Introduction
Retinal detachment (RD) is an important cause of
blindness and visual impairment worldwide (1). There are four major types of RD,
including rhegmatogenous, exudative or serous, tractional and
combined tractional-rhegmatogenous RD (2). Among all risk factors, including severe
myopia, retinal tears, trauma, proliferative retinopathy and
cataract surgery complications, trauma accounts for 11% of cases in
adults affected by RD (3). RD refers
to a severe disorder in which the retina separates from the
underlying support tissue (4).
Extensive death of photoreceptor cells occurs once they are
physically separated from the retinal pigment epithelium (RPE) and
choroidal vessels, a source of oxygen and nourishment for
photoreceptor cells (5). In human RD
caused by trauma, early events of photoreceptor apoptosis have been
detected at 8 h, peaking at 48 h (6)
and dropping to a low level after 7 days (7). However, the irreversible loss of
photoreceptor cells may continue as long as RD persists. As a
result, although retinal surgery can successfully reattach the
retina (8), visual acuity is not
always restored.
Numerous histological and clinical studies have
highlighted the biological process involved in RD-induced
photoreceptor change. It has been demonstrated that
short-wavelength sensitive (S) cones are more susceptible to damage
compared with middle (M)-to long (L)-wavelength sensitive cones
(9). Furthermore, RD seems to
produce more damage to the cones than to the rods (10). In long-term RD, the majority of
surviving cones failed to be labeled by almost all reliable
markers, while rod photoreceptors continued to express most rod
markers as long as they were alive (11). This may explain a faster rod vision
rebuilding and specific color vision defects subsequent to
successful reattachment surgeries. Furthermore, subretinal gliosis
caused by Müller cells is involved in irreversible photoreceptor
loss following RD (12). The
functional outcome of proliferative vitreoretinopathy (PVR) is
determined by the balance between protective and destructive repair
mechanisms (13).
Although the pathophysiology of RD is better
understood at present, the disease management remains an issue.
Traumatic RD in pediatric patients is often found to be more
challenging compared with that in an adult (14), and the anatomical success of surgery
in children is much lower than in adults (15). Additional complications in
long-standing RD, including PVR, cataract and deprivation
amblyopia, may further aggravate the symptoms and lead to atrophy
(16,17). Overactivity of Müller cells may be
the cause of poor outcome in young patients undergoing RD medical
treatment. Therefore, the prevention of PVR is indispensable for
the success of RD repair (13). A
systematic search for preoperative PVR risk factors is essential to
provide valuable insight into a suitable, personalized therapeutic
strategy.
Immunohistochemistry is important in establishing
potential mechanisms and precise cellular responses following RD.
To date, only a limited number of studies on human tissue of
detached retina have been reported (7,9).
Therefore, the aim of the present study was to evaluate the
pathophysiology and reveal the underlying risk factors of a young
patient with long-term RD.
Materials and methods
Ethical statement
The present study adhered to the tenets of the
Declaration of Helsinki. All clinical records and information were
anonymized, and used only for research purposes. Written informed
consent was provided by the guardians on behalf of the minor
involved in the current study. The study procedures were approved
by the Institutional Ethics Committee in Renmin Hospital of Wuhan
University.
Patient information and tissue
collection
A 15-year-old male patient was admitted to Renmin
Hospital of Wuhan University (Wuhan, China) with left eye RD
secondary to blunt trauma in May 2015. The patient had been treated
for RD surgically >10 years earlier. However, it was unknown
whether the RD occurred during the event of trauma or afterwards.
The eye deteriorated to no light perception with undetectable
intraocular pressure (IOP), and gradually resulted in ocular globe
atrophy. By contrast, the corrected visual acuity of the patient's
right eye was 20/20 and the IOP was 18 mmHg (IOP normal range,
10–21 mmHg) (2). Anterior segment
evaluation and B-scan ultrasonography of the eyes were performed.
However, fundoscopy and optical coherence tomography examination on
the left eye could not be successfully accessed due to a
complicated cataract.
Since restoration of vision in the left eye was
unlikely, enucleation of his left atrophic globe was performed for
cosmetic purposes. Upon approval from the Ethics Committee, the
surgical procedure was conducted and the ocular tissue was
collected. In addition, normal ocular tissue from an age- and
sex-matched donor was obtained post mortem from the eye bank of our
hospital to serve as a control in the present study.
