Introduction
Age-related macular degeneration (AMD) is the
leading cause of visual loss after the age of 60 years (1). Although the exact pathogenic mechanism
of AMD is still unknown, numerous reports provide evidence that
oxidative stress plays an important role in the pathophysiology of
AMD. Retinal pigment epithelial (RPE) is a monolayer of
differentiated cells located between the neural retina and Bruch's
membrane, performing essential functions for the maintenance of the
normal visual process (2). During
AMD, excessive oxidative stress occurs, resulting in the
accumulation of reactive oxygen species (ROS), causing damage to
RPE cells (3–5). Excessive ROS causes an oxidative
cascade, mediated in part by ROS-induced activation of NF-ĸB, STAT,
and AP-1 transcription factors leading to oxidative injury to
macromolecules in RPE cells, which ultimately contributes to the
pathogenesis of AMD (6). Thus,
inhibiting H2O2-induced RPE cell injury may
be a therapeutic approach for the treatment of AMD.
Salidroside (SAL) is the major phenylpropanoid
glycoside and pharmacological active constituent of Rhodiola
rosea. Previous studies have been shown that SAL possesses a
wide range of pharmacological functions, including anti-aging,
anti-inflammatory, antioxidant, anti-cancer and neuroprotective
activities (7–11). For example, Zhang et al
(12) reported that pretreatment
with SAL dose-dependently upregulated the production of antioxidant
enzymes and inhibited the elevation of intracellular ROS level in
Abeta-induced human neuroblastoma cells. However, the effects and
mechanisms of SAL on oxidative stress in RPE cells exposed to
hydrogen peroxide (H2O2) remain unclear.
Therefore, the purpose of this study was to investigate its
protective effects and the underlying mechanisms against
H2O2-induced oxidative stress in human RPE
cells. The results indicated that SAL protects RPE cells against
H2O2-induced cell injury through the
activation of Akt/GSK-3β signaling pathway.
Materials and methods
Cell culture
The human RPE cell line ARPE-19 was obtained from
the American Type Culture Collection (ATCC; Manassas, VA, USA) and
maintained in a 1:1 mixture of Dulbecco's modified Eagle's medium
(DMEM) and nutrient mixture F-12 (Life Technologies, Carlsbad, CA,
USA) supplemented with 10% fetal bovine serum (FBS), 100 U/ml
penicillin, 100 µg/ml streptomycin, and 2 mM l-glutamine (Lonza,
Basel, Switzerland). The cells were cultured in a humidified
incubator at 37°C and 5% CO2.
Cell viability assay
Cell viability was evaluated using the MTT assay. In
brief, ARPE-19 cells at a density of 1×104 cells/well
were incubated with or without SAL (12.5–100 µg/ml) for 24 h and
then treated with 200 µM H2O2 for 6 h. Next,
50 µl of MTT solution (5 mg/ml; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was added into each well, and the plate was
incubated for 4 h at 37°C. Then the supernatant was removed and 100
µl DMSO (Sigma-Aldrich; Merck KGaA) was added to dissolve formazan.
The absorbance was read at 490 nm using an enzyme linked
immunosorbent assay plate reader (Olympus, Tokyo, Japan). The
experiment was performed in triplicate.
Cell cytotoxicity assay
The cytotoxicity of treated ARPE-19 cells was
evaluated via determining the activity of lactate dehydrogenase
(LDH) enzyme released into medium with the CytoTox96®
Non-Radioactive Cytotoxicity Assay (Promega, Fitchburg, WI, USA)
according to the manufacturer's instructions. The experiment was
performed in triplicate.
Cell apoptosis assay
After treatment, ARPE-19 cells were harvested by
trypsinization. Then, the cells were centrifuged, washed twice with
PBS and resuspended in 1X Binding Buffer. After adding 5 µl of
Annexin V-FITC solution and 5 µl of PI solution according to the
instructions of Annexin V-FITC apoptosis detection kit (Beyotime
Institute of Biotechnology, Nantong, China), cells were incubated
in the dark for 30 min at room temperature. The experiment was
performed in triplicate.
Detection of ROS
After treatment, ARPE-19 cells were loaded with 5 mM
CellROX orange reagent for 30 min at 37°C. Then, the fluorescence
intensity of CellRox Green in each well was measured using
SpectraMax 5 (Molecular Devices, Downington, PA, USA) following
manufacturer's instructions. The experiment was performed in
triplicate.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from ARPE-19 cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). First-strand cDNA was synthesized with the Prime
Script RT reagent kit (Takara Bio Inc., Otsu, Japan). PCR
amplification was carried out by ABI PRISM 7900 thermocycler using
SYBR Premix Taq (Applied Biosystems, Foster City, CA, USA). The
primers used were as follows: for Bcl-2 forward,
5′-CAAAGGTGGATCAGATTCAAG-3′ and reverse,
5′-GGTGAGCATTATCACCCAGAA-3′; for Bax forward,
5′-TGGCAGCAGTGACAGCAGCG-3′ and reverse, 5′-TACGGAGGTGGAGTGGGTGT-3′;
and for GAPDH forward, 5′-TGACTTCAACAGCGACACCCA-3′ and
5′-CACCCTGTTGCTGTAGCCAAA-3′. The comparative threshold cycle method
(ΔΔCq) was used to determine the levels of gene expression
(13). The experiment was performed
in triplicate.
