The results showed
that severe damage to balance motion ability occurred in MCC rats.
Kerr et al (16) found that
after cerebral concussion had occurred many times, the balance
ability of athletes in high-intensity collision sports
significantly declines. The MWM test revealed that the escape
latency of MCC rats was increased significantly, but the motion
distance in target quadrant was obviously shortened. The above
results indicated that MCC can reduce the learning and memory
abilities of rats. Moreover, many studies in China and foreign
countries have shown that patients with brain injury suffer from
severe cognitive behavior disorders (17,18).
MRS is a method to quantify the tissue metabolism in
organs under noninvasive conditions, which is widely used in the
diagnosis of a variety of metabolic diseases. The detection indexes
mainly include MI, Cho, Cr and NAA (19). In this study, the changes in brain
tissue metabolites in 4MCC rats after 2 weeks were detected via
MRS. The results showed that the NAA and Cr expression levels in
brain tissues in rats with cerebral concussion were significantly
reduced, but Cho and MI expression levels were significantly
increased, suggesting that the cerebral concussion can cause
neuronal damage, leading to a significant increase in the
expression level of glial cells. NAA only exists in mature nerve
cells, and the neuronal loss or death can lead to a decrease in
NAA; Cr is a standard of energy metabolism and exists in neurons
and glial cells, whose expression will be reduced under
pathological conditions; Cho is a precursor of acetylcholine, as
well as a marker of cell membrane density and integrity, which is
abundant in glial cells; MI, as a monosaccharide, is also a marker
of glial cells. Cho and MI expression levels can be significantly
increased in proliferation of glial cells (20). GFAP is a marker of astrocytes, and it
can directly reflect the number of astrocytes. Besides, TUNEL
staining can be used to label the apoptotic cells (21). In this study, GFAP was used to label
the astrocytes, and TUNEL staining was performed to detect the
number of apoptotic cells. The results showed that the numbers of
GFAP-positive cells and TUNEL-positive cells in hippocampal and
septal areas were significantly increased in rats with cerebral
concussion. These results indicated that MCC can significantly
increase the number of dead neural cells, and increase the glial
cell expression, which are consistent with the results of MRS.
Okonkwo et al (22) found
that the number of astrocytes in brain tissues of patients with
Alzheimer's disease and Parkinson's disease will be significantly
increased, and a large number of neurons will die once the damage
to learning and memory abilities occurs. This study also found that
the expression levels of p-tau and Aβ1-40 proteins in
brain tissues of MCC rats were increased significantly. Both p-tau
and Aβ1-40 proteins lead to the neurodegenerative
diseases, which are closely associated with learning and memory
impairment.
In conclusion, MCC can lead to severe damage to
learning, memory and balance motion abilities in rats, whose
mechanism may be that the expression levels of p-tau and A
Aβ1-40 proteins in brain tissues are significantly
increased due to multiple striking, resulting in neuronal apoptosis
and proliferation of a large number of glial cells.
Acknowledgements
Not applicable.
Funding
This study was supported by The National Natural
Science Fund (no. 81360467).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ and ZZ were responsible for animal feeding,
treatment and grouping. HZ, ZW and YZ performed BB test. JY and HS
contributed to MWM test. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
All the animal experiments in the present study were
approved by the Animal Ethics Committee of The First Affiliated
Hospital of Kunming Medical University (Kunming, China), and all
operations strictly followed the regulations of the National
Institute Guide for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Spira JL, Lathan CE, Bleiberg J and Tsao
JW: The impact of multiple concussions on emotional distress,
post-concussive symptoms, and neurocognitive functioning in active
duty United States marines independent of combat exposure or
emotional distress. J Neurotrauma. 31:1823–1834. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gardner AJ, Howell DR, Levi CR and Iverson
GL: Evidence of concussion signs in National Rugby League match
play: A video review and validation study. Sports Med Open.
3:292017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brooks BL, McKay CD, Mrazik M, Barlow KM,
Meeuwisse WH and Emery CA: Subjective, but not objective, lingering
effects of multiple past concussions in adolescents. J Neurotrauma.
30:1469–1475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ford JH, Giovanello KS and Guskiewicz KM:
Episodic memory in former professional football players with a
history of concussion: An event-related functional neuroimaging
study. J Neurotrauma. 30:1683–1701. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tator CH: Concussions and their
consequences: Current diagnosis, management and prevention. CMAJ.
185:975–979. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gardner AJ, Levi CR and Iverson GL:
Observational review and analysis of concussion: A method for
conducting a standardized video analysis of concussion in Rugby
League. Sports Med Open. 3:262017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hehar H, Yeates K, Kolb B, Esser MJ and
Mychasiuk R: Impulsivity and concussion in juvenile rats: Examining
molecular and structural aspects of the frontostriatal pathway.
