Introduction
Epithelial ovarian cancer (EOC) is the most common
cause of mortality among women with gynecological malignancies. The
majority of patients with EOC are diagnosed at an advanced clinical
stage, as they are asymptomatic until the tumor has metastasized on
the surface of the peritoneum. Based on GLOBOCAN estimates, there
were ~238,700 new cases of ovarian cancer and 151,900 cases of
ovarian cancer-associated mortality worldwide in 2012 (1). Despite surgery combined with
chemotherapy as the main strategy for treatment, chemoresistance
exists in 25% of patients, and the majority ultimately experience
relapse (2). Therefore, it is
important to identify a potential novel therapeutic target for
patients with EOC.
Tripartite motif-containing protein 44 (TRIM44)
belongs to the tripartite motif protein family, which comprises
structurally-related proteins and are involved in multiple cellular
processes, including cell proliferation, migration, apoptosis and
cancer cell invasion. Studies have suggested that TRIM44 may have
an oncogenic role in the invasion and metastases of several types
of human cancer, including prostate cancer (3), hepatocellular carcinoma (4), gastric carcinoma (5), non-small cell lung cancer (6) and testicular germ cell tumors (7). However, at present, the precise
function of TRIM44 and its underlying carcinogenic mechanism in
ovarian cancer remain to be fully elucidated.
Therefore, the present study aimed to investigate
the clinical significance of the expression of TRIM44 in patients
with EOC, and performed further experiments to determine whether
TRIM44 may be a potential biomarker for patients with EOC.
Materials and methods
Ethics statement
The present study was approved by the Ethical
Committee of the Harbin Medical University Cancer Hospital (Harbin,
China).
Patient population
In total, 109 patients underwent primary surgery
with the goal of maximal tumor resection followed by standard
combination chemotherapy with carboplatin and paclitaxel between
March 2010 and March 2013. None of the patients had received
neoadjuvant chemotherapy and/or radiation prior to surgery. All
cases were diagnosed according to the Silverberg grading system
(8) and the International Federation
of Gynecology and Obstetrics (FIGO) staging system. The patients'
characteristics, including age, FIGO stage, histological grade,
histological type, and lymph node metastasis, are summarized in
Table I. Normal samples in the
present study were obtained from women undergoing ovariectomy for
benign gynecologic disease at the Department of Gynecology of the
Harbin Medical University Cancer Hospital.
 | Table I.Association analyses between the
expression levels of TRIM44 and the clinicopathological
characteristics of epithelial ovarian cancer. |
Table I.
Association analyses between the
expression levels of TRIM44 and the clinicopathological
characteristics of epithelial ovarian cancer.
|
|
| TRIM44
expression |
|
|---|
|
|
|
|
|
|---|
| Variable | Patients (n) | Low, n (%) | High, n (%) | P-valuea |
|---|
| Age (years) |
|
|
| 0.097 |
| ≤55 | 61 | 12 (19.7) | 49 (80.3) |
|
|
>55 | 48 | 4 (8.3) | 44 (91.7) |
|
| FIGO stage |
|
|
| <0.001 |
| I | 8 | 8 (100.0) | 0 (0.0) |
|
| II | 23 | 2 (8.7) | 21 (91.3) |
|
| III | 75 | 6 (8.0) | 69 (92.0) |
|
| IV | 3 | 0 (0.0) | 3 (100.0) |
|
| Histological
grade |
|
|
| 0.053 |
| G1 | 12 | 4 (33.3) | 8 (66.7) |
|
|
G2/G3 | 97 | 12 (12.4) | 85 (87.6) |
|
| Histological
type |
|
|
| <0.001 |
|
Serous | 92 | 8 (8.7) | 84 (91.3) |
|
|
Mucinous | 7 | 4 (57.1) | 3 (42.9) |
|
|
Endometrioid | 5 | 2 (40.0) | 3 (60.0) |
|
| Clear
cell | 5 | 2 (40.0) | 3 (60.0) |
|
| Lymph node
metastasis |
|
|
| 0.015 |
| No | 83 | 16 (19.3) | 67 (80.7) |
|
| Yes | 26 | 0 (0.0) | 26 (100.0) |
|
Western blot analysis
The protein expression levels were evaluated by
western blot analysis with an anti-TRIM44 antibody. Total proteins
from nine frozen tissue samples were extracted using RIPA buffer
(Beyotime Institute of Biotechnology, Haimen, China). Protein
concentration was determined by a Bradford assay using bovine serum
albumin (Shanghai Ruisai Biotechnology Co., Ltd., Shanghai, China).