Western blot analysis
ARPE-19 cells were homogenized and lysed with RIPA
lysis buffer (Beyotime Institute of Biotechnology). Then, protein
concentrations were measured with a BCA protein assay kit (Pierce,
Rockford, IL, USA). The proteins (30 µg/lane) were subjected to 10%
sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) and
transferred to Immobilon P EMD Millipore (Billerica, MA, USA).
After blocking with 5% non-fat milk in PBS with Tween-20 buffer at
room temperature for 1 h, the blots were incubated for 60 min at
room temperature with primary antibody against the following:
Bcl-2, Bax, Akt, phosphorylated Akt, GSK-3β, phosphorylated GSK-3β
or GAPDH (diluted 1:1,000 in TBST; Santa Cruz Biotechnology, Santa
Cruz, CA, USA). Subsequently, the membranes were incubated with
horseradish-peroxidase-conjugated secondary antibody (1:1,000;
Santa Cruz Biotechnology) for 1 h at room temperature. Detection
was performed using the ECL western blotting detection system
(Thermo Fisher Scientific, Inc.). Each band density was quantified
using Quantity One software (Bio-Rad, Richmond, CA, USA) and
normalized to GAPDH. The experiment was performed in
triplicate.
Statistical analysis
Statistical analysis was made using Prism (GraphPad
Software, San Diego, CA, USA). Data are expressed as mean ±
standard deviation. Significance was determined by one-way analysis
of variance followed by Tukey's post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Pretreatment with SAL markedly
attenuated H2O2-induced loss of cell
viability
To study the effect of SAL on RPE cell viability,
ARPE-19 cells were treated with increasing concentrations of SAL
(12.5, 25, 50 or 100 µg/ml). As shown in Fig. 1A, 100 µg/ml of SAL significantly
decreased cell viability. Cell viability of ARPE-19 cells was not
significantly impaired by concentrations of SAL <100 µg/ml.
Then, we detected the effect of various concentrations of
H2O2 on RPE cell viability. The results
showed that 200 µM H2O2 could significantly
reduce cell viability. Since the effects of 200 and 300 µM
H2O2 were not significantly different, 200 µM
H2O2 was chosen for subsequent experiments
(Fig. 1B). In addition, the effect
of SAL on cell viability in H2O2-treated
ARPE-19 cells was evaluated. The results of MTT assay demonstrated
that the viability of RPE cells treated with 200 µM
H2O2 significantly decreased compared with
the untreated group. However, pretreatment with SAL obviously
increased the viability of RPE cells in a dose-dependent manner
(Fig. 1C). We further analyzed
whether SAL pretreatment could influence
H2O2-induced cellular cytotoxicity. As shown
in Fig. 1D, the exposure to 200 µM
H2O2 greatly increased LDH release. However,
the LDH release gradually down to 274.3, 223.7, and 164.1% with
increasing concentrations of SAL, respectively.