Histological techniques
The size of the eyeball, cornea and the diameter of
the optic nerve of the atrophic and post-mortem eyes were measured
by vernier caliper. Immunohistochemistry was then performed in the
tissue samples as described in a previous study (18). Briefly, the posterior eyecups were
fixed in fresh 4% paraformaldehyde dissolved in 0.1 M PBS, pH 7.4
at room temperature for 12 h. The retina tissue samples were
harvested with 6 mm trephine, followed by graded dehydration by the
sequential immersion of the retinas in 10 and 20% sucrose for 2 h
each time in PBS, pH 7.4 at 4°C and 30% sucrose overnight at 4°C.
The tissues were then embedded in optimal cutting temperature
compound (Tissue-Tek; Sakura Finetek USA, Inc., Torrance, CA, USA),
frozen in liquid nitrogen and vertically cut into 12-µm sections
using a Leica CM1900 cryostat (Leica Microsystems, Wetzlar,
Germany). Retina sections were incubated overnight at 4°C in PBS
containing 0.5% bovine serum albumin (BSA; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 0.2% Triton X-100 plus normal
donkey serum (Abcam, Cambridge, UK). The following day, this
blocking serum was replaced with primary antibodies diluted in 5%
BSA (containing 0.2% Triton X-100) and further incubated overnight
at 4°C. A goat polyclonal antibody against short-wave-sensitive
opsin 1 (OPN1SW; dilution, 1:200; cat. no. sc-14363; Santa Cruz
Biotechnology Inc., Dallas, TX, USA) was used to label S cones. In
addition, a mouse monoclonal antibody against glial fibrillary
acidic protein (GFAP; dilution, 1:500; cat. no. G3893;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used to label
retinal macroglia, including Müller cells and astrocytes. After
three washes with PBS for 5 min at room temperature, the sections
