Introduction
Tuberculous meningitis (TBM) is the most severe type
of extrapulmonary tuberculosis (TB), although it is the least
common type (incidence, 5–15%). Approximately 1/3 of the global
population is infected with latent TB (1). TBM is associated with a high mortality
and morbidity, particularly in developing countries, such as in
China, where TBM has the second highest incidence in the world
(2). In 2012, there were ~8.6
million incidences of TB globally, and among these, 1.3 million
fatalities occurred (3). The
diagnosis of TBM remains challenging due to the nonspecific
clinical presentation of patients. The initial differential
diagnosis includes other bacterial, viral or fungal infections of
the central nervous system (CNS), noninfectious inflammatory
diseases of the meninges (including systemic lupus erythematosus)
and intracranial malignancy (2). The
TBM-induced inflammatory reaction is associated with several
complications, including cerebrovascular disease, cranial nerve
palsy, hydrocephalus and infarction (4). Rapid diagnosis and therapy are
therefore necessary to decrease the high-mortality and severe
sequelae associated with the disease (4). Due to common characteristics with other
diseases and nonspecific clinical presentation, misdiagnosis or
delayed diagnosis of TBM results in increased mortality rate
(2).
The risk factors of the TBM, including dystrophia,
alcoholism, diabetes mellitus and cell-mediated immune mechanism
defects, commonly exist in the patients with human immunodeficiency
virus (HIV) disease, immunosuppressive diseases, and even in
immunocompetent individuals (5).
However, alternative factors, including corticosteroid medication,
diabetes mellitus and chronic hepatitis and cirrhosis have been
associated with the occurrence of TBM in the clinic (6). The standard anti-TBM drugs were applied
at all centers, however, the total length of treatment ranged from
6 to 12 months. The World Health Organization suggests treatment
between 9–12 months is sufficient for successfully treating TBM
(7). The adjuvant prednisolone
therapy method has been applied to a large number of TBM patients,
and significantly decreased the mortality rates of TBM (8).
In the present study, 189 patients with confirmed or
presumed TBM reported over a 6-year period were analyzed
retrospectively. The aims of the current study were to assess the
clinical, laboratory and neuroradiological findings of TBM patients
and to evaluate the prognostic significance of these variables. In
addition, the local epidemiological data were reported from these
cases.
Materials and methods
Patients
The cases included in the present study were
patients >14 years of age (age range, 15–79 years) with
confirmed or highly suspected TBM, who were admitted to the Xiangya
Hospital of Central South University (Changsha, China) during the
6-year period between January 2009 and January 2015. Cases with
pulmonary TB were excluded. Among 213 patients with confirmed or
presumed TBM, 24 were excluded due to insufficient follow-up or
incomplete data (5 cases refused lumbar puncture and imaging
examination). The clinical, laboratory and radiological
characteristics of 189 patients obtained through the retrospective
review of hospital and outpatient follow-up reports were evaluated.
All of the patients provided their prior informed written consent
for the present study.
Diagnosis
The common basic diagnosis of TBM is based on the
typical clinical presentation, neuroimaging characteristics,
cerebrospinal fluid (CSF) examination, and comprehensive judgment
of the response to anti-TB drug treatment (2). A clear diagnosis is based on the
discovery of Mycobacterium tuberculosis in CSF cell smears
or bacterial culture.