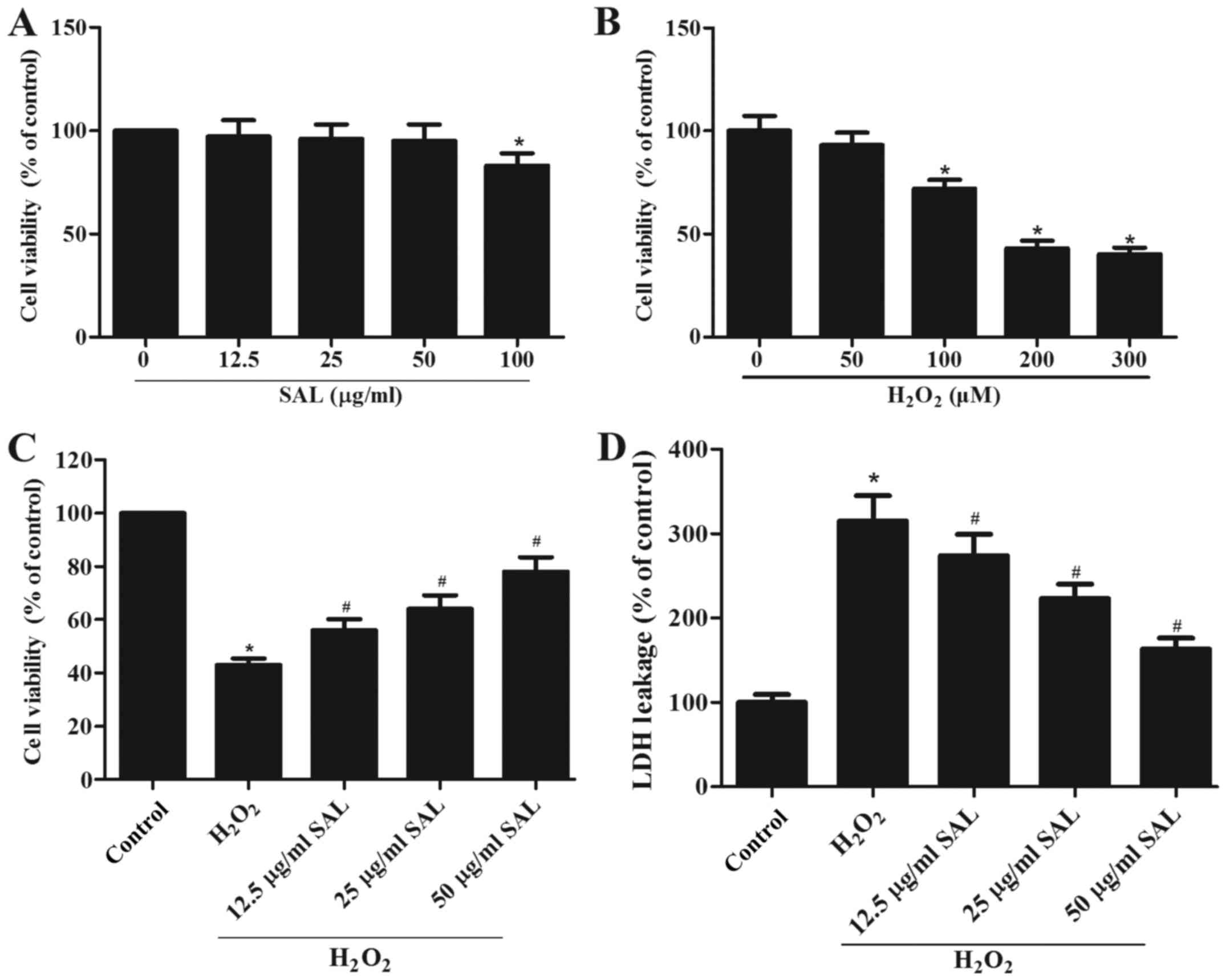 | Figure 1.Pretreatment with SAL markedly
attenuates H2O2-induced loss of cell
viability. (A) ARPE-19 cells were treated with various doses of SAL
(0, 12.5, 25, 50 and 100 µg/ml) for 24 h and the cell viability was
analyzed by an MTT assay. (B) ARPE-19 cells were incubated with
various concentrations of H2O2 (0, 50, 100,
200 and 300 µM) for 24 h and cell viability was detected by an MTT
assay. (C) ARPE-19 cells were incubated with various doses of SAL
(0, 12.5, 25 and 50 µg/ml) for 24 h and then exposed to 200 µM
H2O2 for 24 h. Cell viability was analyzed by
an MTT assay. (D) Cell cytotoxicity was analyzed by an LDH assay.
Data are expressed as the mean ± standard deviation from a minimum
of three independent experiments. *P<0.05 vs. the control group.
#P<0.05 vs. the H2O2 group.
