Introduction
Pre-eclampsia (PE) (1–3) is a
unique and multisystem-related disease in pregnant women, of whom
the incidence rate is approximately 7%. It is clinically
characterized (4,5) by endothelial dysfunction, platelet
aggregation abnormality and hypertension proteinuria, which can
also cause slow growth and development in fetus. PE is an important
cause of fetal death, affecting many families. The current research
on the mechanism of PE is not mature, and two theories have been
recognized: i) Abnormal immune mechanism (6), which means that abnormal immunity of
pregnant women and fetus can induce PE and ii) injury of vascular
endothelial cells (7) means that PE
is a vascular disease caused by oxidative stress due to placental
ischemia-reperfusion injury. Therefore, there are more studies on
oxidative stress in PE patients.
A study indicated that (8) PE occurs in women during pregnancy due
to a variety of regulatory molecules secreted into the blood of
pregnant women, which is caused by the abnormality of trophoblast
infiltration leading to the incorrect implantation of placenta in
the uterus thus resulting in the persistent lack of nutrients such
as O2. C-X-C chemokine receptor type 4 (CXCR4)/CXCR7
(9,10) is an important signaling pathway
during pregnancy, which is highly expressed in the placenta. It is
involved in embryogenesis and implantation of placenta; moreover,
they can also form a dipolymer, aggregate β-inhibitory protein and
activate its related signaling transduction pathways. The study
indicates that CXCR4 and CXCR7 combined with CXCL12 can regulate
signaling pathways such as phosphatidylinositol 3-kinase
(PI3K)/protein kinase B (AKT)/ fructooligosaccharide (FOS) and
Ser/Thr kinase (Raf)/ methyl ethyl ketone (MEK)/extracellular
signal-regulated kinase 1 (ERK1) that participate in the
differentiation of trophoblast cells and formation of placenta.
Therefore, we suspect PE of pregnant women is related to the
abnormal expression of CXCR4/CCR7.
The present study investigated the correlation of
CXCR4/CXCR7 signaling pathway with PE through detecting the
expression levels of CXCR4 and CXCR7 in the serum of women.
Patients and methods
Clinical data
Twenty patients with mild PE and 40 cases with
severe PE enrolled into PE group and 60 cases of normal pregnancy
group during the same period admitted to Wenzhou People's Hospital
from January 2015 to January 2017 were selected. The changes in
expression of serum CXCR4 messenger ribonucleic acid (mRNA) and
CXCR7 mRNA were detected by reverse transcription-polymerase chain
reaction (RT-PCR), and CXCR4/CXCR7 protein concentration in the
serum of women during pregnancy was determined by enzyme-linked
immunosorbent assay (ELISA). Patients were diagnosed as mild and
severe PE accordingly (11). i) Mild
PE: after 20 weeks of gestation, BP ≥140/90 mmHg; urinary protein
≥0.3 g/24 h or random urinary protein (+); symptoms such as upper
abdominal discomfort and headache. ii) Severe PE:BP ≥160/110 mmHg;
urinary protein ≥2.0 g/24 h or random urinary protein (++); Serum
creatinine >1,061 µmol/l; platelet <100 × 109/l;
microangiopathy hemolysis (elevated LDH); elevated serum ALT or
AST; persistent headache or other neurological or visual disorders;
persistent upper abdominal discomfort. The 24 h urinary protein
quantification was tested by routine urine test and defined as:
0.15–0.5 g was microalbuminuria (±), 0.5–1 g was mild proteinuria
(+), and 1–4 g was moderate proteinuria (++), >4 g was severe
proteinuria (+++). The pregnant women enrolled in this study had no
organic inflammation, systemic infection, hypertension or diabetes,
and all of them were delivered by caesarean section. Pre-analytical
routine including Systolic pressure, Diastolic pressure and Random
proteinuria were performed. The study was approved by the Ethics
Committee of Wenzhou People's Hospital (Wenzhou, China) and
informed consent was signed by the patients or their families.