were incubated at 4°C for 2 h with the appropriate secondary
antibodies as follows: Cy3-conjugated donkey anti-goat IgG
(dilution, 1:200; cat. no. SA00009-3) and FITC-conjugated donkey
anti-mouse IgG (dilution, 1:200; cat. no. SA00003-9; both
ProteinTech Group, Inc., Chicago, IL, USA). Nuclear DNA was then
labelled with 4′,6-diamidino-2-phenylindole (DAPI). Images were
captured under a fluorescence microscope (magnification, ×400;
BX53; Olympus Corp., Tokyo, Japan).
Results
Patient eye characteristics
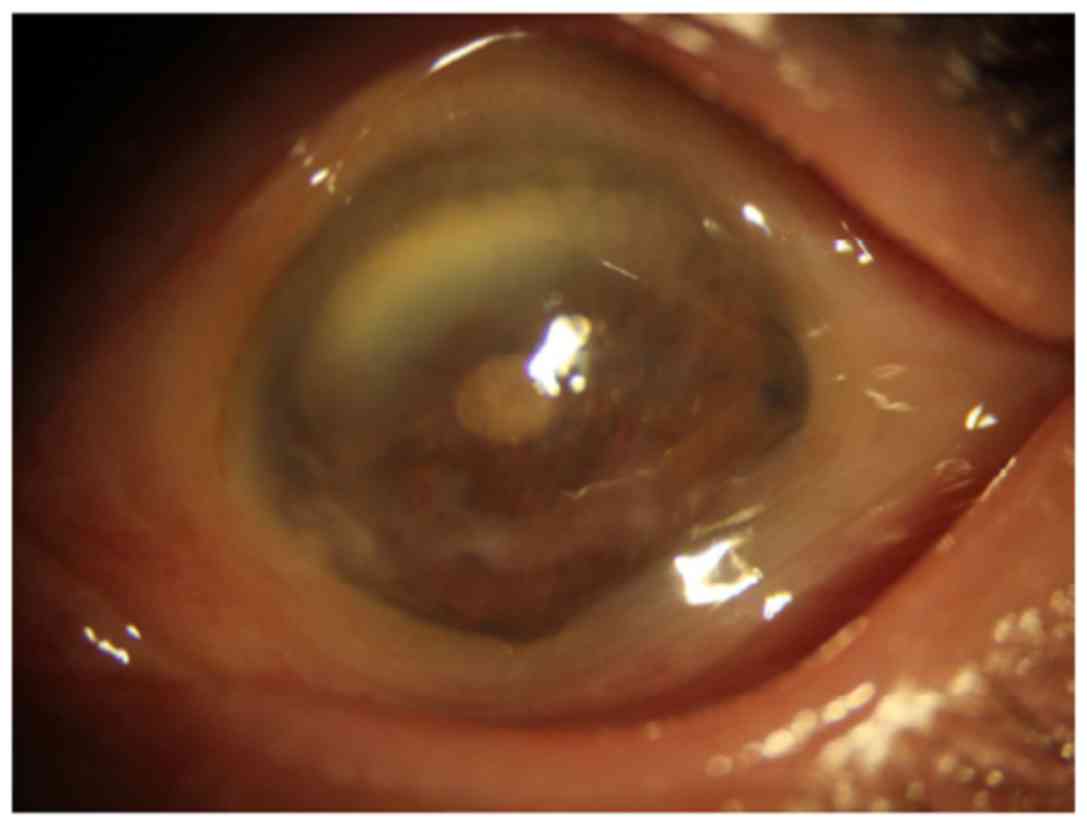
The patient's left eye revealed enophthalmos,
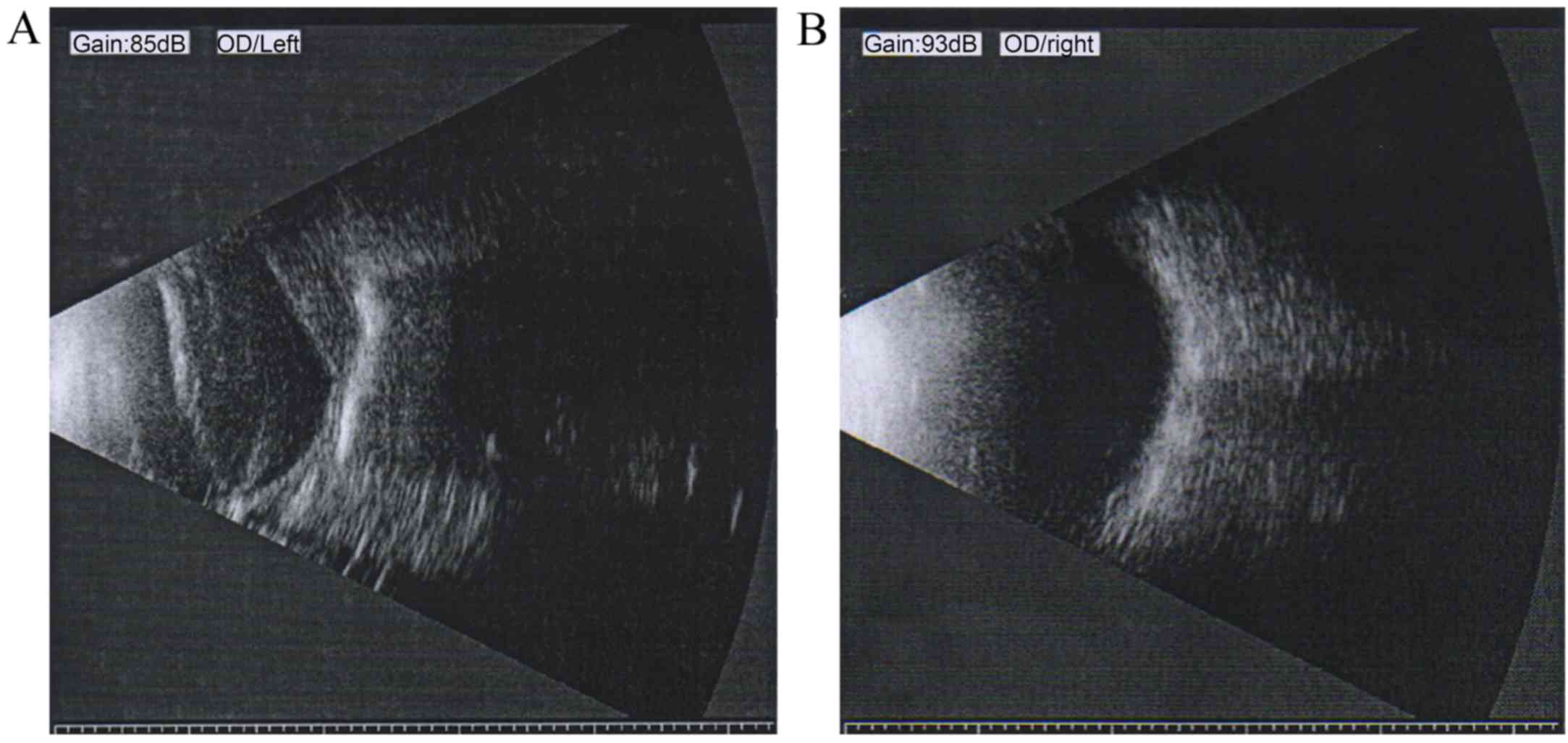
leukoplakia cornea, pupil distortion and lens opacity (Fig. 1). B-scan ultrasonography of the
atrophic eye demonstrated long-term RD, disorganized posterior
contents and irregular posterior contour (Fig. 2A). By contrast, B-scan
ultrasonography of the unaffected right eye of the patient showed a
normal appearance and clear reflection (Fig. 2B).
Eye measurements and
characteristics
The size of the eyeball and cornea, and the diameter
of the optic nerve of the atrophic eye and the matched post-mortem
eye were measured and compared (Table
I). In the atrophic eye, the vertical, horizontal and
anterior-posterior diameters of the eyeball were 19.0, 20.0 and
18.5 mm, respectively. The horizontal and vertical corneal
diameters were 10.0 and 8.5 mm, respectively, and the diameter of
the retrobulbar optic nerve was 2.5 mm. In the healthy ocular eye,
the vertical, horizontal and anterior-posterior diameters of the
eyeball were 24.0, 23.5 and 23.5 mm, respectively. The horizontal
and vertical corneal diameters were 11.5 and 11.0 mm, respectively,
and the diameter of the retrobulbar optic nerve was 3.0 mm. These