SAL, salidroside; H2O2, hydrogen
peroxide. |
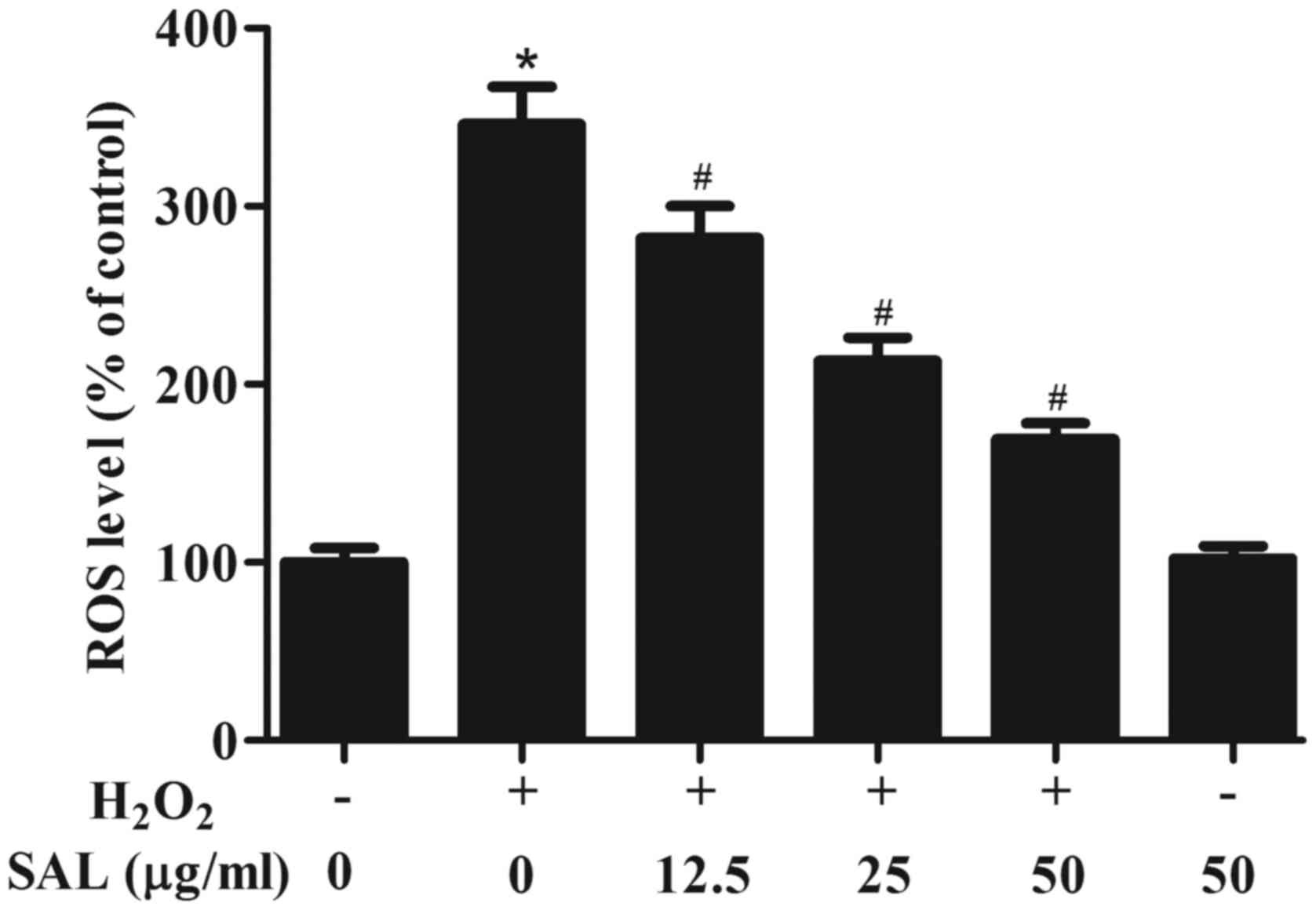
Pretreatment with SAL ameliorated
H2O2-induced oxidative stress in RPE
cells
It is known that oxidative stress plays a pivotal
role in AMD pathogenesis. Thus, we examined the effect of SAL in on
oxidative stress in H2O2-treated ARPE-19
cells. As shown in Fig. 2, as
compared with the control group, the level of intracellular ROS was
significantly increased in H2O2-treated
ARPE-19 cells. However, pretreatment with SAL markedly reduced
H2O2-induced ROS level in ARPE-19 cells. In
addition, SAL alone treatment did not affect ROS level in ARPE-19
cells.