Extraction of ribonucleic acid
Whole blood samples of the patients were collected
in the morning fasting state. The sample was tested in 2 h to
obtain serum by centrifugation at 2,500 × g for 10 min. The serum
total RNA was extracted by TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), according to the
instructions provided by Invitrogen; Thermo Fisher Scientific, Inc.
The concentration and purity of the extracted RNA were analyzed by
an ultraviolet spectrophotometer (Hitachi, Tokyo, Japan), and the
integrity of RNA was analyzed by 3% agarose gel
electrophoresis.
Synthesis of complementary
deoxyribonucleic acid (cDNA)
The RNA was reversely transcribed into cDNA,
referring to the TaqMan® MicroRNA Reverse Transcription
kit instructions (Thermo Fisher Scientific, Inc.). The reaction
included 37°C for 45 min and 95°C for 5 min. The production was
preserved at −20°C.
RT-qPCR
The reaction system was 25 µl in total, including
pre-denaturation at 95°C for 5 min, denaturation at 95°C for 30
sec, annealing at 60°C for 45 sec, extension at 72°C for 3 min, a
total of 35 cycles, finally extension at 72°C for 5 min. The
production of PCR was preserved at 4°C. PCR was carried out by ABI
Prism 7900 PCR instrument (Applied Biosystems; Thermo Fisher
Scientific, Inc.). CXCR4 mRNA: upstream primer
5′-GAGTCGATGCTGATCCCAAT-3′ and downstream primer
5′-GGCAGGTGAAAGGGATGTAG-3′; CXCR7 mRNA: upstream primer
5′-CCTACGTGGTGGTCTTCCTT-3′ and downstream primer
5′-GGCAGGTGAAAGGGATGTAG-3′. U6 was used as the internal reference
of reaction (upstream primer: 5′-CTCGCTTCGGCAGCACA-3; downstream
primer: 5′-AACGCTTCACGAATTTGCGT-3′) (Guangzhou Shangeng Biological
Technology Co., Ltd., Guangzhou, China). A 3-well was duplicated
for all samples, and the result was analyzed by 2−∆∆Cq
(12). The specimen was stored for
use within 7 days.
Detection of CXCR4/CXCR7 protein
The protein levels of CXCR4 and CXCR7 were detected
according to the instructions of ELISA kit (Nanjing Jin Yibai
Biological Technology Co., Ltd., Nanjing, China), including the
collection of serum specimen (preservation at −20°C, the specimen
was stored for use within 7 days), preparation of reagents and
standards, and production of standard curves. The optical density
(OD) value was measured at 450 nm.
Statistical analysis
The data were analyzed by Statistical Product and
Service Solutions (SPSS) 19.0 software (IBM Corp., Armonk, NY,
USA). Enumeration data were analyzed by Chi-square test, and
measurement data are expressed by (mean ± SD). Comparison between
groups was done using One-way ANOVA test followed by SNK test as
its post hoc test. The correlation of expression level of CXCR4
with CXCR7 was analyzed by Pearson's correlation analysis. The
correlation of expression level of CXCR4/CXCR7 with PE was analyzed
by Cox regression. P<0.05 was considered to indicate a
statistically significant difference.
Results
General data
There was no difference in age and number of fetus
between the PE group and normal pregnancy group. All the subjects
enrolled in this study were delivered by caesarean section
(Table I).
 | Table I.Comparison of general data between the
PE group and normal pregnancy group. |
Table I.
Comparison of general data between the
PE group and normal pregnancy group.