The cases reviewed in the current study were revised
based on the standardized clinical case definition that was
reported by Bartzatt (2). Based on
the aforementioned study, the classification of categories was
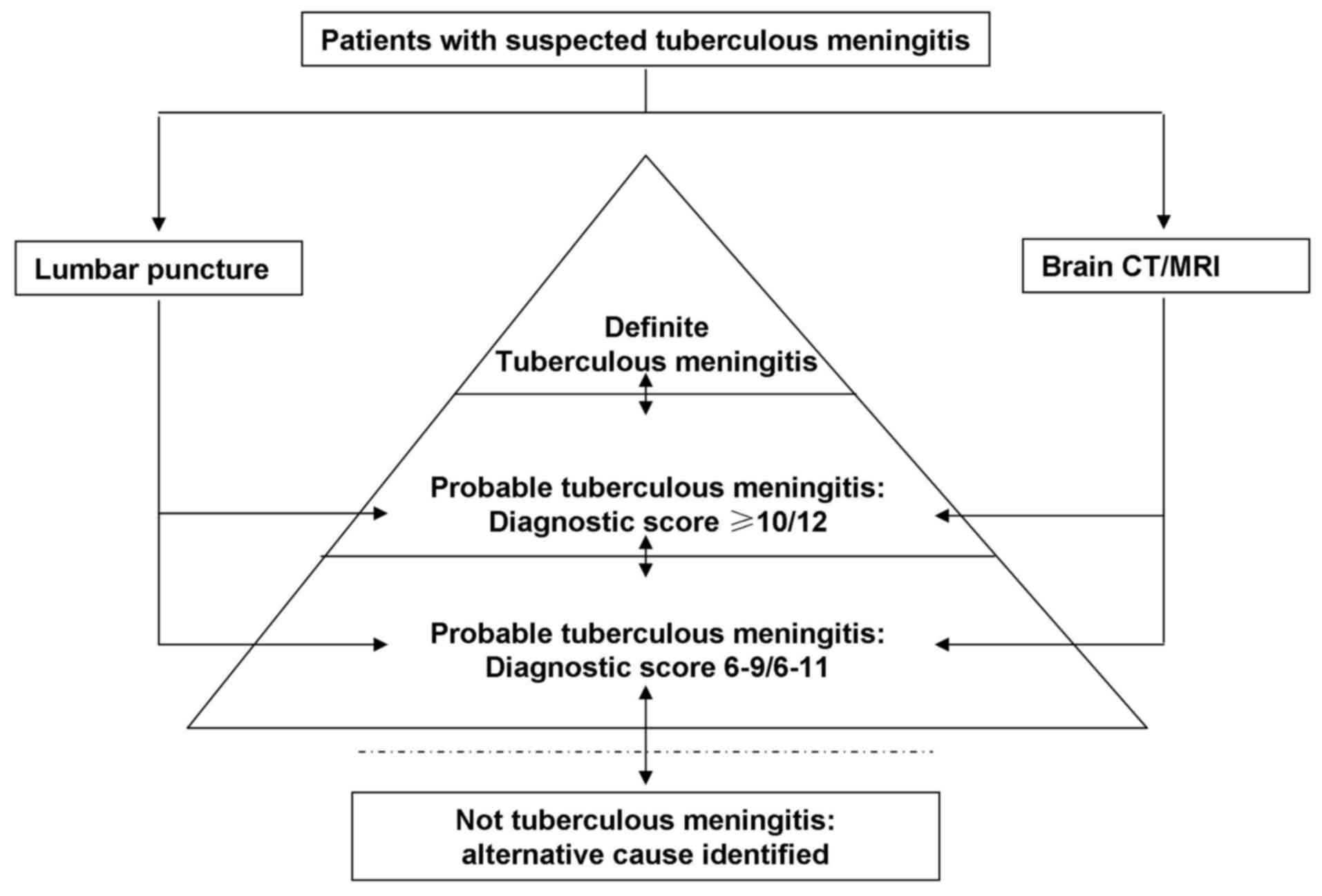
re-structured as shown in Fig. 1.
All patients were classified into the definite, probable, possible
and not TBM diagnosis categories according to Marais et al
(9). Briefly, in patients meeting
the criteria for suspected TBM, lumbar puncture or brain imaging
was conducted.
As subsequent examination results became available,
patients moved up or down the diagnostic pyramid (Fig. 1), and were finally classified as
definite, probable, possible or not TBM according to the diagnostic
criteria. Definite TBM is defined as the detection of CNS
Mycobacterium tuberculosis infection based on
microbiological identification or evidence from commercial nucleic
acid amplification tests. For probable TBM diagnosis, a diagnostic
score of ≥12 is required when imaging is available, or a score of
≥10 when imaging is not available. Possible TBM is diagnosed in
patients with a diagnostic score of 6–11 when imaging is available,
while a score of 6–9 is required when imaging is not available.
Clinical stage
According to the classification of the UK Medical
Research Council (1), the TBM cases
were divided into the following three categories: Stage I,
non-specific symptoms and signs, unconscious or vague
consciousness, and no damage to the function of the nervous system;
stage II, meningeal irritation, mild damage to the function of the
nervous system (such as cranial nerve palsy) and motor dysfunction;
stage III, seizures or convulsions, drowsiness or coma, and severe
neurological dysfunction (including paresis or paralysis).
Other examinations
Patients were also subjected to a lumbar puncture,
CSF cytology detection examinations as described previously
(2,9,10).
Statistical analysis
Continuous variables are expressed as the mean ±
standard deviation, and categorical variables are presented as a
proportion of the total number of patients. Univariate analysis was
conducted using χ2 test or Fisher's exact test for
categorical variables, and Student's t-test or Mann-Whitney U test
for continuous variables, as indicated. All statistical analyses
were performed using GraphPad Prism version 5.0 (GraphPad Software,
Inc., San Diego, CA, USA). Results were expressed as adjusted odds
ratios with the corresponding 95% confidence interval (CI). A
P-value of <0.05 was regarded to indicate a statistically
significant difference.