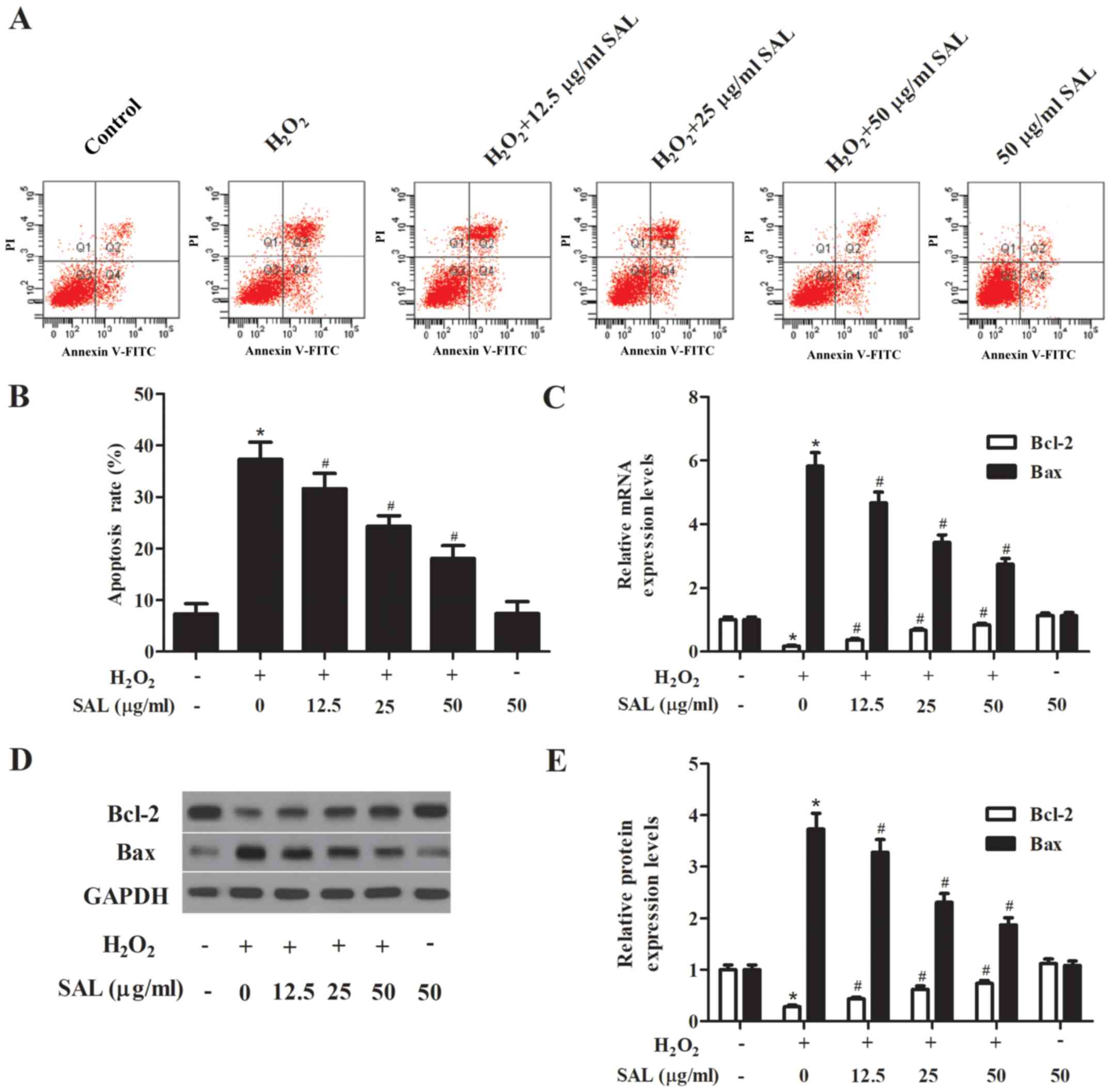
Pretreatment with SAL inhibited
H2O2-induced cell apoptosis in RPE cells
H2O2 has been shown to induce
apoptosis in RPE cells. Thus, we examined the effect of SAL on
ARPE-19 cell apoptosis induced by H2O2. As
shown in Fig. 3, the exposure to 200
µM H2O2 for 24 h would lead to a significant
higher rate of apoptosis, compared with the control cells.
Pretreatment with SAL dramatically reversed
H2O2-induced apoptosis. In addition, we
observed that pretreatment with SAL significantly upregulated the
expression of Bcl-2 and downregulated the expression of Bax, as
compared with the H2O2 group (Fig. 3).
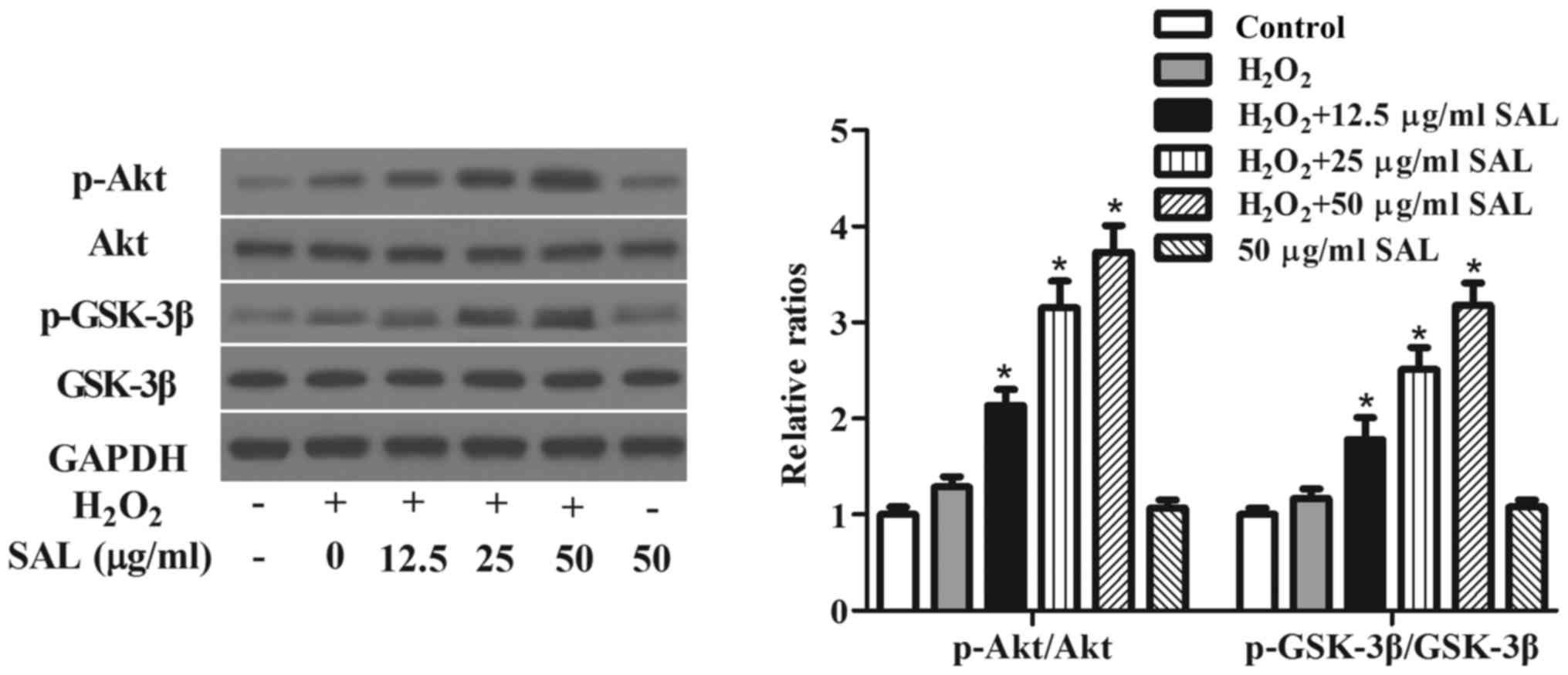
Pretreatment with SAL activated
Akt/GSK-3β signaling pathway in RPE cells
It has been reported that the activation of
Akt/GSK-3β signaling pathway plays an important role in the
progression of AMD (14), we
examined the effects of SAL on Akt/GSK-3β signaling pathway in
H2O2-treated ARPE-19 cells. The results of
western blot analysis indicated that the phosphorylation levels of
Akt and GSK-3β were not significantly activated in
H2O2-stimulated ARPE-19 cells. Pretreatment
with SAL significantly increased the phosphorylation levels of Akt
and GSK-3β in H2O2-treated ARPE-19 cells.
Additionally, SAL alone treatment did not affect the activation of
Akt/GSK-3β pathway (Fig. 4).
Discussion
To our knowledge, we have shown for the first time
that SAL markedly attenuated H2O2-induced
loss of cell viability. SAL also ameliorated
H2O2-induced oxidative stress and cell
apoptosis in RPE cells. Furthermore, pretreatment with SAL