| General data | PE group (n=60) | Normal pregnancy
group (n=60) | P-value |
|---|
| Age (years) | 27.6±4.1 | 28.9±4.8 | 0.798 |
| Pregnancy termination
of time (week) | 37.4±2.1 | 39.8±2.5 | 0.657 |
| Delivery mode | Caesarean
section | Caesarean
section |
|
| Infant weight
(Kg) | 3.1±0.5 | 3.3±0.5 | 0.742 |
| Mild PE patient
(n) | 20 |
|
|
| Severe PE patients
(n) | 40 |
|
|
| Number of fetus |
|
| 0.546 |
| Woman with single
birth (n) | 58 (96.67%) | 59 (98.33%) |
|
| Woman with twins
(n) | 2 (3.33%) | 1 (1.67%) |
|
| Systolic pressure
(mmHg) | 156.52±11.35 | 107.69±11.87 | 0.021 |
| Diastolic pressure
(mmHg) | 94.44±7.47 | 67.80±8.95 | 0.034 |
| Random
proteinuria | +/++ | −/± |
|
There was no difference in pregnancy termination
time between the mild PE patients and normal pregnancy group
(37.9±1.8 vs. 39.8±2.5) (P>0.05). The pregnancy termination time
of patients with severe PE was earlier than that in the normal
pregnancy group (36.9±2.4 vs. 39.8±2.5) (P<0.05).
The systolic pressure, diastolic pressure and mean
arterial pressure (MAP) were higher in patients with mild and
severe PE than those in the normal pregnancy group (P<0.05).
There was a difference in infant weight between the mild and severe
PE patients and normal pregnancy group (P>0.05). The differences
in systolic and diastolic pressure, MAP and infant weight between
patients with mild and severe PE were not detected (P>0.05)
(Table II).
 | Table II.Comparisons of clinical features
between the normal pregnancy group and PE group. |
Table II.
Comparisons of clinical features
between the normal pregnancy group and PE group.
| Clinical
features | Normal pregnancy
group (i) | Mild PE patient
(ii) | Severe PE patient
(iii) |
|---|
| Systolic pressure
(mmHg) | 113.4±9.8 |
137.8±11.2 |
155.2±11.9 |
| Diastolic pressure
(mmHg) |
72.7±7.2 |
91.3±9.7 | 101.2±9.2 |
| MAP (mmHg) |
86.2±7.6 | 106.5±9.4 | 117.9±8.7 |
| Infant weight
(kg) |
3.3±0.5 |
3.4±0.4 |
2.7±0.6 |
|
| i vs. ii | i vs. iii | ii vs. iii |
| Systolic
pressure | P=0.045 | P=0.024 | P=0.267 |
| Diastolic
pressure | P=0.037 | P=0.023 | P=0.358 |
| MAP | P=0.034 | P=0.021 | P=0.432 |
| Infant weight | P=0.876 | P=0.056 | P=0.051 |
The results of RT-qPCR showed that the expression
levels of CXCR4 mRNA and CXCR7 mRNA in the serum of mild and severe
PE patients were distinctly higher than those in the normal
pregnancy group (P<0.05). There were no statistically
significant differences in serum CXCR4 mRNA and CXCR7 mRNA between
patients with severe and mild PE (P>0.05) (Table III).
 | Table III.Comparison of expression levels of
CXCR4 mRNA and CXCR7 mRNA. |
Table III.
Comparison of expression levels of
CXCR4 mRNA and CXCR7 mRNA.
| mRNA | Normal pregnancy
group (i) | Mild PE patients
(ii) | Severe PE patients
(iii) |
|---|
| CXCR4 mRNA | 0.319±0.121 | 0.697±0.134 | 0.740±0.142 |
| CXCR7 mRNA | 0.542±0.079 | 0.791±0.155 | 0.798±0.134 |
|
| i vs. ii | i vs. iii | ii vs. iii |
| CXCR4 mRNA | 0.024 | 0.017 | 0.728 |
| CXCR7 mRNA | 0.031 | 0.032 | 0.987 |
The results of ELISA displayed that the protein
content of CXCR4/CXCR7 in the serum of patients with mild and
severe PE was remarkably higher than that in the normal pregnancy
group (P<0.05). The expression level of CXCR4/CXCR7 protein in
the serum of patients with severe PE was higher than that in those
with mild PE (P<0.05) (Table
IV).
 | Table IV.Comparison of protein expression
levels between CXCR4 and CXCR7 (ng/ml). |
Table IV.
