Introduction
Drug addiction is a costly and disabling condition,
which arises from the combined effects of genetic and environmental
interactions. Addicts are characterized by uncontrollable
drug-taking and drug-seeking behaviors, and have a high likelihood
of relapse for an extensive period after the cessation of drug use
(1). The number of heroin addicts
worldwide has been increasing substantially, and the rate of heroin
users aged >12 years increased from 0.16% between 2002 and 2004
to 0.26% between 2011 and 2013 in the United States (2). Recently, there has been a marked
increase in the use of a novel drug in China, termed
methamphetamine (METH), and at the end of 2012, there were ~0.41
million registered METH addicts nationwide, accounting for 23% of
all drug addicts (3). The rate of
METH use is second only to the rate of heroin use (3). Both heroin and METH addictions may
cause a number of social problems, including the spread of human
immunodeficiency virus (4).
Opioid receptors are important in the development
and maintenance of addiction, dysphoria and reward mechanisms in
the brain (5). Increased methylation
of the µ1 opioid receptor promoter has been identified in the
lymphocytes of former heroin users on methadone maintenance
(6). OPRK1 encodes the κ1 opioid
receptor, which is a member of the opioid family. Genetic
polymorphisms in OPRK1 have been associated with alcohol dependence
(7,8), body weight and opioid withdrawal
symptoms (8).
Epigenetics is defined as the investigation of
heritable changes in gene transcription and phenotypic alterations
that are independent of DNA sequence changes (9). DNA methylation is among the most
commonly studied epigenetic mechanisms (10), and is typically represented by
covalent modifications at the 5-position of cytosine to form
5-methylcytosine (11–13). More recently, several studies have
indicated an epigenetic role in molecular processes in the brain
such as the protein kinase A CREB pathway and calcium-dependent
phosphorylation cascades, which may lead to an addiction to
psychostimulants (14–18). In addition, a previous study
demonstrated that methylation of brain-derived neurotrophic factor
was correlated with heroin and METH addiction (19), suggesting that the blockade of drug
effects on epigenetic markers may be useful in the treatment of
drug addiction. The aim of the present study was to investigate the
potential association between OPRK1 promoter methylation and drug
addiction.
Materials and methods
Subjects and clinical data
A total of 60 drug users including 30 heroin (mean
age, 30.90±0.97 years; 50:50 males:females) and 30 METH addicts
(mean age, 31.03±0.99; 50:50 males:females) were recruited from the
Ningbo Addiction Research and Treatment Center (Ningbo, China) from
June 2012 to June 2013. The control group consisted of 52
individuals that exhibited no significant differences in age or
gender (mean age, 30.90±0.73 years; 27:25 males:females), who were
recruited from Ningbo Blood Bank. Patients with drug addictions
were diagnosed according to the diagnostic and statistical manual
of mental disorders, fourth edition (DSM-IV) (20). Individuals who had a history of
psychiatric disease, severe disease, aged <18 years or >65
years were excluded. All controls were free from any nervous system
diseases and had no history of drug addiction. In addition, the
Profile of Mood State (POMS) interview records of male patients
were collected. The present study was approved by the Ethics
Committees of Ningbo University (Ningbo, China) and Ningbo
Addiction Research and Treatment Center, and written informed
consent was obtained from all participants.
DNA methylation assay
DNA extraction and quality tests were performed as
described previously (21). The
level of DNA methylation was measured using bisulfite
pyrosequencing technology which combined sodium bisulfite DNA
conversion chemistry (EZ DNA Methylation-Gold™ kit), polymerase
chain reaction (PCR) amplification (Zymo Taq™ PCR PreMix; both from
Zymo Research Corporation, Irvine, CA, USA) and sequencing by
synthesis assay (Pyromark Gold Q24 Reagents; Qiagen, Hilden,
Germany) as previously described (22). A fragment containing 7 CpG sites from
OPRK1 promoter was used to represent the DNA methylation of
OPRK1 promoter. Primers for PCR were designed PyroMark Assay
Design software, version 2.0 as described previously (Qiagen)
(23). Each PCR reaction consisted
of 10 µl 2×PCR PreMix (Zymo Taq™ PCR PreMix), 0.5 µl forward primer
(10 µM), 0.5 µl reversed primer (10 µM), 1 µl template DNA, and 8
µl water. PCR was completed as follows, for 45 cycles; 95°C for 30
sec, 58°C for 40 sec and 72°C for 50 sec, followed by 72°C for 7
min. The sequences used for target gene (OPRK1)
amplification were 5′-biotin-TTAGTATTTAAGAGGAAAAGGGAAAGTTGT-3′ for
the forward primer and 5′-CCCCCATCATAACTAAAAATCT-3′ for the reverse
primer, and 5′-GGGAAAGTTGTTGGG-3′ for the sequencing primer for
pyrosequencing. The methylation level was obtained directly via
PyroMark Assay Design software 2.0.