significantly increased the phosphorylation levels of Akt and
GSK-3β in H2O2-treated ARPE-19 cells.
Previous studies reported that
H2O2 can decrease RPE cell viability, which
induce AMD progression (15–17). In line with these previous studies,
our present study confirmed that H2O2
significantly decreased cell viability, as evidenced by the MTT
assay. Meanwhile, we observed that pretreatment with SAL obviously
increased the viability and reduced LDH release in
H2O2-induced ARPE-19 cells in a dose
dependent manner. These observations suggest that SAL protected
human RPE cells from H2O2-induced oxidative
stress through increasing the viability and reducing LDH
release.
Numerous studies have demonstrated that oxidative
stress is a major stimulus in the pathogenesis of AMD (18–20). SAL
is a strong antioxidative supplement in Chinese traditional
medicine. It was confirmed that SAL effectively attenuated the
production of ROS in human umbilical vein endothelial cells
(HUVECs) under conditions of oxidative injury induced by
H2O2 (21).
Another study reported that SAL effectively inhibited oxidative
stress in cardiac H9c2 cells induced by H2O2
insult (22). In accordance with
previous studies, in the present study, we observed that
pretreatment with SAL markedly reduced
H2O2-induced ROS level in ARPE-19 cells.
These data suggest that SAL effectively protected human RPE cells
from H2O2-induced oxidative stress via
antioxidant effect.
Previous studies showed that oxidative stress
induces mitochondrial dysfunction and promotes apoptosis
correlating with increased Bax expression and decreased Bcl-2
expression in human RPE cells (23,24).