Results
Clinical diagnosis
The imaging data were available for 144 cases, with
66 cases presenting abnormal chest X-ray or computed tomography
findings, and 127 cases presenting abnormal brain magnetic
resonance imaging findings among the 144 patients examined.
In the present study, 4 cases were confirmed with
definite TBM. Probable TBM was diagnosed in 65 cases, including 47
cases where imaging was available (diagnostic score, ≥12) and 18
cases where imaging was not available (diagnostic score, ≥10). A
total of 120 patients were classified as possible TBM cases,
including 77 cases where imaging was available (diagnostic score,
6–11) and in 43 cases where imaging was not available (diagnostic
score, 6–9).
Clinical data
A total of 189 cases of definite, probable or
possible TBM were included in the current study, with an age
distribution range of 15–79 years and an average age of 38.38±17.90
years (95% CI, 32.41–44.35 years). As shown in Table I, TBM was more prevalent in men, and
the male to female ratio was 1.36:1 (109:80 patients). The mean
duration of follow-up was 5±3.3 months (range, 3–12 months). The
number of patients in the current study with acute onset was 88
(46.6%) cases, while subacute onset was observed in 67 (35.4%)
cases and chronic onset in 34 (20.0%) cases. The average hospital
stay of probable or possible TBM patients was 14.87±10.76 days (95%
CI, 11.25–18.43 days). All patients presented with symptoms for
>1 week, and the main complaints included fever (78.3%),
headache (89.2%) and neurological complications (including focal
neurological deficits, disturbance of consciousness and personality
changes) in almost 40% of patients. In total, 6.3% of patients were
in stage I, 69.3% were in stage II and 24.3% were in stage III
(Table I). Furthermore, there were
34 patients with the increased intracranial pressure, who were
treated by applying symptomatic and supportive treatment.
 | Table I.Clinical characteristics of probable
or possible TBM cases. |
Table I.
Clinical characteristics of probable
or possible TBM cases.
| Characteristics | n (%) |
|---|
| Sex |
|
| Male | 109 (57.7) |
|
Female | 80 (42.3) |
| Age range, years |
|
| ≤18 | 27 (14.3) |
|
18–35 | 67 (35.4) |
|
35–65 | 84 (44.4) |
|
>65 | 11 (5.8) |
| Onset type |
|
|
Acute | 88 (46.6) |
|
Sub-acute | 67 (35.4) |
|
Chronic | 34 (20.0) |
| Clinical stage |
|
| Stage
I | 12 (6.3) |
| Stage
II | 131 (69.3) |
| Stage
III | 46 (24.3) |
| Symptoms |
|
|
Headache | 168 (89.2) |
|
Fever | 148 (78.3) |
|
Nausea/vomiting | 128 (67.7) |
| Lack of
appetite/weakness | 77 (40.7) |
| Changes
in personality | 71 (37.6) |
| Weight
loss | 52 (27.5) |
| Night
sweats | 47 (24.9) |
| Stiff
neck | 138 (73.0) |
| Cranial
nerve palsy | 43 (22.8) |
| Blurred
consciousness | 91 (48.1) |
|
Convulsions | 33 (17.5) |
|
Paraplegia/paresis | 29 (15.3) |
Laboratory data
The purified protein derivative (PPD) skin test was
negative in 106 cases and positive in 83 cases. All patients
underwent CSF cytology detection, and the CSF findings on admission
were as follows: A 13.9% of white blood cells and the quantity of
CSF was >500/mm3. Acid-fast bacilli (AFB) culture or
smear detection was conducted in 173 cases, with only four cases
(2.3%) showing positive staining for Mycobacterium
tuberculosis in an AFB smear and no positive cases in AFB
culture test. In addition, 7 (18.9%) cases presented positive
results among 37 patients who underwent polymerase chain reaction
detection of Mycobacterium tuberculosis DNA (Table II). Additionally, the TB antibody
was examined in the CSF of 153 cases, with only 27 cases (17.6%)
demonstrating positive results.
 | Table II.Laboratory characteristics in the
cerebrospinal fluid of tuberculous meningitis cases (n=189). |
Table II.