Construction of recombinant
plasmids
One long fragment (OPRK1 L) and one short fragment
(OPRK1 S) from the OPRK1 promoter region were selected for a
luciferase reporter gene assay (24). The OPRK1 L fragment contained the
pyrosequenced sequence, while the OPRK1 S fragment did not. The
following primers were used to amplify OPRK1 L: Forward,
5′-CGGGGTACCGGCCGGTGCCTAGAATT-3′ and reverse,
5′-CTAGCTAGCGCCTGACCCTCACTCCCT-3′. The following primers were used
to amplify OPRK1 S: Forward, 5′-CGGGGTACCGGCCGGTGCCTAGAATT-3′ and
reverse, 5′-CTAGCTAGCCAAGCCCACGACGAACAC-3′. The PCR reaction was
performed using Taq DNA Polymerase TQ2100 (Omega Bio-Tek, Inc.,
Norcross, GA, USA) according to manufacturer's instructions. PCR
conditions used were as follows: 95°C for 10 min and then 35 cycles
of 98°C for 10 sec, 65°C for 15 sec and 72°C for 1 min; followed by
a further 10 min extension at 72°C. The pGL3-Basic vector (Promega
Corporation, Madison, WI, USA) lacked the eukaryotic promoter and
enhancer sequence that allowed maximum flexibility in cloning
putative regulatory sequences. A gel extraction kit (Omega Bio-Tek,
Inc.) was used to gel purify the PCR products according to the
manufacturer's protocol, which were subsequently quantified using a
Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Then the purified PCR products were digested
with NheI (5′-G^CTAGC-3′) and Kpn1 (5′-GGTAC^C-3′;
New England Biolabs, Inc., Ipswich, MA, USA) and cloned into a
pGL3-Basic vector, which had luc+ gene using a DNA ligation
kit (Takara Bio, Inc., Otsu, Japan) following the manufacturer's