Proteins (50 µg/lane) were electrophoretically separated on 5%
sodium dodecyl sulfate-polyacrylamide gels and transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked in 5% fat-free milk at room
temperature for 1 h. Primary antibodies against TRIM44 (cat. no.
NBP2-31681; 1:300; Novus Biologicals, LLC, Littleton, CO, USA) and
β-actin (cat. no. WL01845; 1:1,000; Wanleibio Co., Ltd., Harbin,
China) were diluted in buffer and incubated with the samples at 4°C
overnight. The membranes were then incubated with secondary
antibodies from and visualized with reagents from the
Phototope®-HRP Western Blot Detection System (cat. no.
7071; Cell Signaling Technology, Inc., Danvers, MA, USA). The
experiment was performed in triplicate.
Immunohistochemistry and
evaluation
Immuno histochemistry was performed using an
anti-TRIM44 antibody following standard methods. The tissue
specimens were paraffin-embedded and tissue blocks were cut into
5-µm-thick sections. Following deparaffinization and rehydration,
the sections were incubated in 0.3% hydrogen peroxide at room
temperature. Blocking with peroxidase was performed for 10 min.
TRIM44 antigen retrieval was performed by heating the sections in a
stainless autoclave. Following washing in PBS, the sections were
incubated with the anti-TRIM44 antibody (Abcam, Cambridge, UK) at a
dilution of 1:100, and then placed into a humidor overnight at 4°C.
The slides were counterstained with hematoxylin for 2 min.
According to the number of positive tumor cells, the
staining was scored as follows: ‘0’ if <10% of the tumor cells
were positively stained, ‘1’ if 10–33%, and ‘2’ if 34–66%, ‘3’ if
67–100%. The staining intensity was scored as follows: Score 0
(negative), score 1 (weak) and score 2 (moderate) in at least five
different high-power fields with a light microscope. The protein
expression levels of TRIM44 were classified semi-quantitatively
based on the total combined score of the positive-staining tumor
cell percentage and the staining intensity. The sum of the staining
intensity score and the percentage of positive staining was used to
evaluate expression levels, where <3 indicated low expression
and ≥3 indicated high expression (5).
The immunohistochemistry scoring procedure was
performed twice by two independent pathologists who were
experienced in assessing immunohistochemistry and had no knowledge
of the clinicopathological information of the slides.
Statistical analyses
Pearson's χ2 square test was used for
determining proportional differences in univariate analyses. The
survival curve was generated using the Kaplan-Meier and the
log-rank test. The logistic regression model was used for
univariate and multivariate analyses. All tests were two-sided and
P≤0.05 was considered to indicate a statistically significant
difference. Statistical analysis was performed using SPSS 21.0
statistical software (IBM SPSS, Armonk, NY, USA).
Results
Clinicopathological features of
patients with EOC
The present study analyzed a total of 109 patients,
who underwent primary surgery with the goal of maximal tumor
resection followed by standard combination chemotherapy with
carboplatin and paclitaxel. The clinicopathological features of the
patients enrolled in the study are summarized in Table I. Among them, 12 (11%) patients were
diagnosed with grade 1 (G1) EOC and 97 patients (89%) were
diagnosed with grade 2 and grade 3 (G2/G3) EOC. According to the
American Joint Committee on Cancer (AJCC) tumor staging criteria,
eight (7.3%) patients were stage I, 23 (21.1%) patients were stage
II, 75 (68.8%) patients were stage III, and three (2.8%) patients
were stage IV. There were 26 (23.9%) patients with distant
metastases at the time of diagnosis. The 109 patients were further
grouped into four histological types, which included 92 patients
with serous type, seven patients with mucinous type, five patients
with endometrioid type and five patients with the clear cell type.
The survival analysis of all patients with EOC was followed up
until 11 January 2017. The mean follow-up duration was 36.28 months
(range, 3–77 months).