Laboratory characteristics in the
cerebrospinal fluid of tuberculous meningitis cases (n=189).
| Characteristics | n (%) |
|---|
| White blood cell
count/mm3 |
|
| ≤100 | 69 (36.9) |
|
100–500 | 92 (49.2) |
|
≥500 | 26 (13.9) |
| Intracranial
pressure (mmH2O) |
|
|
≤180 | 63 (33.7) |
|
180–250 | 78 (41.7) |
|
≥250 | 46 (24.6) |
| Blood glucose level
(mmol/l) |
|
|
<0.60 | 165 (88.2) |
|
≤0.30 | 117 (62.6) |
| Protein level
(g/l) |
|
|
≤0.45 | 21 (11.2) |
|
0.45-2 | 122 (65.2) |
| ≥2 | 44 (23.5) |
| Acid-fast bacilli
culture Mycobacterium tuberculosis positivity (n=173) | 4 (2.3) |
Imaging data
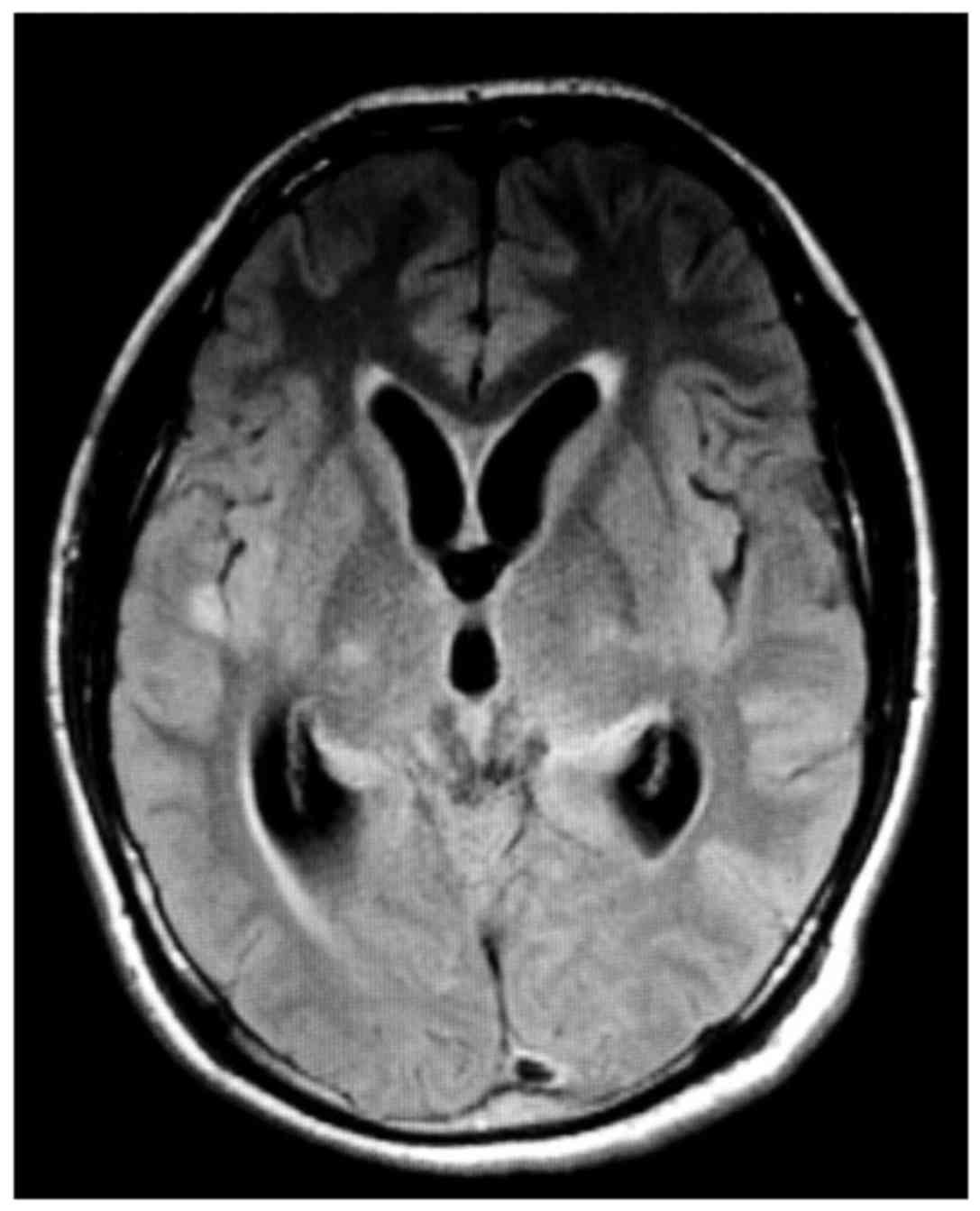
In the imaging data of TBM patients, longer T1 and
T2 signals were observed in the temporal lobe and thalamus
(Fig. 2). According to the image, a
large part of the brain nodules underwent meningeal enhancement.
Furthermore, the cerebral ventricle and associated vessels were
moderately expanded.