protocol. The total volume for each double digestion reaction was
50 µl including 5 µl buffer, 1 µl NheI (20,000 U/ml), 1 µl
Kpn1 (20,000 U/ml), 30 µl PCR product or 5 µl pGL3 plasmid
DNA (as the control) and water up to 50 µl. The double digestion
was performed in 37°C water bath for 15 min. All the primers were
synthesized by Sangon Biotech Co., Ltd. (Shanghai, China).
Luciferase reporter gene assay
Human HEK293T cells (American Type Culture
Collection, Manassas, VA, USA) were transfected with recombinant
plasmids and used in the luciferase reporter gene assay. The
protocol was performed as described previously (25).
Statistical analysis
Statistical analysis was performed using PASW
statistics 18.0 software (SPSS, Inc., Chicago, IL, USA). The data
were presented as mean ± standard deviation. The mean values of two
groups were compared using a Student's t-test. The Pearson's
correlation test was used to determine potential associations
between OPRK1 promoter methylation and the length and frequency of
drug use in male patients. P<0.05 was considered to indicate a
statistically significant difference.
Results
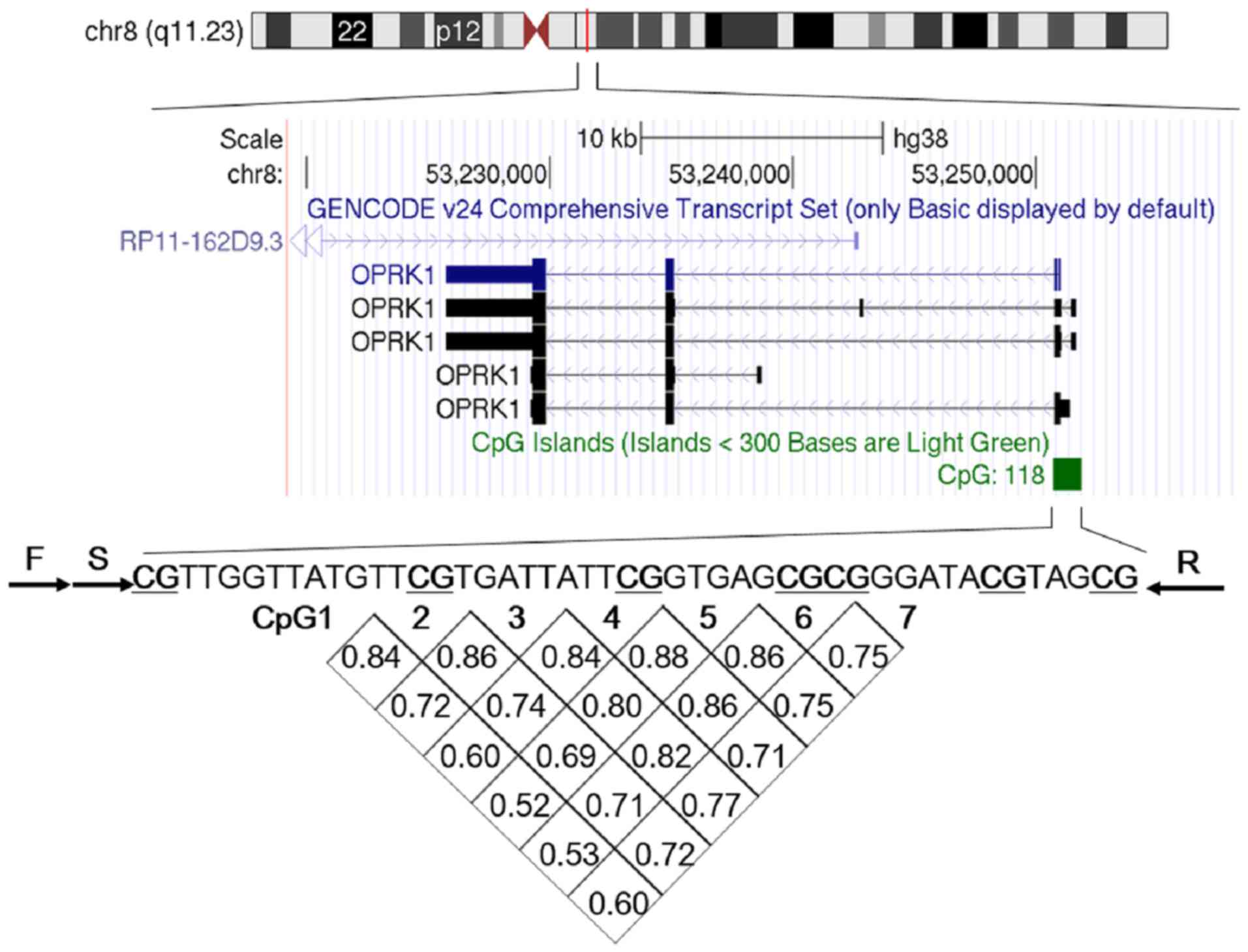
Association of 7 CpG sites
A fragment from the OPRK1 promoter containing seven
5′-C-phosphate-G-3′ (CpG) sites was bisulphite pyrosequenced to
detect the level of OPRK1 promoter methylation, as depicted in
Fig. 1. Within these seven sites,
methylation of a given site was significantly correlated with
methylation of any other site (r>0.50, P<0.05; Fig. 1). Therefore, the average level of