Comparison of protein expression
levels between CXCR4 and CXCR7 (ng/ml).
| Protein | Normal pregnancy
group (i) | Mild PE patients
(ii) | Severe PE patients
(iii) |
|---|
| CXCR4 protein | 11.083±2.445 | 18.275±7.582 | 25.765±4.660 |
| CXCR7 protein |
3.048±0.673 |
4.468±1.130 |
5.707±1.576 |
|
| i vs. ii | i vs. iii | ii vs. iii |
| CXCR4 protein | 0.036 | 0.021 | 0.029 |
| CXCR7 protein | 0.044 | 0.032 | 0.040 |
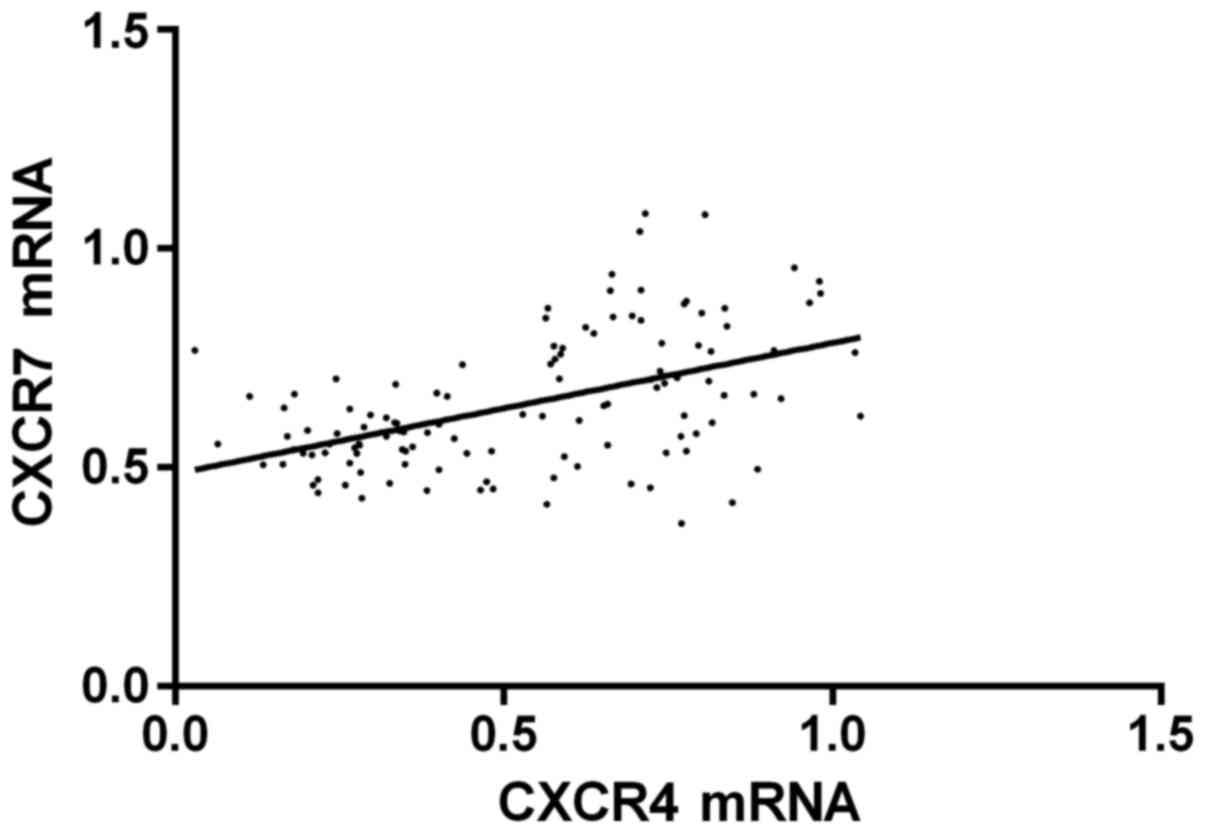