Treatment
Conventional 3HRZE/9HRZ treatment was administered
to all the patients as described previously (1,10), while
symptomatic and supportive treatment was provided for reducing
intracranial pressure, preventing arachnoid adhesions and reducing
the CSF protein and sugar content. Patients with severe brain edema
were administered glucocorticoid therapy. Following regular and
rational anti-TB treatment, as well as supportive treatment, these
patients achieved an improved clinical outcome, the improved
clinical outcome rate is 87.3%. In total, 7 cases presented
hydrocephalus, and the fatality rate was only 1.58% (3/189).
Prognosis
The comprehensive treatment of the disease resulted
in significant improvement in 145 cases, accounting for 76.7% of
cases. In general, the cases were followed-up for 3–12 months and
no recurrence was observed. There were 3 cases that succumbed to
the disease with deteriorated, while 12 cases presented severe
complications (6.5%; including focal neurological deficits,
disturbance of consciousness and personality changes) and 14 cases
were discharged automatically or transferred for other reasons are
unknown.
In addition, the fever reported in patients of the
current retrospective study was mostly medium (38–39°C) and high
(40–41°C), with only 43 cases (29.1%) presenting peak fever
temperature (37–38°C).
Discussion
According to a published study, the number of annual
incidence of TB in China is currently ~100 million, accounting for
14.3% of the global TB incidence and ranking as the second highest
incidence in the world (10). The
results of the ‘The fifth national tuberculosis epidemiological
survey in 2010’ (11) revealed that
the prevalence of TB in China has declined very slowly. In the
population aged ≥15 years, the TB prevalence rate was reduced from
466/100,000 in 2000 to 459/10 million in 2010 (4). In China, the TB infection rate was 4.5%
of the total population, and the incidence of TBM accounted for ~1%
of this rate.
TBM is a common infectious disease of the CNS, and
results in high disability and mortality rates among severe
infectious diseases. The main etiology of TBM is the infection with
the TB bacterium, which is transported through the blood and
directly infects the meninges, in which case TBM is a partial
performance of the whole body blood line of disseminated TB.
Another secondary consequence of TBM is CNS inflammation caused by
invading Mycobacterium tuberculosis, which usually involves
parts of body other than the lungs, including pleura, lymph nodes
and genitourinary tract (10).
The clinical inflammation course of TBM can be
divided into acute (≤14 days), sub-acute (15–30 days) and chronic
onset (≥31 days). The clinical manifestations of TBM are
non-characteristic, and common symptoms include headache (50–80%),
fever (60–95%), loss of appetite and weight loss (60–80%), and
vomiting (30–60%). Common signs are a stiff neck (40–80%), altered
mental status (30–60%), cranial nerve damage (30–50%), mental
abnormalities (10–30%), hemiplegia (10–20%), paraplegia (5–10%) and
seizures (5% in adults; 50% in children) (12).
The retrospective analysis of suspected TBM patients
conducted in the current study indicated that 82% of cases
presented with acute and sub-acute onset. This high rate of
diagnosis at the earlier stages of TBM may be associated with the
gradual increase in the concern of the population over their
health, as well as the increased awareness of TBM in recent
decades. In addition, the Department of Neurology in the Xiangya
Hospital of Central South University is the leading and
professional institute for TBM treatment in China, and therefore
may treat more patients with TBM symptoms (13). Patients with TBM often exhibit common
symptoms, including fever, headache, cough and TB prior to the
onset. It is widely considered that TBM patients commonly present
low-grade fever (2,10,11),
however, the fever reported in patients of the current
retrospective study was mostly medium and high, with only 43 cases
(29.1%) presenting peak fever temperature (37–38°C), which is not
consistent with previous studies. This inconsistency in
observations may be associated with the mutation of
Mycobacterium tuberculosis and the high reproduction of Rich
foci. In the present study, 138 (73.0%) cases had stiff neck, while
clinical fever was reported in 148 cases (78.3%), headache in 168
cases (89.2%) and vomiting in 128 patients (67.7%) (Table I). Therefore, in order to prevent
misdiagnosis, clinical symptoms (including headache and fever)
should be taken into account during TBM diagnosis.
TBM diagnosis is based primarily on CSF smear
detection or CSF culture of isolated Mycobacterium
tuberculosis. However, the positive rate of detection for the
smear and culture tests is low (14). CSF smears for acid-fast staining
etiological diagnosis is the most efficient method compared with
the traditional methods, however, the smear microscopy for
AFB-positive rate in the present study was 2.3%, which was lower
than the rates of 37 to 87% reported in the other studies (9,11,14).