Correlation between the expression
levels of TRIM44 and the clinicopathological characteristics of
EOC
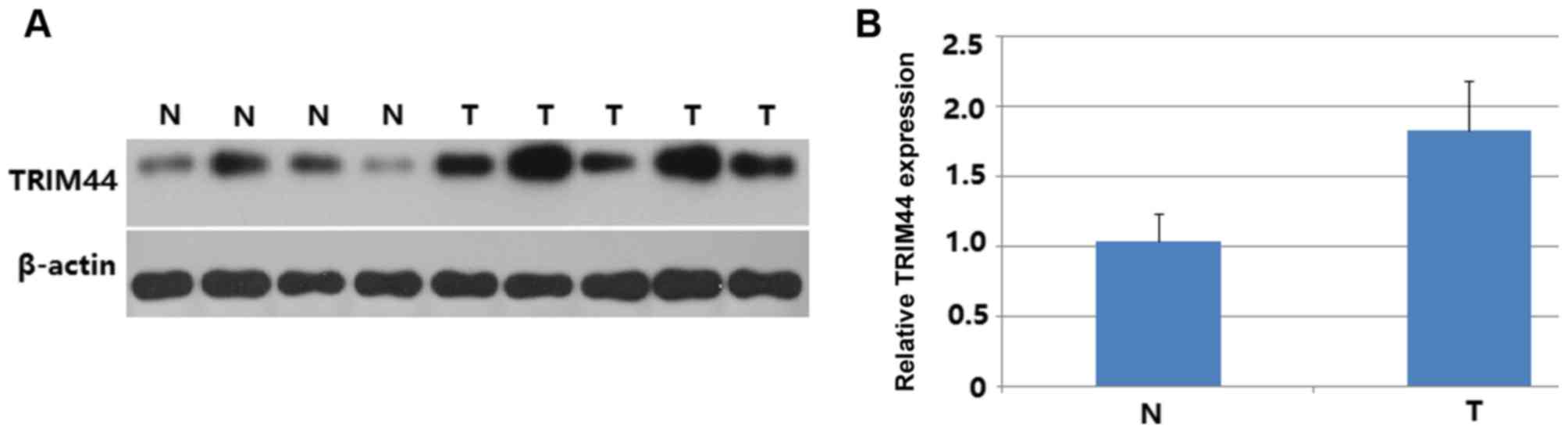
The protein expression of TRIM44 in EOC and normal
ovarian tissues was measured by western blot analysis. As shown in
Fig. 1, the protein expression of
TRIM44 in EOC tissues was significantly higher than that in normal
tissues (P<0.001; Fig. 1A and B).
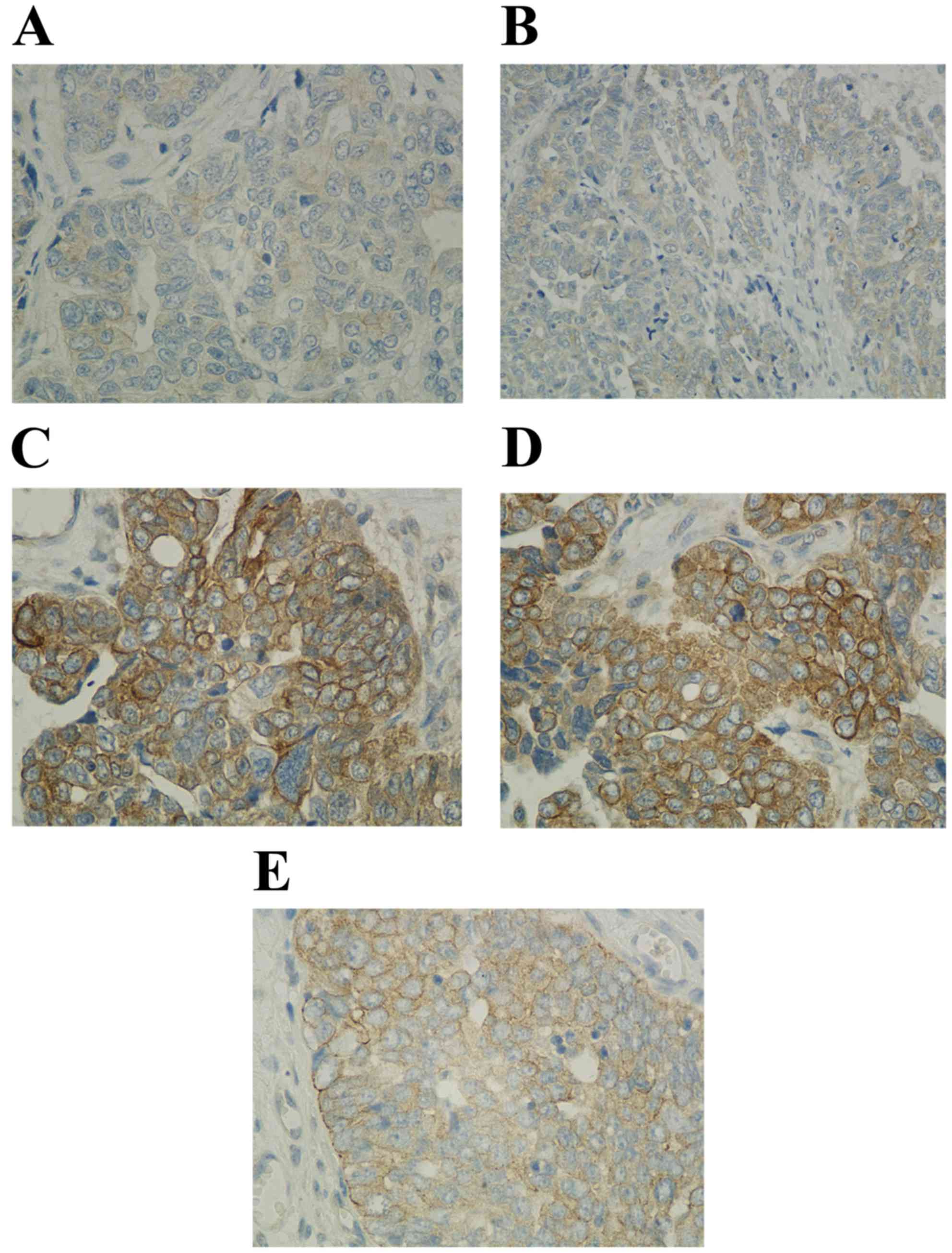
The expression of TRIM44 was further detected using
immunohistochemistry. The results of the immunohistochemical
staining are shown in Fig. 2A-E. A
high level of immunoreactivity for TRIM44 was found in the plasma
of EOC tissues. As shown in Table I,
there was significant correlation between the expression of TRIM44
and AJCC stage (P<0.001), histological grade (P=0.053),
histological type (P<0.001) and lymph node metastasis (P=0.015).