CpG1-7 methylation was used for subsequent analyses in the present
study.
DNA methylation analysis of the
different groups
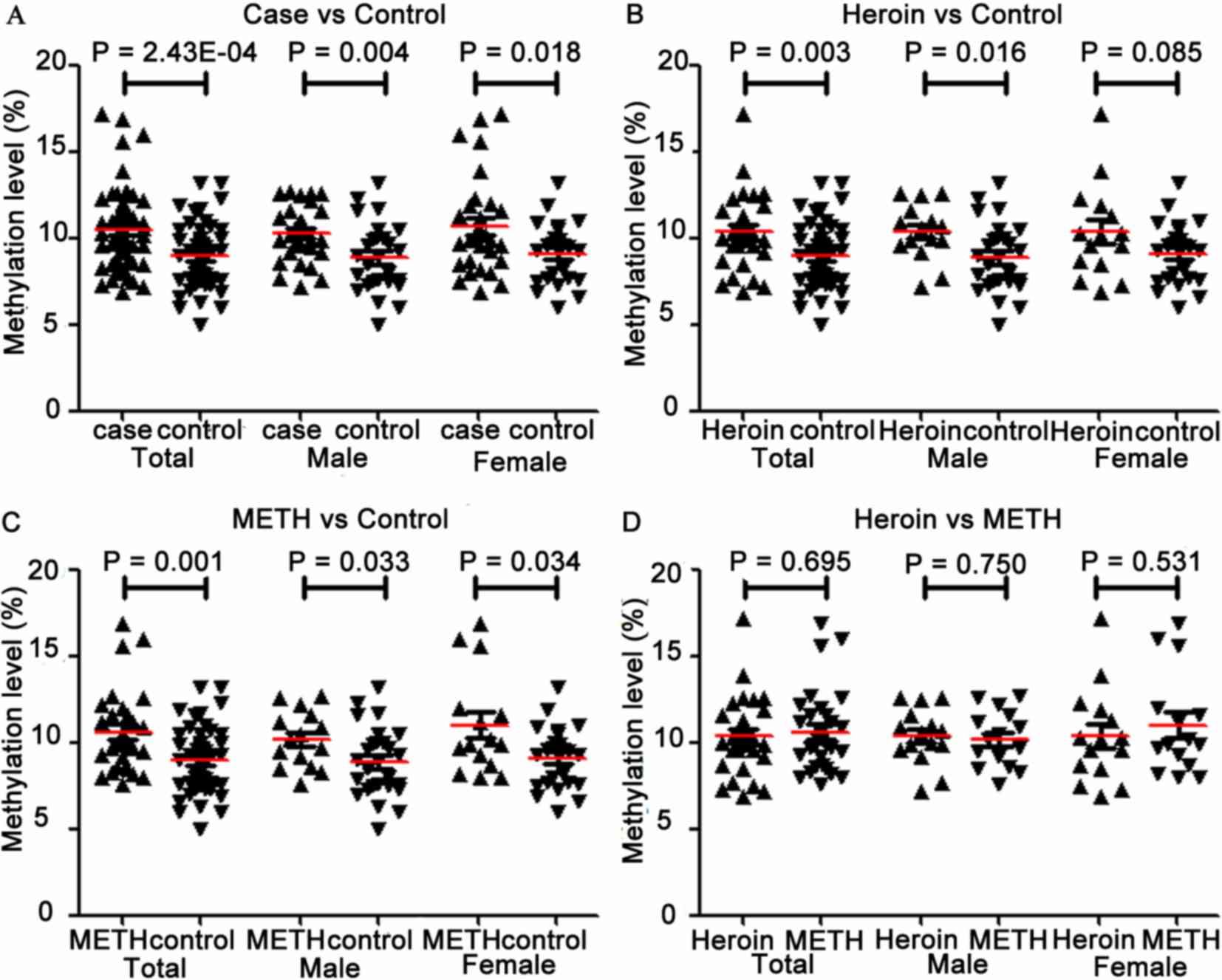
As depicted in Table
I and Fig. 2A, OPRK1 promoter
methylation was significantly increased in addicts compared with
control subjects, and this was also confirmed in subgroup
association tests based on gender (total, P=2.43×10−4;
male, P=0.004; female, P=0.018). Furthermore, increased levels of
methylation were observed in the heroin and METH groups when
compared with controls (heroin, P=0.003; METH, P=0.001; Table I and Fig.
2B and C). Similar trends were observed in the subgroup
comparisons based on gender in each drug group (for heroin: male,
P=0.016 and female, P=0.085; for METH: male, P=0.033 and female,
P=0.034; Table I and Fig. 2B and C). The non-significant
association between OPRK1 methylation and heroin addiction in
females may be due to a small sample size (n=30). OPRK1 promoter
methylation did not differ significantly between heroin and METH
addicts, which was confirmed in gender subgroup comparisons (all
P>0.5; Table I and Fig. 2D).
 | Table I.Comparisons of OPRK1 promoter
methylation levels among different groups. |
Table I.
Comparisons of OPRK1 promoter
methylation levels among different groups.
|
| P-value |
|---|
|
|
|
|---|
| Groups | CpG1 | CpG2 | CpG3 | CpG4 | CpG5 | CpG6 | CpG7 | Average |
|---|
| Cases vs. controls
(total) | 0.611 | 0.004a | 0.005a |
1.65×10-7a |
3.04×10-5a |
5.51×10-5a | 0.025a |
2.43×10-4a |
|
Male | 0.569 | 0.016a | 0.013a |
4.93×10-5a | 0.004a | 0.003a | 0.120 | 0.004a |
|