results demonstrated that the ocular globe from the patient was
atrophic and smaller in size compared with the size of the control
eye.
 | Table I.Measurements of atrophic and the age-
and sex-matched control eye. |
Table I.
Measurements of atrophic and the age-
and sex-matched control eye.
|
| Eyeball diameter
(mm) | Cornea diameter
(mm) |
|
|---|
|
|
|
|
|
|---|
| Samples | Vertical | Horizontal |
Anterior-posterior | Vertical | Horizontal | Retrobulbar optic
nerve diameter (mm) |
|---|
| Atrophic eye | 19.0 | 20.0 | 18.5 | 10.0 | 8.5 | 2.5 |
| Control eye | 24.0 | 23.5 | 23.5 | 11.5 | 11.0 | 3.0 |
When the atrophic eye was dissected vertically to
obtain an eyecup, opaque vitreous with pigment deposits was
observed, while the pale retina was fully detached from the
posterior eyecup. Proliferation was clearly present as yellow- or
white-colored linear strands in the atrophic eye. In comparison to
the atrophic eye, when the matched post mortem eye was dissected,
transparent gel-like vitreous humor and healthy retina was observed
without any PVR signs.
Immunofluorescence analysis
results
To further examine the RD-induced retina
degeneration, immunofluorescence analysis was conducted in the
retina tissue samples. The control retina had an intact laminar
structure and organized nuclear layers as demonstrated by DAPI
staining (Fig. 3A). In addition, the
ratio of the outer nuclear layer (ONL) to the inner nuclear layer
(INL) thickness was approximately 2:1, while the INL and ganglion
cell layer (GCL) were tightly packed. Conversely, the atrophic
retina displayed decreased thickness of ONL, distorted laminar
structure and unorganized nuclear layers (Fig. 3A). The ONL/INL thickness ratio was
approximately 1:2, while the INL and GCL were less tightly
packed.