Thwaites et al (15)
conducted Ziehl-Neelsen staining in CSF samples from 132 TBM
patients and reported 77 cases of AFB detection, with a detection
rate of up to 58%. The study observed that the detection of a large
quantity of CSF (>6 ml) was a direct method to discover the AFB
smear independent factors, while an average of 30-min microscopy
time and repeated testing helps to improve the detection rate
(15). In addition, the length of
the TBM course, CSF and neutrophils ratio, CSF lactate content, and
CSF and plasma glucose ratio are associated with the AFB detection
rate (15). Furthermore, a previous
meta-analysis indicated that nucleic acid amplification testing in
the diagnosis of TBM had a sensitivity and specificity of 56 and
98%, respectively (16). The small
amount of CSF samples in the present study may be the main reason
for the low rate of positive AFB observed.
It has been reported that >2/3 of TBM patients
have increased CSF pressure (220–500 mm H2O), therefore,
it's critical for the TBM diagnosis to exclude the other factors
that affect the CSF pressure, including mental stress, suffocation,
coughing, and increased chest and abdominal cavity pressure. In
addition, the intracranial pressure is always low in the presence
of sub-arachnoid adhesions or obstruction (16). In the present study, 66.3% of
patients exhibited increased intracranial pressure (>180 mm
H2O), however, a previous study demonstrated ~7.5%
patients with intracranial pressure of >100 mm H2O
(11). Total of 59 patients with
lower intracranial pressure than normal, and even 4 patients'
pressure could not be tested which considered with varying degrees
of high obstruction. It should be noted the intracranial
tuberculoma or abscess oppression, venous sinus thrombosis and
subarachnoid hemorrhage condition are observed when intracranial
pressure is high (>400 mm H2O). If the above higher
intracranial pressures (>400 mm H2O) occurred, the
doctor closely monitor these patients. The CSF in patients with TBM
in the acute phase commonly exhibit an increased number of
neutrophils, which are converted into hybrid cells (derived from
stem cells that have an important role in organogenesis, tissue
regeneration and cancer formation) in the subacute phase, and an
increased number of lymphocytes is detected in the tissue repair
stage (15,16). CSF cytological studies (15,16) have
demonstrated that the increased neutrophils in 20–40% patients were
usually changed to lymphocyte dominance in the subsequent 24–48 h.
In the present study, this neutrophilic predominance was present in
16.4% of patients on admission and changed into lymphocytic
predominance within a few days. However, all CSF cytology tests
demonstrated a few interference factors, and doctors should pay
more attention to distinguish from the purulent meningitis in the
clinic.
The CSF protein levels in TBM patients increased in
165 out of 189 cases (87.3%) in the present study, with ≥5 g/l in 5
patients (2.6%) considered to be due to spinal canal obstruction.
Compared with cryptococcal meningitis (15), CSF protein content in TBM was
increased to a greater extent, and protein levels of >2 g/l
suggested a greater possibility of TBM. In addition, another
important basis for the diagnosis of TBM was the fact that the CSF
glucose generally exhibited a moderate reduction. There were 153
out of the 189 patients (80.9%) who presented decreased glucose
level, which was similar to the findings of previous studies
(10,16). Although the retrospective analysis of
glucose content demonstrated reduction in 92 patients (48.7%), as
blood sugar and ion factors were not excluded, the absolute glucose
content of CSF in TBM in the present study was not fully
determined. However, the reasons for not excluding blood sugar and
ion factor in the present study included: The patients' condition
was mild or in the early stage, the patient exhibited alternative
underlying diseases and their respective long-term drug treatments
affected the laboratory markers, in intravenous saline or glucose
levels. However, in the present study, the atypical cases of CSF
caused due to the variability of Mycobacterium tuberculosis
were significantly increased. Consequently, too much dependence on
testing cell count, glucose and chloride changes for diagnosing TBM
typically results in misdiagnosis of TBM (16,17).
Furthermore, as demonstrated by the statistical results of the
present study, the positive rates of patients with TBM for PPD skin
test and PPD antibody tests were lower compared with the number of
negative rates, which may be due to the fact that the study
patients were adults, and these tests may have a higher positive
rate in children.
The performance of head computed tomography (CT) and
magnetic resonance imaging (MRI) in TBM patients is relatively
similar, although MRI is superior to CT, with the most important
change reflected in the asymmetric cisterna ambiens, Sylvian
cistern and the abnormal anatomy region on the brain (18). The degree of enhancement during MRI
cisternography is superior to that in CT, which may be associated
with meningeal infiltration (18).