Female | 0.895 | 0.073 | 0.115 | 0.001 | 0.003a | 0.003a | 0.112 | 0.018a |
| Heroin vs. controls
(total) | 0.383 | 0.038 | 0.056 |
8.06×10-7a | 0.001a | 0.002a | 0.087 | 0.003a |
|
Male | 0.341 | 0.074 | 0.080 |
5.12×10-5a | 0.022a | 0.011a | 0.136 | 0.016a |
|
Female | 0.772 | 0.336 | 0.351 | 0.004a | 0.029a | 0.114a | 0.354 | 0.085 |
| METH vs. controls
(total) | 0.966 | 0.004a | 0.005a |
2.32×10-4a |
1.10×10-4a |
6.59×10-5a | 0.039a | 0.001a |
|
Male | 0.959 | 0.040a | 0.034a | 0.006a | 0.015a | 0.034a | 0.278 | 0.033a |
|
Female | 0.944 | 0.047a | 0.102 | 0.009a | 0.003 | 0.005a | 0.070 | 0.034a |
| Heroin vs. METH
(total) | 0.404 | 0.545 | 0.429 | 0.704 | 0.506 | 0.597 | 0.679 | 0.695 |
|
Male | 0.373 | 0.832 | 0.706 | 0.173 | 0.919 | 0.695 | 0.834 | 0.750 |
|
Female | 0.739 | 0.569 | 0.498 | 0.654 | 0.478 | 0.440 | 0.478 | 0.531 |
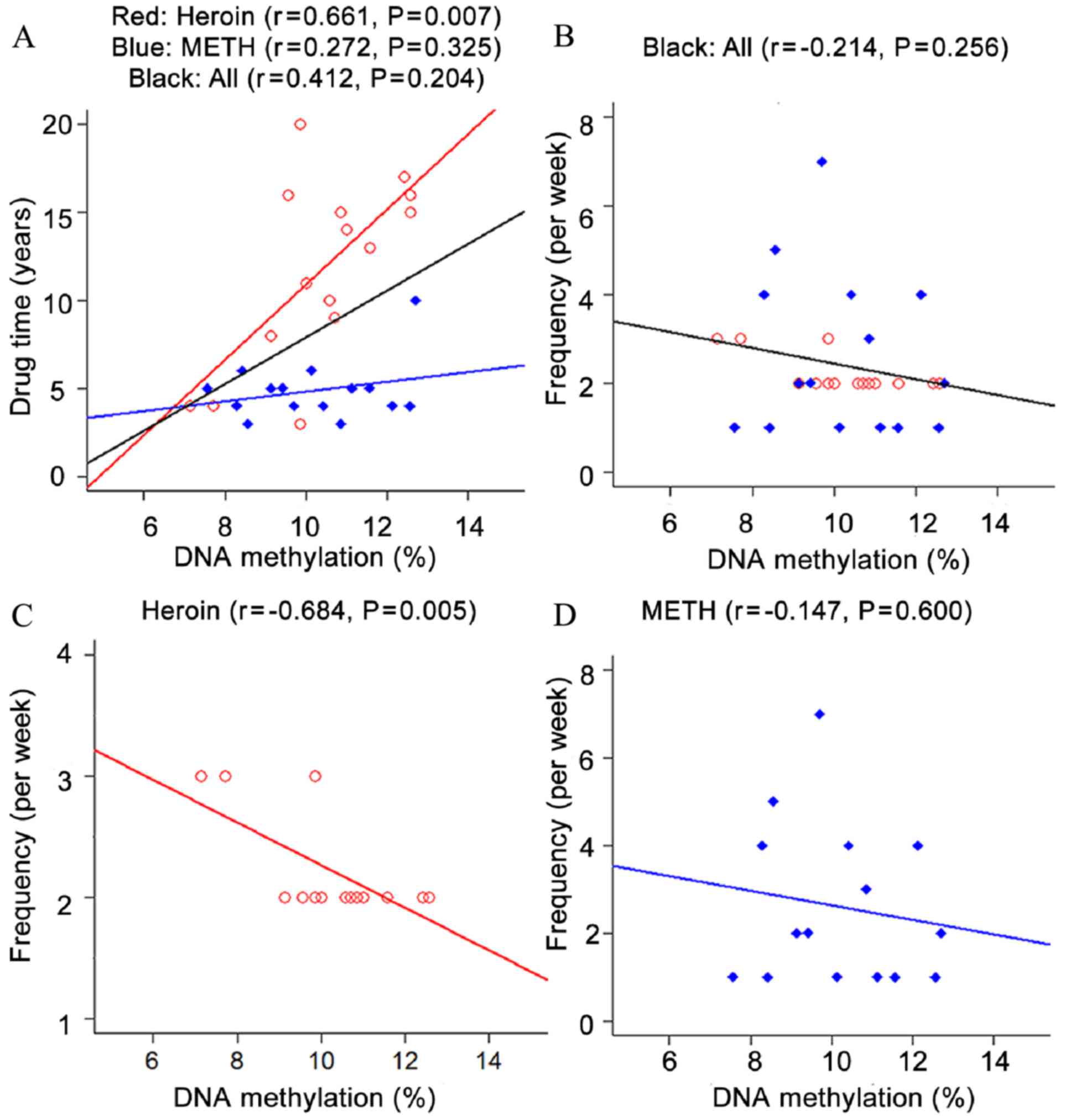
Correlation analysis between DNA
methylation and pheno-typic indices in males
Correlation tests between OPRK1 promoter methylation
and phenotypic indices in male subjects were also performed in the
present study. The length and frequency of drug use were selected
as relevant factors in assessing the situation of addicts. The
results indicated that OPRK1 promoter methylation was significantly
associated with the length and frequency of drug use in male heroin
addicts (length: r=0.661, P=0.007; frequency: r=−0.684, P=0.005;
Fig. 3). No significant association
was observed between the phenotypes, including length, frequency
and OPRK1 methylation in METH addicts in addition to total addicts
(P>0.05; Fig. 3).