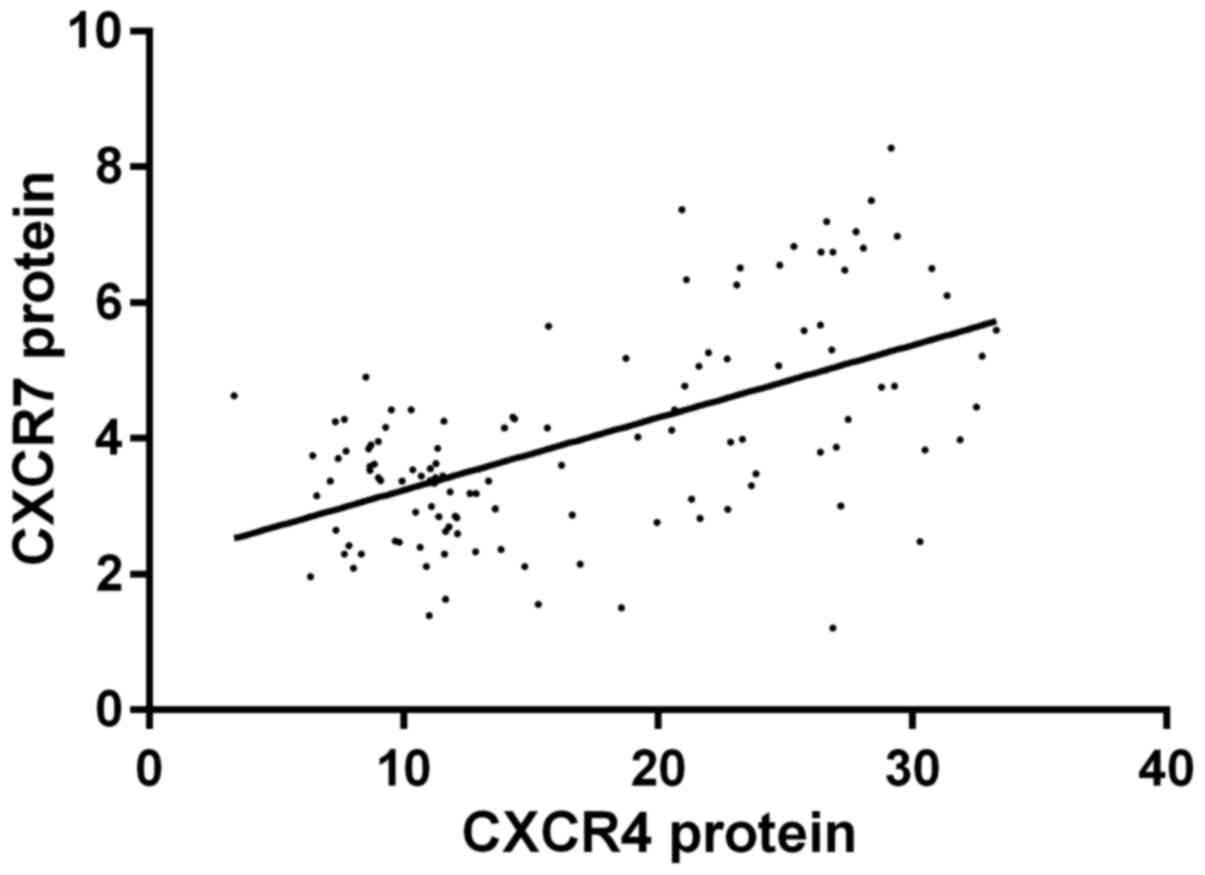
Pearson's correlation analysis indicated that the
expression level of CXCR4 mRNA was positively correlated with CXCR7
mRNA (r=0.501, P<0.001); the expression level of CXCR4 protein
was positively correlated with CXCR7 protein (r=0.572, P<0.001);
COX regression analysis revealed that CXCR4/CXCR7 was related to
the formation of PE, and 95% confidence intervals (CI) were [2.124
(1.557–4.479), P=0.002; 2.315 (1.231–3.524), P=0.002] (Figs. 1 and 2).