MRI scanning can clearly demonstrate early-stage or small lesions,
as well as reflect the size and shape of the lesions and different
tissue components of the lesion, whereas CT is better at displaying
calcification (18). It has been
reported that positive rate of TBM in CT examination is as high as
80%, while the rate in MRI scans is even higher; thus, performing
CT or MRI examination is essential for the diagnosis of TBM
(11,15,16). In
the current study, 144 patients underwent head imaging studies, of
which 127 cases (88.3%) presented abnormal findings. The positive
detection rate in the present study was lower compared with that
reported in other studies (14,15), and
this discrepancy may be due to the following main reasons: i)
Patients were admitted at the early onset of the disease and
alterations in the brain had not yet developed; ii) patients did
not undergo head MRI or CT enhancement examination due to economic
or other problems; and iii) the reviewer did not have a clear
understanding to the TBM imaging changes. In the present study, it
is recommended that dynamically imaging examination should be
performed for TBM diagnosis. Imaging also identified patients with
hydrocephalus in 7 cases (4.86%), as observed by retrospective
analysis. Previous research has demonstrated that brain effusion
serves a certain role in TBM prognosis, commonly resulting in
poorer patient prognosis (18).
Long-term brain effusion leads to increased intracranial pressure,
which oppresses the nerve in the skull base and brain parenchyma,
resulting in facial paralysis, blindness, strabismus, mental
retardation, aphasia, paralysis or other consequences (11,16).
Thus, early detection and appropriate management of brain effusion
can improve the prognosis.
Clinically, the treatment of TBM usually involves
anti-TB therapy supplemented by symptomatic and supportive
treatment. Anti-TB treatment is required to comply with the
principle of early supervision, combined supervision, regular
supervision, adequate supervision and the full supervision
(11). During treatment, a
particular drug that is able to go through the blood-brain barrier
and reach higher blood concentration in the CSF should be selected
for TBM. For the initial treatment of patients with TBM mainly
includes the HREP and O/HRESO programs in China, consistent with
the strategies reported in other countries (1,11). If
the patient develops resistance, then second-line anti-TB drugs are
used, such as isoniazid aminosalicylate tablets and prothionamide
plus amikacin therapy (9,15).
During hospital admission in the present study, 6.3%
of the cases were stage I, 69.3% were stage II and 24.3% were stage
III (Table I). At this time, 37.6%
of the patients presented altered mental status, 17.5% had
convulsions, 48.1% presented blurred consciousness, and 15.3%
presented paresis/paraplegia. In addition, 23% of patients suffered
from an underlying disease, including gastritis, hepatitis,
diabetes, coronary heart disease, trauma, malignancy and
alcoholism. Particularly, factors such as diabetes, malignancy and
alcoholism are known to serve a role in TBM development; therefore,
they may also be considered to contribute to a poor prognosis
(19). However, the mortality rate
in the present study was 1.58%, which is lower in comparison with
other studies in China and abroad (11,15,16).
This may be associated with the following factors: i) The disease
duration of patients in the current study was mostly in the early
or middle period, and the symptoms were mild; ii) the patient
cohort included mostly young men, whose immune system was normal,
with only a few cases with human immunodeficiency virus infection
or other autoimmune diseases; and finally; and iii) once TBM was
considered, the patients were administered anti-TB therapy along
with symptomatic and supportive treatment. Furthermore, it should
be noted that certain patients that survived requested discharge
due to economic or other reasons.
Although the present study identified various
significant findings, there were also certain limitations. Firstly,
the most significant limitation is the TB-spot test, which was not
performed in all the patients. The TB-spot test is widely used
worldwide, and thus this test should be used for TB infection
diagnosis in future studies. Furthermore, the current study did not
include cases with other meningitis types, caused by the bacteria
or a virus. The present study is only a preliminary analysis of TBM
in Chinese patients, and in following studies, cases that involved
other meningitis types caused by bacteria will be used as the
control group, in order to clarify the diagnosis of TBM.
In the present study, 189 patients with confirmed or
presumed TBM reported over a 6-year period were analyzed
retrospectively. The present study examined the clinical,
laboratory and neuroradiological findings of patients with TBM, and
evaluated the prognostic significance of these variables. In
addition, the local epidemiological data were reported from these
cases. In conclusion, the pathogenesis and clinical manifestations
of TBM were variable. In patients with TBM, the typical alterations
of CSF and brain imaging are rarely identified, therefore, the
diagnosis for TBM must be made by combining these with with the
findings of laboratory tests, which may subsequently reduce the
misdiagnosis rate. Thus, the diagnosis of infection of the CNS
should be considered along with the clinical manifestations, CSF
examination and treatment effect. Comprehensively investigating
clinical manifestations, carefully reviewing radiology data and
examining microbiological and pathological evidence of TB are
extremely important for establishing the correct diagnosis. In
certain cases, a therapeutic trial of anti-TB therapy is required
in order to avoid delaying the treatment and adversely affecting
the patient's condition. Furthermore, the present data combined
with the British Infection Society guidelines (12) and the standardized clinical case
definition for TB, maybe used to develop effective and applicable
TBM treatment.