Bcl-2 is a key anti-apoptotic member of the Bcl-2 family that
regulates the intrinsic apoptosis pathway. In addition, it was
reported that SAL increased the ratio of Bcl2/Bax in
H2O2-induced retinal endothelial cells
(25). Similarly, in the present
study, we observed that pretreatment with SAL upregulated the
expression of Bcl-2 and downregulated the expression of Bax in
H2O2-treated ARPE-19 cells. These data
suggest that SAL effectively protected human RPE cells from
H2O2-induced oxidative stress via
anti-apoptotic effect.
The Akt/GSK-3β signaling pathway plays a crucial
role in a variety of cellular processes such as cell proliferation
and apoptosis (26–28). Akt activation enhances RPE cell
survival and thus may protect RPE cells from oxidant-induced cell
death in the pathogenesis of AMD (29). Previous studies demonstrated that
adding H2O2 to RPE cells caused Akt
activation (29,30). In the present study, we observed that
Akt phosphorylation was moderately enhanced in the stimulation of
H2O2. In addition, pretreatment with SAL
significantly increased the phosphorylation levels of Akt and
GSK-3β in H2O2-treated ARPE-19 cells. These
results suggest that SAL protected RPE cells against
H2O2-induced cell injury through the
activation of Akt/GSK-3β signaling pathway.
In conclusion, this study demonstrated that SAL
could stimulate the recovery of RPE cells under oxidative stress
through the activation of Akt/GSK-3β signaling. These data suggest
that SAL may be a potential therapeutic strategy for the prevention
and therapy of AMD.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DT conceived and designed the experiments. YY and DL
performed the experiments. DL analyzed the data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Friedman DS, O'Colmain BJ, Muñoz B, Tomany
SC, Mccarty C, de Jong PT, Nemesure B, Nemesure B, Mitchell P and
Kempen J; Eye Diseases Prevalence Research Group, : Prevalence of
age-related macular degeneration in the United States. Arch
Ophthalmol. 122:564–572. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Strauss O: The retinal pigment epithelium
in visual function. Physiol Rev. 85:845–881. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dong A, Xie B, Shen J, Yoshida T, Yokoi K,
Hackett SF and Campochiaro PA: Oxidative stress promotes ocular
neovascularization. J Cell Physiol. 219:544–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu L, Hackett SF, Mincey A, Lai H and
Campochiaro PA: Effects of different types of oxidative stress in
RPE cells. J Cell Physiol. 206:119–125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Glotin AL, Calipel A, Brossas JY, Faussat
AM, Tréton J and Mascarelli F: Sustained versus transient ERK1/2
signaling underlies the anti- and proapoptotic effects of oxidative
stress in human RPE cells. Invest Ophthalmol Vis Sci. 47:4614–4623.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beatty S, Koh H, Phil M, Henson D and
Boulton M: The role of oxidative stress in the pathogenesis of
age-related macular degeneration. Surv Ophthalmol. 45:115–134.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun L, Isaak CK, Zhou Y, Petkau JC, O K,
Liu Y and Siow YL: Salidroside and tyrosol from Rhodiola protect
H9c2 cells from ischemia/reperfusion-induced apoptosis. Life Sci.
91:151–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li D, Fu Y, Zhang W, Su G, Liu B, Guo M,
Li F, Liang D, Liu Z, Zhang X, et al: Salidroside attenuates
inflammatory responses by suppressing nuclear factor-κB and mitogen
activated protein kinases activation in lipopolysaccharide-induced
mastitis in mice. Inflamm Res. 62:9–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mao GX, Wang Y, Qiu Q, Deng HB, Yuan LG,
Li RG, Song DQ, Li YY, Li DD and Wang Z: Salidroside protects human
fibroblast cells from premature senescence induced by H(2)O(2)
partly through modulating oxidative status. Mech Ageing Dev.
131:723–731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu X, Zhang X, Qiu S, Yu D and Lin S:
Salidroside induces cell-cycle arrest and apoptosis in human breast
cancer cells. Biochem Bioph Res Commun. 398:62–67. 2010. View Article : Google Scholar
|
|
11
|
Shi TY, Feng SF, Xing JH, Wu YM, Li XQ,
Zhang N, Tian Z, Liu SB and Zhao MG: Neuroprotective effects of
Salidroside and its analogue tyrosol galactoside against focal
cerebral ischemia in vivo and H2O2-induced neurotoxicity in vitro.