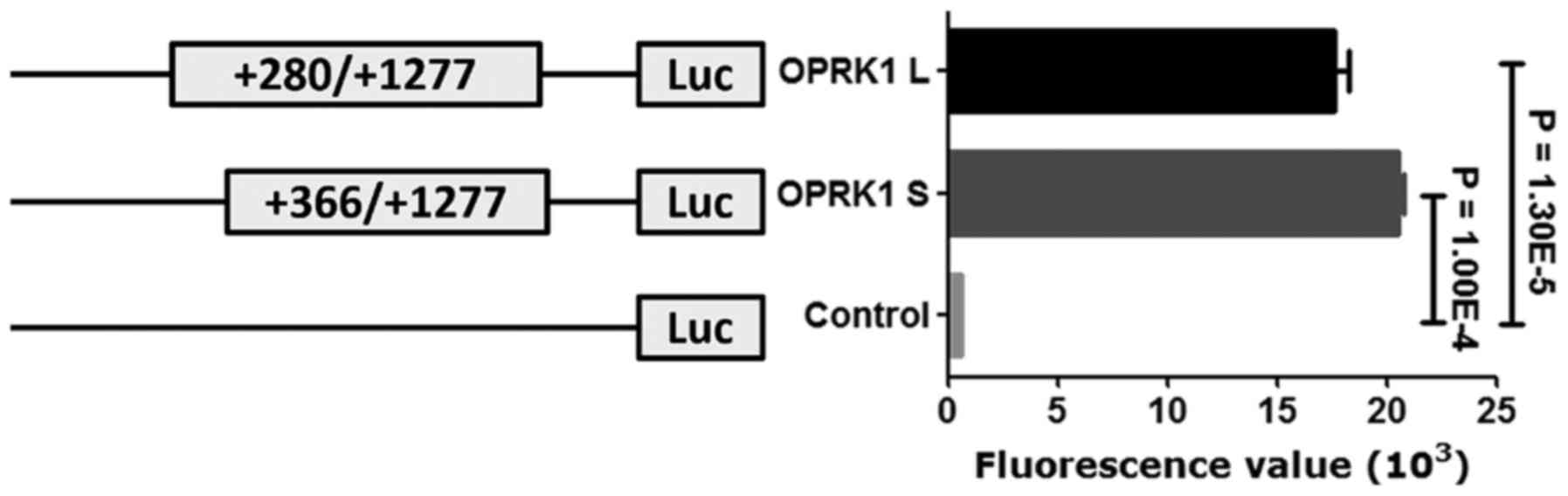
Functional analysis of target
fragment
Furthermore, a subsequent luciferase reporter gene
assay demonstrated that both two OPRK1 promoter fragments (OPRK1 L
and OPRK1 S) significantly enhanced luciferase gene activity when
compared with a control construct (OPRK1 L vs. OPRK1 S, fold
change=0.86, P=0.013; OPRK1 L vs. control, fold change=32.19,
P=1.30E-5; OPRK1 S vs. control, fold change=37.53, P=1.00E-4;
Fig. 4).
Discussion
To elucidate the underlying molecular mechanism of
OPRK1 in drug addiction, OPRK1 promoter methylation was compared
between drug addicts and controls. The results of the present study
demonstrated that OPRK1 promoter methylation was significantly
higher in addicts than in control subjects. Significant
correlations are also identified between OPRK1 promoter methylation
and phenotypic indices (length and frequency of drug use) in male
heroin addicts. In addition, two OPRK1 promoter fragments (OPRK1 L
and OPRK1 S) were able to regulate gene expression.
Heroin is converted back into morphine after it
enters the brain (26). Morphine
binds to opioid receptors expressed on the surface of cells, which
are particularly involved in the perception of pain and reward
mechanisms (27). In the brain,
morphine binds to µ opioid receptors, resulting in euphoric,
analgesic and anxiolytic effects (26). OPRK1 is critical in drug addiction,
and OPRK1 agonists have inhibitory effects on addictions to
alcohol, cocaine and opiate (28).
Furthermore, several genetic variants of OPRK1 have been associated
with heroin addiction (8,29,30).
Previous studies have indicated that heritable factors may be
important in addictive behavior (8,31), and
suggest that higher levels of OPRK1 promoter methylation may be
associated with heroin addiction. The results of the present study
indicate that OPRK1 promoter methylation affects the propensity of
individuals to exhibit addictive behaviors.
OPRK1 may influence the stress-induced reinstatement
of cocaine-seeking (31,32). In a previous study, OPRK1 agonist
administration was effective in decreasing cocaine-seeking and
self-administration (33). The
influence of OPRK1 in drug addiction may principally be due a
marked increase in OPRK1 expression in the nucleus accumbens, which
is considered to be most strongly associated with addiction in the
brain (34). Numerous other factors
have also been associated with heroin addiction, including the
method of administration (35) and
heroin purity (36). Furthermore, a
prolonged duration of heroin usage has been associated with heroin
addiction and mortality (37). In
the present study, OPRK1 promoter methylation was associated with a
longer duration and lower frequency of drug use in male heroin
addicts.
METH is a potent central nervous stimulant, and is
com-monly used as a recreational drug (38). METH may affect the processing and
function of monoamines through its effects on the dopamine
transporter, monoamine oxidases and vesicular monoamine
transporter-2, and inhibition of these molecules by METH has been
associated with increased levels of presynaptic cytosolic dopamine
and euphoria (39). In the present
study, significantly higher OPRK1 promoter methylation was observed
in METH addicts when compared with controls, indicating that OPRK1
promoter methylation may serve a pivotal role in METH addiction. In
addition, OPRK1 promoter hypermethylation may increase the risk of
neurological disease through its regulation of gene expression
(25). The present in vitro
assay verified that OPRK1 promoter fragments (including OPRK1 S and
OPRK1 L) were able to regulate gene expression. OPRK1 S exhibits a
greater efficacy, compared with OPRK1 L, which indicates that the
target fragment may repress the expression of the backward gene.
Further functional experiments are required to elucidate the
detailed mechanism that controls this expression.
However, the present study had several limitations.