Furthermore, the positive expression rate of TRIM44 in patients
with late-stage (stage III and IV) disease was 92.3% (72/78), which
was significantly higher than the rate in patients with early-stage
(stage I and II) disease of 67.7% (21/31) (P<0.001). Patients
with serous type (91.3%, 84/92) tended to express higher levels of
TRIM44, compared with the other three histological types (mucinous,
42.9%, 3/7; endometrioid, 60%, 3/5; and clear cell, 60%, 3/5)
(<0.001). In addition, the expression level of TRIM44 in
patients with lymph node metastasis (100%, 26/26) was significantly
higher, compared with the level in patients without lymph node
metastasis (80.7%, 67/83) (P=0.015). The positive expression rate
of TRIM44 in patients with moderately or poorly differentiated
disease (grade G2/3; 87.6%, 85/97) was significantly higher than
that in patients with well-differentiated disease (grade G1; 66.7%,
8/12) (P=0.053). No significant correlation existed between the
expression of TRIM44 and patient age (P=0.097). The results of the
immunohistochemical analysis of the expression of TRIM44 in EOC are
summarized in Table I.
Prognostic value of the expression of
TRIM44 in patients with EOC
To investigate the prognostic value of the
expression of TRIM44 in patients with EOC, Kaplan-Meier analysis
and univariate survival analysis of overall survival and
disease-free survival rates were performed for the 109 patients
with EOC. The 109 patients with EOC were firstly classified into
two patient subgroups: Patients with a high expression of TRIM44
(n=93) and patients with a low expression of TRIM44 (n=16)
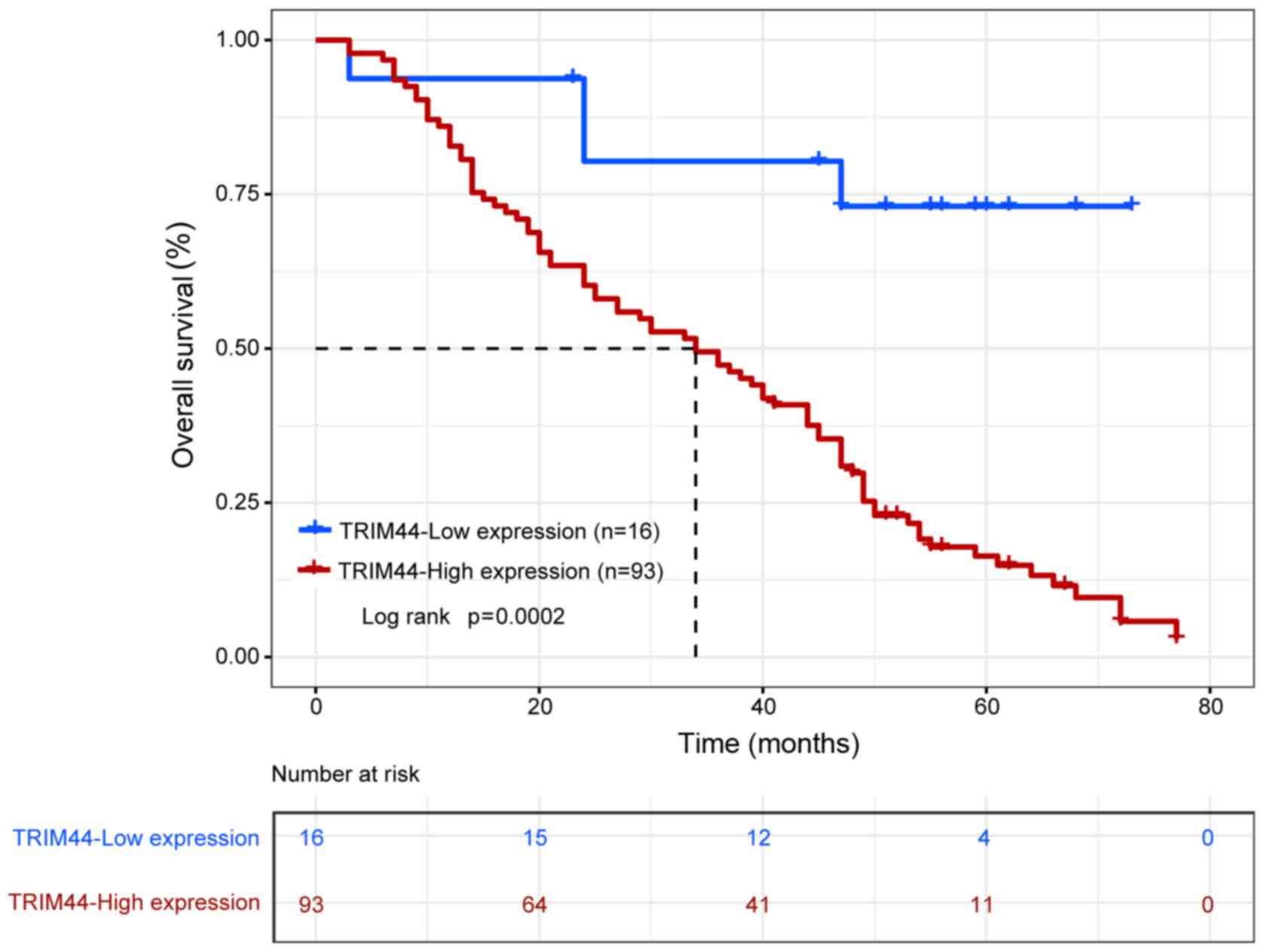
according to the expression levels of TRIM44. The Kaplan-Meier
analysis showed that overall survival rate between patients with a