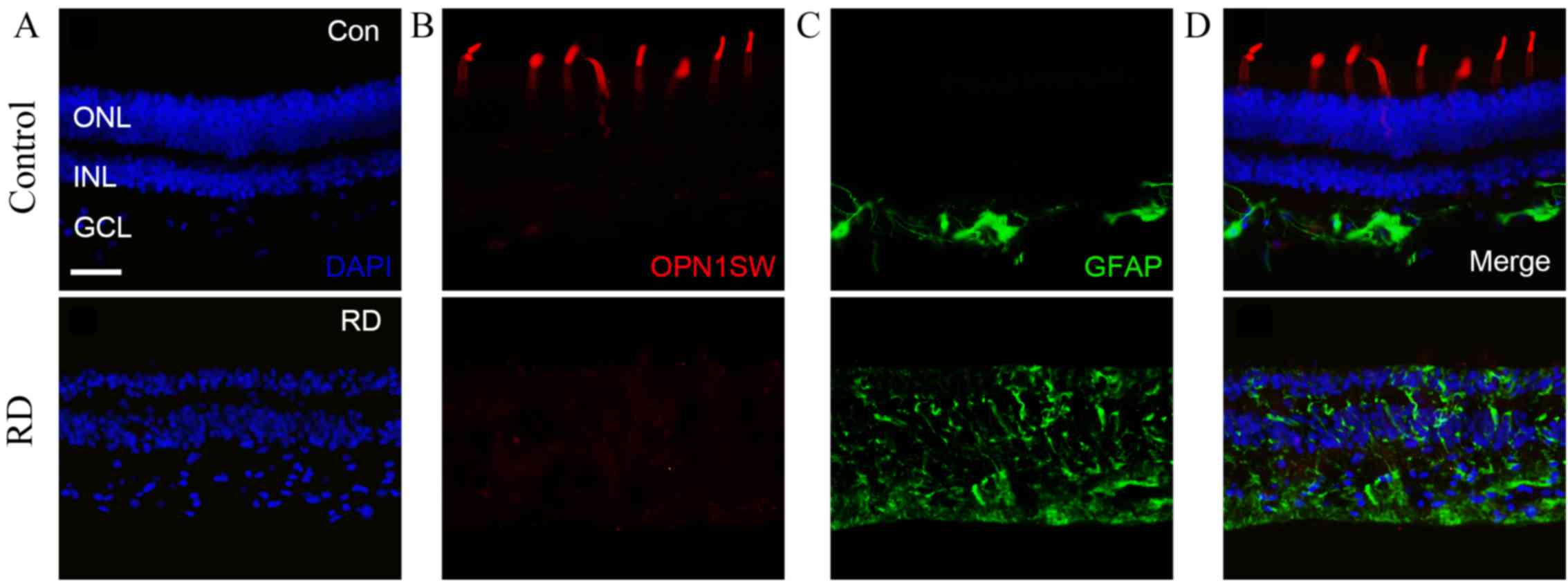 | Figure 3.Expression profiles of OPN1SW and GFAP
in the control and atrophic retina. (A) DAPI staining demonstrated
the organized and distorted retina in the control and atrophic
eyes. (B) OPN1SW staining revealed intact S cone outer segment in
the control, but absent expression in the atrophic retina. (C) GFAP
staining showed restricted signal of activated Müller cells in the
control eye, whereas expanded signal of activated Müller cells was
detected in the atrophic retina. (D) Merged images for the control
and atrophic retinal tissues. Scale bar=50 µm. CON, control; RD,
retinal detachment; ONL, outer nuclear layer; INL, inner nuclear
layer; GCL, ganglion cell layer; DAPI,
4′,6-diamidino-2-phenylindole; OPN1SW, short-wave-sensitive opsin
1; GFAP, glial fibrillary acidic protein. |
To identify changes of cones in the atrophic retina,
outer segments of S cones were stained with OPN1SW, as reported
previously (19). In the control
retina, outer segment of S cones were detected by dense OPN1SW
staining (Fig. 3B). However, the
OPN1SW signal in the atrophic retina was absent (Fig. 3B). Furthermore, GFAP staining was
performed to access the gliosis of Müller cells (20) in the atrophic retina. In the control
retina, sporadic GFAP staining that was likely due to astrocytes
appeared primarily in the inner retina, with compressed, thin and
spiky staining concentrated at the inner limiting membrane
(Fig. 3C). By contrast, in the
atrophic retina, evidently increased GFAP immunoactivity from
Müller cell processes was detected, suggesting that increased glial
cell reactivity was prominent over the entire atrophic retina
(Fig. 3C). Merged images (Fig. 3D) revealed the disruption of the
nuclear structure, loss of the out segment of S cone and the Müller
cell gliosis in the atrophic retina.