PLoS One. 10:e01398422015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Webster KM, Wright DK, Sun M, Semple BD,
Ozturk E, Stein DG, O'Brien TJ and Shultz SR: Progesterone
treatment reduces neuroinflammation, oxidative stress and brain
damage and improves long-term outcomes in a rat model of repeated
mild traumatic brain injury. J Neuroinflammation. 12:2382015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gioia GA: Multimodal evaluation and
management of children with concussion: Using our heads and
available evidence. Brain Inj. 29:195–206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barker T, Russo SA, Barker G, Rice MA Jr,
Jeffrey MG, Broderick G and Craddock TJA: A case matched study
examining the reliability of using ImPACT to assess effects of
multiple concussions. BMC Psychol. 5:142017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Benson BW, Meeuwisse WH, Rizos J, Kang J
and Burke CJ: A prospective study of concussions among National
Hockey League players during regular season games: The NHL-NHLPA
Concussion Program. CMAJ. 183:905–911. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brooks BL, Mannix R, Maxwell B, Zafonte R,
Berkner PD and Iverson GL: Multiple past concussions in high school
football players: Are there differences in cognitive functioning
and symptom reporting? Am J Sports Med. 44:3243–3251. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mychasiuk R, Farran A, Angoa-Perez M,
Briggs D, Kuhn D and Esser MJ: A novel model of mild traumatic
brain injury for juvenile rats. J Vis Exp. 94:13–28. 2014.
|
|
14
|
Prins ML, Hales A, Reger M, Giza CC and
Hovda DA: Repeat traumatic brain injury in the juvenile rat is
associated with increased axonal injury and cognitive impairments.
Dev Neurosci. 32:510–518. 2010.PubMed/NCBI
|
|
15
|
Khuman J, Meehan WP III, Zhu X, Qiu J,
Hoffmann U, Zhang J, Giovannone E, Lo EH and Whalen MJ: Tumor
necrosis factor alpha and Fas receptor contribute to cognitive
deficits independent of cell death after concussive traumatic brain
injury in mice. J Cereb Blood Flow Metab. 31:778–789. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kerr ZY, Snook EM, Lynall RC, Dompier TP,
Sales L, Parsons JT and Hainline B: Concussion-related protocols
and preparticipation assessments used for incoming student-athletes
in National Collegiate Athletic Association member institutions. J
Athl Train. 50:1174–1181. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huh S, Kim TW, Yang JH, Moon MH, Kim SY
and Ko HY: Pharmacotherapy prescription trends for
cognitive-behavioral disorder in patients with brain injury in
Korea. Ann Rehabil Med. 42:35–41. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berthier ML, Kulisevsky JJ, Gironell A and
Lopez OL: Obsessivecompulsive disorder and traumatic brain injury:
behavioral, cognitive, and neuroimaging findings. Neuropsychiatry
Neuropsychol Behav Neurol. 14:23–31. 2001.PubMed/NCBI
|
|
19
|
Johnson B, Gay M, Zhang K, Neuberger T,
Horovitz SG, Hallett M, Sebastianelli W and Slobounov S: The use of
magnetic resonance spectroscopy in the subacute evaluation of
athletes recovering from single and multiple mild traumatic brain
injury. J Neurotrauma. 29:2297–2304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin AP, Ramadan S, Stern RA, Box HC,
Nowinski CJ, Ross BD and Mountford CE: Changes in the
neurochemistry of athletes with repetitive brain trauma:
Preliminary results using localized correlated spectroscopy.
Alzheimers Res Ther. 7:132015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hazrati L-N, Tartaglia MC, Diamandis P,
Davis KD, Green RE, Wennberg R, Wong JC, Ezerins L and Tator CH:
Absence of chronic traumatic encephalopathy in retired football
players with multiple concussions and neurological symptomatology.
Front Hum Neurosci. 7:2222013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okonkwo DO, Yue JK, Puccio AM,
Panczykowski DM, Inoue T, McMahon PJ, Sorani MD, Yuh EL, Lingsma
HF, Maas AI, et al Transforming Research and Clinical Knowledge in
Traumatic Brain Injury (TRACK-TBI) Investigators, : GFAP-BDP as an
acute diagnostic marker in traumatic brain injury: Results from the
prospective transforming research and clinical knowledge in
traumatic brain injury study. J Neurotrauma. 30:1490–1497. 2013.
View Article : Google Scholar : PubMed/NCBI
|