Firstly, the sample size was relatively small, which may have
affected the outcomes of data analysis, particularly regarding the
subgroup analyses. Secondly, the POMS records of female addicts
were not available, and thus correlations between OPRK1 methylation
and the length and frequency of drug use require further
investigation in female addicts. Finally, the present study only
assessed the DNA methylation of an OPRK1 promoter fragment, which
may not be representative of the whole gene.
In conclusion, the present study suggested that
OPRK1 promoter hypermethylation was associated with an increased
risk of heroin and METH addiction. Furthermore, OPRK1
hypermethylation was associated with a longer duration and lower
frequency of drug use in male heroin addicts. Future studies using
larger samples and other populations are now required to confirm
the observations of the present study.
Acknowledgments
The present study was supported by the National Key
R&D Program of China (grant no. 2017YFC1310400), the Natural
Science Foundation of Zhejiang (grant no. LY18H090008), the Ningbo
Natural Science Foundation (grant nos. 2017A610217 and 2017A04),
the Nature Science Foundation of China (no. 81371469) and the K.C.
Wong Magna Fund of Ningbo University.
References
|
1
|
Yager LM, Garcia AF, Wunsch AM and
Ferguson SM: The ins and outs of the striatum: Role in drug
addiction. Neuroscience. 301:529–541. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jones CM, Logan J, Gladden RM and Bohm MK:
Vital signs: Demographic and substance use trends among heroin
users - United States, 2002–2013. MMWR Morb Mortal Wkly Rep.
64:719–725. 2015.PubMed/NCBI
|
|
3
|
Wang R, Ding Y, Bai H, et al: Illicit
heroin and methamphetamine use among methadone maintenance
treatment patients in dehong prefecture of Yunnan province, China.
PloS one. 10:e01334312015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang R, Ding Y, Bai H, Duan S, Ye R, Yang
Y, Wang J, Tang R, Gao M and He N: Illicit heroin and
methamphetamine use among methadone maintenance treatment patients
in dehong prefecture of yunnan province, China. PLoS One.
10:e01334312015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guerrero M, Urbano M, Brown SJ, Cayanan C,
Ferguson J, Cameron M, Devi LA, Roberts E and Rosen H: Optimization
and characterization of an opioid kappa receptor (OPRK1)
antagonistProbe Reports from the NIH Molecular Libraries Program.
Bethesda (MD): 2010
|
|
6
|
Nielsen DA, Yuferov V, Hamon S, Jackson C,
Ho A, Ott J and Kreek MJ: Increased OPRM1 DNA methylation in
lymphocytes of methadone-maintained former heroin addicts.
Neuropsychopharmacology. 34:867–873. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Faisal M, Waseem D, Ismatullah H and Taqi
MM: A molecular prospective provides new insights into implication
of PDYN and OPRK1 genes in alcohol dependence. Comput Biol Med.
53:250–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang SC, Tsou HH, Chung RH, Chang YS, Fang
CP, Chen CH, Ho IK, Kuo HW, Liu SC, Shih YH, et al: The association
of genetic polymorphisms in the κ-opioid receptor 1 gene with body
weight, alcohol use, and withdrawal symptoms in patients with
methadone maintenance. J Clin Psychopharmacol. 34:205–211. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jablonka E: Epigenetic variations in
heredity and evolution. Clin Pharmacol Ther. 92:683–688. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smith ZD and Meissner A: DNA methylation:
Roles in mammalian development. Nat Rev Genet. 14:204–220. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeltsch A and Jurkowska RZ: New concepts
in DNA methylation. Trends Biochem Sci. 39:310–318. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Branco MR, Ficz G and Reik W: Uncovering
the role of 5-hydroxymethylcytosine in the epigenome. Nat Rev
Genet. 13:7–13. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kinney SR and Pradhan S: Regulation of
expression and activity of DNA (cytosine-5) methyltransferases in
mammalian cells. Prog Mol Biol Transl Sci. 101:311–333. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cadet JL, Brannock C, Jayanthi S and
Krasnova IN: Transcriptional and epigenetic substrates of
methamphetamine addiction and withdrawal: Evidence from a
long-access self-administration model in the rat. Mol Neurobiol.
51:696–717. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jayanthi S, McCoy MT, Chen B, Britt JP,
Kourrich S, Yau HJ, Ladenheim B, Krasnova IN, Bonci A and Cadet JL:
Methamphetamine downregulates striatal glutamate receptors via
diverse epigenetic mechanisms. Biol Psychiatry. 76:47–56. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krasnova IN, Chiflikyan M, Justinova Z,
McCoy MT, Ladenheim B, Jayanthi S, Quintero C, Brannock C, Barnes
C, Adair JE, et al: CREB phosphorylation regulates striatal
transcriptional responses in the self-administration model of
methamphetamine addiction in the rat. Neurobiol Dis. 58:132–143.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Robison AJ and Nestler EJ: Transcriptional
and epigenetic mechanisms of addiction. Nat Rev Neurosci.