high expression of TRIM44 and patients with a low expression of
TRIM44 was significantly different (P=0.0002; Fig. 3). Patients expressing a high level of
TRIM44 exhibited poorer overall survival rate than patients
expressing a low level of TRIM44 (P<0.001; Table II). In addition, the difference in
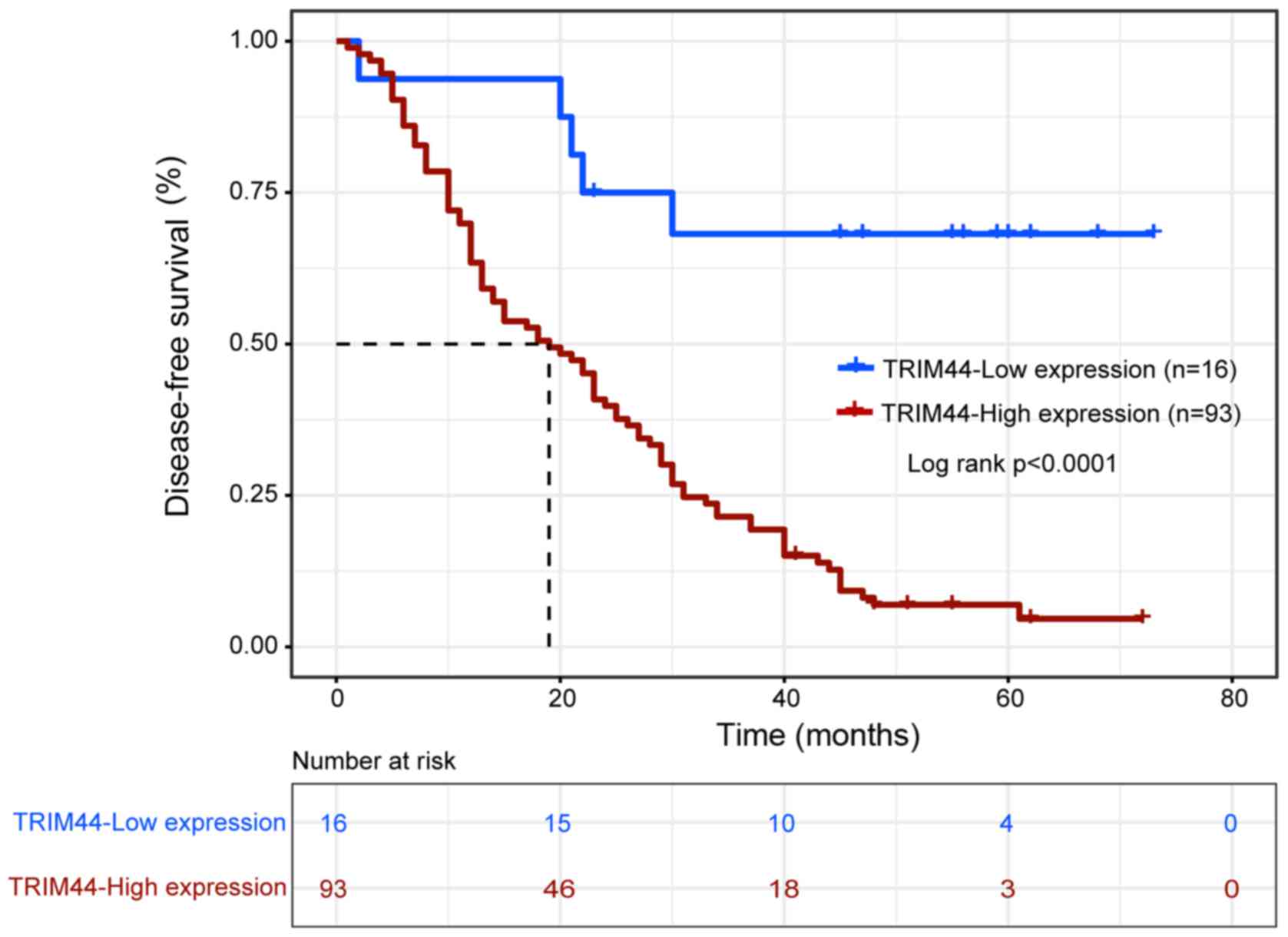
disease-free survival rate between patients with a high expression
of TRIM44 and patients with a low expression of TRIM44 was also
significantly different (P<0.0001; Fig. 4). Patients with a high level of
TRIM44 had a shorter disease-free survival rate than patients with
a low expression of TRIM44 (P<0.001; Table II).
 | Table II.Univariate survival analysis of OS and
DFS rates in 109 patients with epithelial ovarian cancer. |
Table II.
Univariate survival analysis of OS and
DFS rates in 109 patients with epithelial ovarian cancer.