Discussion
In the present study, a case of atrophic human
retina caused by traumatic RD that occurred 10 years earlier was
reported. Immunohistochemical analysis of retinal sections revealed
disruption of the nuclear layer structure, loss of the S cone outer
segment and hypertrophic Müller cells in the atrophic tissue.
Combined with the findings of previous studies (10,11), the
current report provides further insight that may assist in the
examination of RD in children in clinical practice.
Photoreceptor cell death is the ultimate cause of
irreversible vision loss in RD. In previous studies, photoreceptor
cell death involving Fas-receptor activation and caspase-dependent
apoptosis has been widely investigated (21). Non-apoptotic types of cell death,
including autophagy and necrosis, have also been reported recently
(22,23). A photoreceptor shift from autophagy
to apoptosis is caused by photoreceptor-RPE separation in RD;
however, the underling mechanisms remain unknown (24). Other areas, such as the regulatory
role of mitochondria and the inflammation mechanisms during RD,
also warrant further investigation (5).
In the current study, OPN1SW and GFAP staining were
performed to highlight the outer segment of S cone and the gliosis
in atrophic retina. Previously, the loss of S cone and the
activation of glial cells have been reported in human retinal
detachment (4,7,9,24). The degeneration of the rod was not
part of the present study, but it has previously been described in
retinal detachment (9). Future work
may explore the changes of the rod and other types of cones,
including M and L cone, in long-term RD. In addition, other changes
in RD including synaptic terminals of photoreceptors, mitochondria
or various retinal neurons may be confirmed by future studies.
Findings may contribute to analysis of pathological changes in
long-term RD (2,3). The combined analysis and targeting of
photoreceptor death and Müller cell gliosis may facilitate the
design of neuroprotective strategies for photoreceptors following
RD.
PVR remains one of the most common causes of failed
RD surgery. Müller cells respond to various pathologic stimuli in
the retina, including retinitis pigmentosa, Leber congenital
amaurosis, ischemia, RD, glaucoma and diabetic retinopathy
(25–27). In addition, proliferating glia may
serve both beneficial and harmful roles in RD (28). Detachment of the retina activates
Müller cells to secrete cytokines and ATP, which do not only
protect the photoreceptors, but also promote inflammation, retinal
ischemia, cell proliferation and tissue remodeling (13,29).
Müller cell nuclei also produce intermediate filaments as a ‘track’
for their migration into the outer retina (12). This event, known as gliosis, perturbs
the cellular layers, particularly the ONL. In an experimental RD
model, the growth of Müller cells formed a fibrotic layer into the
subretinal space, which completely inhibited the regeneration of
the cone outer segment (30).
Although Müller glial cells have the potential to proliferate and
generate neurons, these responses are insufficient to repair a
damaged retina (31).
The level or the ‘beneficial window’ of Müller cell
activation may vary between pediatric and adult patients, thus
requiring different management strategies. In a human study,
retinas of elderly subjects demonstrated higher GFAP
immunoreactivity and increased glial filaments when compared with
the younger subjects (32).
Furthermore, exacerbated glial response was observed in the aged
mouse hippocampus following controlled cortical impact injury
(33). In an experimental model of
ischemia/reperfusion, retinas of old rats were more susceptible to
cell death induced by secondary glial mechanisms in comparison with
those of the younger rats (34).
Thus, age is an important factor to be considered in the subretinal
gliosis following RD.
The present study reported interesting clinical
observations and immunohistochemical evaluation of RD tissue in a
young male patient. The loss of the photoreceptor nucleus was
observed in the atrophic eye, as well as the expansion of the INL;
as a result, the ONL/INL ratio was reduced. The INL expansion can
be attributed to not only the hypertrophy of Müller cell, but also
the interaction between Müller cell processes and retinal neurons.