Discussion
The present study investigated the correlation of
CXCR4/CXCR7 signaling pathway with PE through detecting the
expression levels of CXCR4 and CXCR7 in the serum of 120 women
during pregnancy. The results showed that the expression levels of
CXCR4 mRNA and CXCR7 mRNA in the serum of PE patients were
distinctly higher than those in the normal pregnancy group
(P<0.05), and the expression levels of proteins were higher in
the PE patients than those in the normal pregnancy group. Thus, it
could be indicated that CXCR4/CXCR7 was related to the formation of
PE, and the correlation coefficient was (r=0.563, P=0.01). The
results of Karakus et al (13) indicated that CXCR4 polymorphism was
related to the development of PE. The study by Lu et al
(9) also demonstrated that CXCR4 and
CXCR7 were associated with trophoblast apoptosis, which may be
related to the formation and development of severe PE. Their
results were consistent with our results, further confirming the
correlation of CXCR4/CXCR7 with PE.
The result of this study indicated that there was no
difference in the expression of CXCR4/CXCR7 between mild and severe
PE patients, so we cannot confirm whether CXCR4/CXCR7 has a
correlation with progression of PE, which may be related to the
small number of the selected samples.
The human placenta is the most important fetal
development organ during pregnancy, regulating the dynamic gene
expression associated with placental and fetal development. The
balanced expression of various genes in the placenta can maintain
pregnancy, including fetal development. However, the abnormal
expression of genes in the placenta can cause obstetric diseases
such as PE, which has a great adverse effect on fetal growth and
survival (14). PE accounts for 2.7%
of all fetal perinatal death factors, 12% of fetal intrauterine
growth restriction factors and 19% of preterm birth factors
(15). Therefore, the prevention,
diagnosis and treatment of PE are very important issues.
The study by Bramham et al (16) displayed that plasma growth factor
(PlGF) may help to guide clinical decisions for admission and
delivery in PE patients. The research by Stepan et al
(17) also verified that utilization
of the soluble fms-like tyrosine kinase-1 (sFlt-1)/PlGF ratio may
help optimize care through ameliorating the management of women
suspected of PE. In our results, CXCR4/CXCR7 may play a supporting
role in the diagnosis of PE. At present, the more popular and
effective research on the prevention and treatment of PE is
low-dose aspirin treatment in pregnant women with high risk of PE,
which may reduce the risk of PE (18,19). A
limitation of the present study is that the reference gene U6 may
differ from the CXCR4/7 mRNAs in processing and stability. The
stability of different mRNA structures is different (20).
In conclusion, the expression level of CXCR4/CXCR7
may be closely related to the formation of PE, which seriously
affects the quality of life of infants. Attention should be paid to
prevention, diagnosis and treatment of PE by pregnant women and
clinicians.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ analyzed and interpreted the patient data, and
was a major contributor in writing the manuscript. HZ performed the
experiments. JZ participated in the experiments and the design of
the study. HC participated in the analysis and discussion of the
data. XC was a major contributor in designing the methods. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Wenzhou People's Hospital (Wenzhou, China). Signed informed
consents were obtained from the patients or their families.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen Q, Sousa JD, Snowise S, Chamley L and
Stone P: Reduction in the severity of early onset severe
preeclampsia during gestation may be associated with changes in
endothelial cell activation: A pathological case report. Hypertens
Pregnancy. 35:32–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McGinnis R, Steinthorsdottir V, Williams
NO, Thorleifsson G, Shooter S, Hjartardottir S, Bumpstead S,
Stefansdottir L, Hildyard L, Sigurdsson JK, et al: FINNPEC
Consortium; GOPEC Consortium: Variants in the fetal genome near
FLT1 are associated with risk of preeclampsia. Nat Genet.
49:1255–1260. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li XL, Chen TT, Dong X, Gou WL, Lau S,
Stone P and Chen Q: Early onset preeclampsia in subsequent
pregnancies correlates with early onset preeclampsia in first
pregnancy. Eur J Obstet Gynecol Reprod Biol. 177:94–99. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mol BWJ, Roberts CT, Thangaratinam S,
Magee LA, de Groot CJM and Hofmeyr GJ: Pre-eclampsia. Lancet.