12:623–637. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
McQuown SC and Wood MA: Epigenetic
regulation in substance use disorders. Curr Psychiatry Rep.
12:145–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu X, Ji H, Liu G, Wang Q, Liu H, Shen W,
Li L, Xie X, Zhou W and Duan S: A significant association between
BDNF promoter methylation and the risk of drug addiction. Gene.
584:54–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
American Psychiatric Association:
Diagnostic and Statistical Manual of Mental Disorders. 4th edition.
American Psychiatric Association; Washington, DC: 1994
|
|
21
|
Shen W, Liu Y, Li L, Zhang Y and Zhou W:
Negative moods correlate with craving in female methamphetamine
users enrolled in compulsory detoxification. Subst Abuse Treat Prev
Policy. 7:442012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kreutz M, Hochstein N, Kaiser J, Narz F
and Peist R: Pyrosequencing: Powerful and quantitative sequencing
technology. Curr Protoc Mol Biol. 104:Unit 7.15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang L, Wang Y, Ji H, Dai D, Xu X, Jiang
D, Hong Q, Ye H, Zhang X, Zhou X, et al: Elevation of peripheral
BDNF promoter methylation links to the risk of Alzheimer's disease.
PLoS One. 9:e1107732014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Edenberg HJ, Wang J, Tian H, Pochareddy S,
Xuei X, Wetherill L, Goate A, Hinrichs T, Kuperman S, Nurnberger JI
Jr, et al: A regulatory variation in OPRK1, the gene encoding the
kappa-opioid receptor, is associated with alcohol dependence. Hum
Mol Genet. 17:1783–1789. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ji H, Wang Y, Liu G, Xu X, Dai D, Chen Z,
Zhou D, Zhou X, Han L, Li Y, et al: OPRK1 promoter hypermethylation
increases the risk of Alzheimer's disease. Neurosci Lett.
606:24–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gharagozlou P, Hashemi E, DeLorey TM,
Clark JD and Lameh J: Pharmacological profiles of opioid ligands at
kappa opioid receptors. BMC Pharmacol. 6:32006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Klous MG, Van den Brink W, Van Ree JM and
Beijnen JH: Development of pharmaceutical heroin preparations for
medical co-prescription to opioid dependent patients. Drug Alcohol
Depend. 80:283–295. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bruchas MR, Xu M and Chavkin C: Repeated
swim stress induces kappa opioid-mediated activation of
extracellular signal-regulated kinase 1/2. Neuroreport.
19:1417–1422. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wen S, Wang C, Berg A, Li Y, Chang MM,
Fillingim RB, Wallace MR, Staud R, Kaplan L and Wu R: Modeling
genetic imprinting effects of DNA sequences with multilocus
polymorphism data. Algorithms Mol Biol. 4:112009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Levran O, Londono D, O'Hara K, Nielsen DA,
Peles E, Rotrosen J, Casadonte P, Linzy S, Randesi M, Ott J, et al:
Genetic susceptibility to heroin addiction: A candidate gene
association study. Genes Brain Behav. 7:720–729. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gerra G, Leonardi C, Cortese E, D'Amore A,
Lucchini A, Strepparola G, Serio G, Farina G, Magnelli F, Zaimovic
A, et al: Human kappa opioid receptor gene (OPRK1) polymorphism is
associated with opiate addiction. Am J Med Genet B Neuropsychiatr
Genet. 144B:771–775. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Black SM, Ellard S, Meehan RR, Parry JM,
Adesnik M, Beggs JD and Wolf CR: The expression of cytochrome
P450IIB1 in Saccharomyces cerevisiae results in an increased
mutation frequency when exposed to cyclophosphamide.
Carcinogenesis. 10:2139–2143. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schenk S, Partridge B and Shippenberg TS:
U69593, a kappa-opioid agonist, decreases cocaine
self-administration and decreases cocaine-produced drug-seeking.
Psychopharmacology (Berl). 144:339–346. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mash DC and Staley JK: D3 dopamine and
kappa opioid receptor alterations in human brain of
cocaine-overdose victims. Ann N Y Acad Sci. 877:507–522. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gossop M, Griffiths P, Powis B, Williamson
S and Strang J: Frequency of non-fatal heroin overdose: Survey of
heroin users recruited in non-clinical settings. BMJ. 313:4021996.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Darke S, Ross J, Zador D and Sunjic S:
Heroin-related deaths in New South Wales, Australia, 1992–1996.
Drug Alcohol Depend. 60:141–150. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Darke S and Zador D: Fatal heroin
‘overdose’: A review. Addiction. 91:1765–1772. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Scott JC, Woods SP, Matt GE, Meyer RA,
Heaton RK, Atkinson JH and Grant I: Neurocognitive effects of
methamphetamine: A critical review and meta-analysis. Neuropsychol
Rev. 17:275–297. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cruickshank CC and Dyer KR: A review of
the clinical pharmacology of methamphetamine. Addiction.
104:1085–1099. 2009. View Article : Google Scholar : PubMed/NCBI
|