|
|
| OS |
| DFS |
|
|---|
|
|
|
|
|
|
|
|---|
| Variable | n | Monthsa | 95% CI | P-valueb | Monthsa | 95% CI | P-valueb |
|---|
| Age (years) |
|
|
| 0.130 |
|
| 0.028 |
| ≤55 | 61 | 43±3 | 37–49 |
| 32±3 | 27–38 |
|
|
>55 | 48 | 34±3 | 27–41 |
| 22±3 | 17–27 |
|
| FIGO stage |
|
|
| <0.001 |
|
| <0.001 |
| I | 8 | 57±10 | 38–76 |
| 58±10 | 39–76 |
|
| II | 23 | 48±5 | 38–58 |
| 35±5 | 27–44 |
|
| III | 75 | 35±2 | 30–40 |
| 23±2 | 19–26 |
|
| IV | 3 | 15±5 | 5–25 |
| 11±5 | 2–20 |
|
| Histological
grade |
|
|
| 0.108 |
|
| 0.102 |
| G1 | 12 | 49±6 | 36–61 |
| 36±6 | 24–48 |
|
|
G2/G3 | 97 | 38±2 | 33–42 |
| 27±2 | 22–31 |
|
| Histological
type |
|
|
| 0.697 |
|
| 0.200 |
|
Serous | 92 | 38±2 | 34–43 |
| 26±2 | 22–30 |
|
|
Mucinous | 7 | 36±10 | 17–56 |
| 32±10 | 12–52 |
|
|
Endometrioid | 5 | 40±9 | 24–57 |
| 37±11 | 16–58 |
|
| Clear
cell | 5 | 45±11 | 23–67 |
| 45±11 | 22–67 |
|
| Lymph node
metastasis |
|
|
| 0.002 |
|
| 0.014 |
| No | 83 | 41±3 | 35–46 |
| 30±3 | 25–36 |
|
|
Yes | 26 | 33±3 | 27–39 |
| 20±2 | 15–24 |
|
| TRIM44 |
|
|
| <0.001 |
|
| <0.001 |
| Low
expression | 16 | 60±6 | 49–72 |
| 56±6 | 43–69 |
|
| High
expression | 93 | 36±2 | 31–40 |
| 23±2 | 20–27 |
|
Independent prognostic value of the
expression of TRIM44
The present study performed multivariate Cox
regression analysis to examine whether the predicative value of the
expression of TRIM44 is independent of tumor stage in predicting
prognosis in EOC. The multivariate analysis showed that a high
expression of TRIM44 was an independent prognostic factor
associated with overall survival rate (HR=3.662, 95% CI
1.313–10.215, P=0.013) and disease-free survival rate (HR=4.021,
95% CI 1.586–10.192, P=0.003), respectively (Table III). Therefore, these results
indicated that the predictive value of the expression of TRIM44 was
independent of other clinicopathological factors.
 | Table III.Multivariate analysis of overall
survival and disease-free survival rates in 109 patients with
epithelial ovarian cancer. |
Table III.
Multivariate analysis of overall
survival and disease-free survival rates in 109 patients with
epithelial ovarian cancer.
| Variable | HR | 95% CI |
P-valuea | HR | 95% CI |
P-valuea |
|---|
| FIGO stage | 1.858 | 1.165–2.963 | 0.009 | 1.835 | 1.187–2.836 | 0.006 |
| TRIM44 | 3.662 | 1.313–10.215 | 0.013 | 4.021 | 1.586–10.192 | 0.003 |
Discussion
In the present study, the expression of TRIM44 in
109 EOC specimens was investigated using immunohistochemistry of
the protein levels. To the best of our knowledge, the present study
provides one of the most comprehensive reports on the correlation
between the expression of TRIM44 and the clinicopathological
features of EOC in the literature. The results of the present study
indicated that the expression of TRIM44 was significantly higher in
cases assigned a high FIGO stage (P<0.001). The expression of
TRIM44 was also significantly increased in patients with cancer
exhibiting lymph node metastasis (P=0.015). To examine the
association between the expression of TRIM44 and patient prognosis,
the present study first analyzed the protein expression of TRIM44
in EOC and found a prognostic correlation. The data demonstrated
that patients with a high expression of TRIM44 had significantly
poorer overall survival and disease-free survival rates, compared
with patients with a low expression of TRIM44. A multivariate
analysis showed that the expression of TRIM44 was an independent
prognostic factor for overall survival and disease-free survival
rates in patients with EOC. Consistent with previous reports
examining the role of TRIM44 in the progression of other tumor
types, the group with a high protein expression of TRIM44 also
exhibited a poor prognosis (6,7,9).
Previous reports have investigated the pathogenesis
by which TRIM44 promotes cancer development. A previous study
indicated that a high expression of TRIM44 was associated with poor
prognosis and that TRIM44 may be involved in cell proliferation,
migration, and anti-apoptotic effects in testicular germ cell
tumors (7). Zhu et al
(4) demonstrated that the
overexpression of TRIM44 enhanced the invasive and migratory
capacities of hepatocellular carcinoma cells. Kashimoto et
al (5) suggested that TRIM44 may
be crucial in tumor cell proliferation through its overexpression,
and indicated its usefulness as a predictor and potential
therapeutic target in gastric cancer.