Acknowledgements
Not applicable.
Funding
The present study was funded by a grant from the
Fundamental Research Funds for Central Universities of Central
South University (grant no. 2011ssxt213).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ML, WW, QZ and XY analyzed and interpreted the
patient data. ML, YL, HY performed the experiments. ML and XY were
the major contributors in writing the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All of the patients provided their prior informed
written consent for the use of their samples in the present
study.
Consent for publication
All of the patients provided their prior written
informed consent for the present study.
Competing interests
The author declared no conflict of interests.
References
|
1
|
Chin H: Tuberculous meningitis: Diagnostic
and therapeutic challenges. Neurol Clin Pract. 4:199–205. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bartzatt R: Tuberculosis infections of the
central nervous system. Cent Nerv Syst Agents Med Chem. 11:321–327.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
World Health Organization, : Global
Tuberculosis Report 2013. http://www.who.int/tb/publications/global_report/en/index.htmlJanuary
10–2014
|
|
4
|
Christense AS, Andersen AB, Thomsen VO,
Andersen PH and Johansen JS: Tuberculous meningitis in Denmark: A
review of 50 cases. BMC Infect Dis. 11:472011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuchong C, Fubin C, Jianghan C, Fenglian
W, Nan X, Minghui Y, Yalin S and Zhizhong Z: Cryptoccosis in China
(1985–2010): Review of cases from Chinese population.
Mycopathologia. 173:329–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qu J, Zhou T, Zhong C, Deng R and Lü X:
Comparison of clinical features and prognostic factors in
HIV-negative adults with cryptococcal meningitis and tuberculous
meningitis: A retrospective study. BMC Infect Dis. 17:512017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
World Health Organization, : Treatment of
tuberculosis guidelines (4th edition). http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf
|
|
8
|
Prasad K and Singh MB: Corticosteroids for
managing tuberculous meningitis. Cochrane Database Syst Rev:
CD002244. 2008. View Article : Google Scholar
|
|
9
|
Marais S, Thwaites G, Schoeman JF, Török
ME, Misra UK, Prasad K, Donald PR, Wilkinson RJ and Marais BJ:
Tuberculous meningitis: A uniform case definition for use in
clinical research. Lancet Infect Dis. 10:803–812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lopez-Varela E and García-Basteiro AL:
World health organization guidenlines for children tuberculosis
management: Successes achieved and challenges ahead. Pediatr Infect
Dis J. 33:1310–1311. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Techinical Guidance Group of the Fifth
National TB Epi-demiological Survery and the Office of the Fifth
National TB Epidemiological Survey, : The Fifth National
Tuberculosis Epidemiological Survey in 2010. Chin J
Antituberculosis. 34:485–508. 2012.
|
|
12
|
Thwaites G, Fisher M, Hemingway C, Scott
G, Solomon T and Innes J; British Infection Society, : British
Infection Society guidelines for the diagnosis and treatment of
tuberculosis of the central nervous system in adults and children.
J Infect. 59:167–187. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang W, Hu Z and Li T: A case of
tuberculous meningitis with atypical multiple lesions. West Indian
Med J. 63:789–790. 2014.
|
|
14
|
Mathai A, Radhakrishnan VV, George SM and
Sarada C: A newer approach for the laboratory diagnosis of
tuberculous meningitis. Diagn Microbiol Infect Dis. 39:225–228.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thwaites GE, Chau TT and Farrar JJ:
Improving the bacteriological diagnosis of tuberculous meningitis.
J Clin Microbiol. 42:378–379. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pai M, Flores LL, Pai N, Hubbard A, Riley
LW and Colford JM Jr: Diagnostic accuracy of nucleic acid
amplification tests for tuberculous meningitis: A systematic review
and meta-analysis. Lancet Infect Dis. 3:633–643. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thwaites GE and Tran TH: Tuberculous
meningitis: Many questions, too few answers. Lancet Neurol.
4:160–170. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kalita J, Misra UK and Ranjan P:
Tuberculous meningitis with pulmonary miliary tuberculosis: A
clinicoradiological study. Neurol India. 52:194–196.
2004.PubMed/NCBI
|
|
19
|
Garg RK, Malhotra HS and Gupta R: Spinal
cord involvement in tuberculous meningitis. Spinal Cord.
53:649–657. 2015. View Article : Google Scholar : PubMed/NCBI
|