387:999–1011. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rana S, Karumanchi SA and Lindheimer MD:
Angiogenic factors in diagnosis, management, and research in
preeclampsia. Hypertension. 63:198–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Levron Y, Dviri M, Segol I, Yerushalmi GM,
Hourvitz A, Orvieto R, Mazaki-Tovi S and Yinon Y: The ‘immunologic
theory’ of preeclampsia revisited: a lesson from donor oocyte
gestations. Am J Obstet Gynecol. 211(383): e1–e5. 2014.
|
|
7
|
Meeme A, Buga GA, Mammen M and Namugowa A:
Endothelial dysfunction and arterial stiffness in pre-eclampsia
demonstrated by the EndoPAT method. Cardiovasc J Afr. 28:23–29.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chaiworapongsa T, Chaemsaithong P, Yeo L
and Romero R: Pre-eclampsia part 1: Current understanding of its
pathophysiology. Nat Rev Nephrol. 10:466–480. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu J, Zhou WH, Ren L and Zhang YZ: CXCR4,
CXCR7, and CXCL12 are associated with trophoblastic cells apoptosis
and linked to pathophysiology of severe preeclampsia. Exp Mol
Pathol. 100:184–191. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Zuylen WJ, Ford CE, Wong DD and
Rawlinson WD: Human cytomegalovirus modulates expression of
noncanonical Wnt receptor ROR2 to alter trophoblast migration. J
Virol. 90:1108–1115. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
August P and Sibai BM: Preeclampsia:
Clinical Features and DiagnosisLockwood CJ and Barss VA: UpToDate.
Waltham, MA: 2015, https://www.uptodate.com/contents/preeclampsia-clinical-features-and-diagnosis
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. METHODS. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Karakus S, Bagci B, Bagci G, Sancakdar E,
Yildiz C, Akkar O and Cetin A: SDF-1/CXCL12 and CXCR4 gene
variants, and elevated serum SDF-1 levels are associated with
preeclampsia. Hypertens Pregnancy. 36:124–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Myatt L, Redman CW, Staff AC, Hansson S,
Wilson ML, Laivuori H, Poston L and Roberts JM: Global Pregnancy
CoLaboratory: Strategy for standardization of preeclampsia research
study design. Hypertension. 63:1293–1301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dodd JM, O'Brien C and Grivell RM:
Preventing pre-eclampsia - are dietary factors the key? BMC Med.
12:1762014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bramham K, Seed PT, Lightstone L,
Nelson-Piercy C, Gill C, Webster P, Poston L and Chappell LC:
Diagnostic and predictive biomarkers for pre-eclampsia in patients
with established hypertension and chronic kidney disease. Kidney
Int. 89:874–885. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stepan H, Herraiz I, Schlembach D,
Verlohren S, Brennecke S, Chantraine F, Klein E, Lapaire O, Llurba
E, Ramoni A, et al: Implementation of the sFlt-1/PlGF ratio for
prediction and diagnosis of pre-eclampsia in singleton pregnancy:
Implications for clinical practice. Ultrasound Obstet Gynecol.
45:241–246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Käehne LV and Lundin ICR: Treatment with
low-dose acetylsalicylic acid can reduce risk of pre-eclampsia in
high-risk pregnant women. Ugeskr Laeger. 179:20172017.(In
Danish).
|
|
19
|
Rolnik D, Wright D, Poon L, O'Gorman N,
Syngelaki A, de Paco Matallana C, Akolekar R, Cicero S, Janga D,
Singh M, et al: OC01. 04: Aspirin versus placebo in pregnancies at
high-risk of preterm pre-eclampsia: A multicentre, double-blind,
placebo-controlled trial. Ultrasound Obstet Gynecol. 50:1–47. 2017.
View Article : Google Scholar
|
|
20
|
Rouskin S, Zubradt M, Washietl S, Kellis M
and Weissman JS: Genome-wide probing of RNA structure reveals
active unfolding of mRNA structures in vivo. Nature. 505:701–705.
2014. View Article : Google Scholar : PubMed/NCBI
|