As Müller cells may use intermediate filaments as a ‘track’ for
migration, retinal neurons may possibly be stretched and migrate
along the same ‘track’. Further studies on the interaction between
retinal neurons and Müller cells at the molecular and cellular
levels may provide valuable insight into preventing photoreceptor
degeneration and vision loss.
In conclusion, the present study provided an unusual
opportunity to examine the characteristics of long-term traumatic
RD in a young patient. It is suggested that greater cooperation
between basic researchers and clinicians is required to match the
different clinical scenarios, including age and etiology, with the
biological markers of RD. Therapies to prolong photoreceptor
survival may be greatly beneficial in improving the visual
outcomes. Medicine treatment to prevent reactive gliosis would also
be highly desirable in children with RD.
Acknowledgements
The authors would like to thank Qinqin Deng for the
critical review of the manuscript.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81470628).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZC, YX and YS conceived and designed the
experiments. SL and YC performed the experiments. SL and YS
analyzed the data and prepared the manuscript. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The study procedures were approved by the
Institutional Ethics Committee in Renmin Hospital of Wuhan
University. Written informed consent was provided by the guardians
on behalf of the minor involved in the current study. The present
study adhered to the tenets of the Declaration of Helsinki.
Patient consent for publication
Written informed consent for the publication of any
associated data and accompanying images was obtained from the
guardians on behalf of the minor involved in the current study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Qi Y, Zhang FY, Peng GH, Zhu Y, Wan GM,
Wang WZ, Ma J and Ren SJ: Characteristics and visual outcomes of
patients hospitalized for ocular trauma in central China:
2006–2011. Int J Ophthalmol. 8:162–168. 2015.PubMed/NCBI
|
|
2
|
Ghazi N and Green WR: Pathology and
pathogenesis of retinal detachment. Eye (Lond). 16:411–421. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haimann MH, Burton TC and Brown CK:
Epidemiology of retinal detachment. Arch Ophthalmol. 100:289–292.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lo AC, Woo TT, Wong RL and Wong D:
Apoptosis and other cell death mechanisms after retinal detachment:
Implications for photoreceptor rescue. Ophthalmologica. 226 Suppl
1:S10–S17. 2011. View Article : Google Scholar
|
|
5
|
Murakami Y, Notomi S, Hisatomi T, Nakazawa
T, Ishibashi T, Miller JW and Vavvas DG: Photoreceptor cell death
and rescue in retinal detachment and degenerations. Prog Retin Eye
Res. 37:114–140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang CJ, Lai WW, Edward DP and Tso MO:
Apoptotic photoreceptor cell death after traumatic retinal
detachment in humans. Arch Ophthalmol. 113:880–886. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arroyo JG, Yang L, Bula D and Chen DF:
Photoreceptor apoptosis in human retinal detachment. Am J
Ophthalmol. 139:605–610. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brazitikos PD: The expanding role of
primary pars plana vitrectomy in the treatment of rhegmatogenous
noncomplicated retinal detachment. Semin Ophthalmol. 15:65–77.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nork TM, Millecchia LL, Strickland BD,
Linberg JV and Chao GM: Selective loss of blue cones and rods in
human retinal detachment. Arch Ophthalmol. 113:1066–1073. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Anderson DH, Guerin CJ, Erickson PA, Stern
WH and Fisher SK: Morphological recovery in the reattached retina.
Invest Ophthalmol Vis Sci. 27:168–183. 1986.PubMed/NCBI
|
|
11
|
Rex TS, Fariss RN, Lewis GP, Linberg KA,
Sokal I and Fisher SK: A survey of molecular expression by
photoreceptors after experimental retinal detachment. Invest
Ophthalmol Vis Sci. 43:1234–1247. 2002.PubMed/NCBI
|
|
12
|
Lewis GP, Chapin EA, Luna G, Linberg KA
and Fisher SK: The fate of Muller's glia following experimental
retinal detachment: Nuclear migration, cell division, and
subretinal glial scar formation. Mol Vis. 16:1361–1372.