Several studies have demonstrated that TRIM44 is
involved in cancer progression and metastasis. A previous study
demonstrated that the silencing of TRIM44 may inhibit the
proliferation, migration and invasion of human papillary thyroid
cancer cells through suppression of the Wnt/β-catenin signaling
pathway (10). Kawabata et al
demonstrated that TRIM44 knockdown attenuated the tumor necrosis
factor-α-dependent phosphorylation of the p65 subunit of nuclear
factor (NF)-κB and inhibitor of NF-κBα in breast cancer cells
(11). Another study inferred that
the knockdown of TRIM44 inhibited the proliferation and invasion of
prostate cancer cells through inactivation of the phosphoinositide
3-kinase/Akt signaling pathway (3).
A study in China found that TRIM44 may promote proliferation and
metastasis in non-small cell lung cancer via the mammalian target
of rapamycin signaling pathway (6).
The present study was limited to several known
clinicopathological factors that were examined for association with
the protein expression of TRIM44. Further investigations are
required to evaluate this expression with other risk factors for
EOC.
The present study demonstrated that overexpression
of TRIM44 protein can be used as an independent prognostic factor
for assessing disease progression and poor prognosis. These results
suggest that TRIM44 may be an attractive therapeutic target for the
treatment of EOC. However, these findings require confirmation in a
larger study.
Acknowledgements
The authors would like to thank Dr Huike Yang
(Department of Anatomy, Harbin Medical University) for the
statistical analysis.
Funding
This study was supported by grants of the Abroad
Science Foundation of Heilongjiang Province (grant no. LC2012C14).
The funders had no role in study design, data collection and
analysis, decision to publish, or preparation of the
manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RM conceived and designed the experiments; SL, HY,
HJ and JZ performed the experiments and analyzed the data; RM wrote
the manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was completed in compliance with
the Helsinki Declaration and was approved by the Ethical Committee
of the Harbin Medical University Cancer Hospital. The data
collection and analysis were performed without disclosing patients'
identities.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TRIM44
|
tripartite motif-containing 44
|
|
EOC
|
epithelial ovarian cancer
|
|
FIGO
|
International Federation of Gynecology
and Obstetrics
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tan Y, Yao H, Hu J and Liu L: Knockdown of
TRIM44 inhibits the proliferation and invasion in prostate cancer
cells. Oncol Res. 25:1253–1259. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu X, Wu Y, Miao X, Li C, Yin H, Yang S,
Lu X, Liu Y, Chen Y, Shen R, et al: High expression of TRIM44 is
associated with enhanced cell proliferation, migration, invasion,
and resistance to doxorubicin in hepatocellular carcinoma. Tumour
Biol. 37:14615–14628. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kashimoto K, Komatsu S, Ichikawa D, Arita
T, Konishi H, Nagata H, Takeshita H, Nishimura Y, Hirajima S,
Kawaguchi T, et al: Overexpression of TRIM44 contributes to
malignant outcome in gastric carcinoma. Cancer Sci. 103:2021–2026.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xing Y, Meng Q, Chen X, Zhao Y, Liu W, Hu
J, Xue F, Wang X and Cai L: TRIM44 promotes proliferation and
metastasis in non-small cell lung cancer via mTOR signaling
pathway. Oncotarget. 7:30479–30491. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamada Y, Takayama KI, Fujimura T,
Ashikari D, Obinata D, Takahashi S, Ikeda K, Kakutani S, Urano T,
Fukuhara H, et al: A novel prognostic factor TRIM44 promotes cell
proliferation and migration, and inhibits apoptosis in testicular
germ cell tumor. Cancer Sci. 108:32–41. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Silverberg SG: Histopathologic grading of
ovarian carcinoma: A review and proposal. Int J Gynecol Pathol.
19:7–15. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ong CA, Shapiro J, Nason KS, Davison JM,
Liu X, Ross-Innes C, O'Donovan M, Dinjens WN, Biermann K, Shannon
N, et al: Three-gene immunohistochemical panel adds to clinical
staging algorithms to predict prognosis for patients with
esophageal adenocarcinoma. J Clin Oncol. 31:1576–1582. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou Z, Liu Y, Ma M and Chang L: Knockdown
of TRIM44 inhibits the proliferation and invasion in papillary
thyroid cancer cells through suppressing the Wnt/β-catenin
signaling pathway. Biomed Pharmacother. 96:98–103. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawabata H, Azuma K, Ikeda K, Sugitani I,
Kinowaki K, Fujii T, Osaki A, Saeki T, Horie-Inoue K and Inoue S:
TRIM44 is a poor prognostic factor for breast cancer patients as a
modulator of NF-κB signaling. Int J Mol Sci. 18:E19312017.
View Article : Google Scholar : PubMed/NCBI
|