Neurotox Res. 21:358–367. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, Yu H, Zhao X, Lin X, Tan C, Cao G
and Wang Z: Neuroprotective effects of salidroside against
beta-amyloid-induced oxidative stress in SH-SY5Y human
neuroblastoma cells. Neurochem Int. 57:547–555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baek SM, Yu SY, Son Y and Hong HS:
Substance P promotes the recovery of oxidative stress-damaged
retinal pigmented epithelial cells by modulating Akt/GSK-3β
signaling. Mol Vis. 22:1015–1023. 2016.PubMed/NCBI
|
|
14
|
Cao X, Liu M, Tuo J, Shen D and Chan CC:
The effects of quercetin in cultured human RPE cells under
oxidative stress and in Ccl2/Cx3cr1 double deficient mice. Exp Eye
Res. 91:15–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cia D, Vergnaud-Gauduchon J, Jacquemot N
and Doly M: Epigallocatechin gallate (EGCG) prevents H2O2-induced
oxidative stress in primary rat retinal pigment epithelial cells.
Curr Eye Res. 39:944–952. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koskela A, Reinisalo M, Hyttinen JM,
Kaarniranta K and Karjalainen RO: Pinosylvin-mediated protection
against oxidative stress in human retinal pigment epithelial cells.
Mol Vis. 20:760–769. 2014.PubMed/NCBI
|
|
17
|
Liang FQ and Godley BF: Oxidative
stress-induced mitochondrial DNA damage in human retinal pigment
epithelial cells: A possible mechanism for RPE aging and
age-related macular degeneration. Exp Eye Res. 76:397–403. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Beatty S, Koh H, Phil M, Henson D and
Boulton M: The role of oxidative stress in the pathogenesis of
age-related macular degeneration. Surv Ophthalmol. 45:115–134.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Klein R, Myers CE, Cruickshanks KJ,
Gangnon RE, Danforth LG, Sivakumaran TA, Iyengar SK, Tsai MY and
Klein BE: Markers of inflammation, oxidative stress, and
endothelial dysfunction and the 20-year cumulative incidence of
early age-related macular degeneration: The beaver dam eye study.
JAMA Ophthalmol. 132:446–455. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu MC, Shi HM, Wang H and Gao XF:
Salidroside protects against hydrogen peroxide-induced injury in
HUVECs via the regulation of REDD1 and mTOR activation. Mol Med
Rep. 8:147–153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu Y, Shi YP, Wu D, Ji YJ, Wang X, Chen
HL, Wu SS, Huang DJ and Jiang W: Salidroside protects against
hydrogen peroxide-induced injury in cardiac H9c2 cells via PI3K-Akt
dependent pathway. Dna Cell Biol. 30:809–819. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujihara M, Nagai N, Sussan TE, Biswal S
and Handa JT: Chronic cigarette smoke causes oxidative damage and
apoptosis to retinal pigmented epithelial cells in mice. PLoS One.
3:e31192008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mao H, Seo SJ, Biswal MR, Li H, Conners M,
Nandyala A, Jones K, Le YZ and Lewin AS: Mitochondrial oxidative
stress in the retinal pigment epithelium leads to localized retinal
degeneration. Invest Ophthalmol Vis Sci. 55:4613–4627. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi K, Wang X, Zhu J, Cao G, Zhang K and
Su Z: Salidroside protects retinal endothelial cells against
hydrogen peroxide-induced injury via modulating oxidative status
and apoptosis. Biosci Biotechnol Biochem. 79:1406–1413. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qiao Z, Xu YW and Yang J: Eupatilin
inhibits the apoptosis in H9c2 cardiomyocytes via the Akt/GSK-3β
pathway following hypoxia/reoxygenation injury. Biomed
Pharmacother. 82:373–378. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lv D, Bai Z, Yang L, Li X and Chen X:
Lipid emulsion reverses bupivacaine-induced apoptosis of h9c2
cardiomyocytes: PI3K/Akt/GSK-3β signaling pathway. Environ Toxicol
Pharmacol. 42:85–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ramírez-Sánchez J, Simões Pires EN,
Nuñez-Figueredo Y, Pardo-Andreu GL, Fonseca-Fonseca LA, Ruiz-Reyes
A, Ochoa-Rodríguez E, Verdecia-Reyes Y, Delgado-Hernández R, Souza
DO and Salbego C: Neuroprotection by JM-20 against oxygen-glucose
deprivation in rat hippocampal slices: Involvement of the
Akt/GSK-3β pathway. Neurochem Int. 90:215–223. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang P, Peairs JJ, Tano R and Jaffe GJ:
Oxidant-mediated Akt activation in human RPE cells. Invest
Ophthalmol Vis Sci. 47:4598–4606. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan T, Bi H and Wang Y: Wogonin modulates
hydroperoxide-induced apoptosis via PI3K/Akt pathway in retinal
pigment epithelium cells. Diagn Pathol. 9:1542014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang K, Jiang Y, Wang W, Ma J and Chen M:
Escin activates AKT-Nrf2 signaling to protect retinal pigment
epithelium cells from oxidative stress. Biochem Bioph Res Commun.
468:541–547. 2015. View Article : Google Scholar
|