2010.PubMed/NCBI
|
|
13
|
Garweg JG, Tappeiner C and Halberstadt M:
Pathophysiology of proliferative vitreoretinopathy in retinal
detachment. Surv Ophthalmol. 58:321–329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wenick AS and Barañano DE: Evaluation and
management of pediatric rhegmatogenous retinal detachment. Saudi J
Ophthalmol. 26:255–263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soliman MM and Macky TA: Pediatric
rhegmatogenous retinal detachment. Int Ophthalmol Clin. 51:147–171.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Häring G and Wiechens B: Long-term results
after scleral buckling surgery in uncomplicated juvenile retinal
detachment without proliferative vitreoretinopathy. Retina.
18:501–505. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moisseiev J, Vidne O and Treister G:
Vitrectomy and silicone oil injection in pediatric patients.
Retina. 18:221–227. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen YY, Liu SL, Hu DP, Xing YQ and Shen
Y: N-methyl-N-nitrosourea-induced retinal degeneration in mice. Exp
Eye Res. 121:102–113. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gaillard F, Kuny S and Sauvé Y:
Topographic arrangement of S-cone photoreceptors in the retina of
the diurnal Nile grass rat (Arvicanthis niloticus). Invest
Ophthalmol Vis Sci. 50:5426–5434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lewis GP, Erickson PA, Kaska DD and Fisher
SK: An immunocytochemical comparison of Muller cells and astrocytes
in the cat retina. Exp Eye Res. 47:839–853. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Trichonas G, Murakami Y, Thanos A,
Morizane Y, Kayama M, Debouck CM, Hisatomi T, Miller JW and Vavvas
DG: Receptor interacting protein kinases mediate retinal
detachment-induced photoreceptor necrosis and compensate for
inhibition of apoptosis. Proc Natl Acad Sci USA. 107:21695–21700.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dong K, Zhu H, Song Z, Gong Y, Wang F,
Wang W, Zheng Z, Yu Z, Gu Q, Xu X and Sun X: Necrostatin-1 protects
photoreceptors from cell death and improves functional outcome
after experimental retinal detachment. Am J Pathol. 181:1634–1641.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Besirli CG, Chinskey ND, Zheng QD and
Zacks DN: Autophagy activation in the injured photoreceptor
inhibits fas-mediated apoptosis. Invest Ophthalmol Vis Sci.
52:4193–4199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chinskey ND, Zheng QD and Zacks DN:
Control of photoreceptor autophagy after retinal detachment: The
switch from survival to death. Invest Ophthalmol Vis Sci.
55:688–695. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bringmann A, Iandiev I, Pannicke T, Wurm
A, Hollborn M, Wiedemann P, Osborne NN and Reichenbach A: Cellular
signaling and factors involved in Muller cell gliosis:
Neuroprotective and detrimental effects. Prog Retin Eye Res.
28:423–451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Singh RK, Kolandaivelu S and Ramamurthy V:
Early alteration of retinal neurons in Aipl1-/-animals. Invest
Ophthalmol Vis Sci. 55:3081–3092. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jacobson SG and Cideciyan AV: Treatment
possibilities for retinitis pigmentosa. N Engl J Med.
363:1669–1671. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bringmann A, Pannicke T, Grosche J,
Francke M, Wiedemann P, Skatchkov SN, Osborne NN and Reichenbach A:
Müller cells in the healthy and diseased retina. Prog Retin Eye
Res. 25:397–424. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reichenbach A and Bringmann A: New
functions of Müller cells. Glia. 61:651–678. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lewis GP and Fisher SK: Müller cell
outgrowth after retinal detachment: Association with cone
photoreceptors. Invest Ophthalmol Vis Sci. 41:1542–1545.
2000.PubMed/NCBI
|
|
31
|
Goldman D: Müller glial cell reprogramming
and retina regeneration. Nat Rev Neurosci. 15:431–442. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ramirez JM, Ramirez AI, Salazar JJ, de Hoz
R and Trivino A: Changes of astrocytes in retinal ageing and
age-related macular degeneration. Exp Eye Res. 73:601–615. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sandhir R, Onyszchuk G and Berman NE:
Exacerbated glial response in the aged mouse hippocampus following
controlled cortical impact injury. Exp Neurol. 213:372–380. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim KY, Ju WK and Neufeld AH: Neuronal
susceptibility to damage: Comparison of the retinas of young, old
and old/caloric restricted rats before and after transient
ischemia. Neurobiol Aging. 25:491–500. 2004. View Article : Google Scholar : PubMed/NCBI
|