Introduction
Non-alcoholic fatty liver disease (NAFLD) is the
most common form of liver disease, and encompasses a spectrum of
liver conditions, including simple steatosis, steatohepatitis and
end-stage liver disease (1,2). NAFLD is now the liver disease
associated with the highest mortality rate, as a consequence of
increased risk of cardiovascular disease and hepatocellular
carcinoma (3). NAFLD is also
associated with metabolic diseases, including diabetes mellitus,
obesity and hypertension (4,5). In a 5-year retrospective review,
participants with NAFLD had higher risks of impaired fasting
glucose and type 2 diabetes mellitus (T2DM) compared with
NAFLD-free controls (2).
NAFLD is generally asymptomatic at presentation and
frequently identified among individuals with conditions including
obesity, T2DM, metabolic syndrome and pathological alterations of
liver tissues (1). NAFLD is
primarily characterized by accumulation of triacylglycerol in the
liver (2). Ingestion of high-fat
foods is a key inducer of excessive fat accumulation in the liver,
resulting in insulin resistance (IR), dyslipidemia and NAFLD
(3,6,7). Current
treatments for NAFLD include weight reduction by lifestyle change,
insulin sensitizer agents, lipid-lowering drugs and antioxidants
(5,6,8,9). Antidiabetic drugs, which improve IR,
have a notable effect on NAFLD and slow down the progression of
symptoms (4,5).
Sirtuin 1 (SIRT1), a mammalian sirtuin, is an
NAD+-dependent protein deacetylase, which functions as
an important regulator of energy hemostasis in response to nutrient
availability (10). Adenosine
monophosphate-activated protein kinase (AMPK) acts as a cellular
metabolic switch in regulating fatty acid synthesis and oxidation,
maintaining the balance of metabolism in cells and the body
(11). AMPK relies on SIRT1 activity
to regulate the gene expression of fatty acid metabolism, and AMPK
and SIRT1 are important in maintaining energy hemostasis and
regulating fatty acid metabolism (12,13).
Dipeptidyl peptidase-4 (DPP-4) is a serine protease
that contributes to inactivation of incretin hormones, including
glucagon-like peptide-1 (GLP-1) (14,15).
DPP-4 inhibitors have been developed as oral anti-hyperglycemic
agents (16). DPP-4 inhibitors
increase GLP-1 levels and inhibit glucagon release, which in turn
enhances insulin secretion, and ameliorates liver enzymes and
hepatocyte ballooning in non-alcoholic steatohepatitis patients
with T2DM (15–18). Sitagliptin, a recently developed
DPP-4 inhibitor, has been widely used to treat T2DM and has also
been evaluated in diabetic patients with NAFLD symptoms (6,18,19).
However, the effect of sitagliptin on reducing fatty liver in NAFLD
patients requires further investigation and its mechanism remains
unknown.
In the present study, a rat model of NAFLD was
established by administration of a high-fat diet (HFD), and the
effect of sitagliptin on the progression of NAFLD was evaluated.
With this model, the preventive and therapeutic efficacy of
sitagliptin on lipid accumulation in blood and liver was evaluated.
Furthermore, the underlying mechanisms involving the SIRT1/AMPK
signaling pathway were investigated.
Materials and methods
Animals
The following animal studies were approved by the
Animal Care and Ethics Committee of Putuo Hospital Affiliated to
Shanghai University of Traditional Chinese Medicine (Shanghai,
China) and performed in accordance with the Guide for the Care and
Use of Laboratory Animals (20).
Six-week old male Sprague-Dawley rats (~200 g) were obtained from
Shanghai Laboratory Animal Center (Shanghai, China) and bred under
25°C and 60% relative humidity with a 12-h light/dark cycle and
unlimited food and water supplied. Rats were randomly divided into
2 groups: Normal control (NC) group (n=16, fed with normal diet: 10
kcal% fat, 20 kcal% protein and 70 kcal% carbohydrate; cat. no.
D12450B) and high fat (HF) group (n=26, fed with HFD: 45 kcal% fat,
20 kcal% protein and 35 kcal% carbohydrate; cat. no. D12451) (both
Research Diets, Inc., New Brunswick, NJ, USA) (21). After 12 weeks of feeding, 6 rats from
each group were randomly selected and analyzed in order to confirm
the establishment of the NAFLD model in the HF group compared with
the NC group (22). Other rats in
the HF group were then divided into 2 subgroups:
Sitagliptin-treated group (HF + XI) (n=10, HFD-fed and 100
mg/kg/day sitagliptin) and HF only group (n=10, HFD-fed and an
equal volume of saline) (23–25).
Rats were treated through gavage every day for the next 8 weeks.
During the experiments, body weight and food intake were monitored
twice per week. At week 20, rats were fasted overnight and
sacrificed. Blood samples were collected from the abdominal aorta.
Half of the liver tissues were excised, flash frozen in liquid
nitrogen, and stored at −80°C until further processing. The other
halves of the liver tissues were fixed in 10% formalin solution for
24 h at room temperature.
Serum analysis
Sera were separated from blood samples by
centrifugation at 1,500 × g at 20°C for 15 min after coagulation.
Alanine transferase (ALT), aspartate aminotransferase (AST),
triglycerides (TG), total cholesterol (TC), fasting blood glucose
(FBG) and free fatty acid (FFA) in the serum were analyzed using an
automatic biochemical analyzer (Dimension® RxL
Max®; Siemens Healthineers, Erlangen, Germany). The
fasting serum insulin level was measured using a rat insulin ELISA
kit (cat. no. RAB0904-1KT; Sigma-Aldrich; Merck, KGaA, Darmstadt,
Germany). Homeostatic model assessment of IR (HOMA-IR) was
calculated as previously described (26).
Liver lipid test and histological
analysis
Liver tissues were weighed and homogenized in PBS
(20 ml/g of tissue). Lipids were then extracted from the liver
tissue lysates using a chloroform/methanol (2:1) mixture (21). TG was determined using the Serum
Triglyceride Determination kit (TR0100, Sigma-Aldrich; Merck
KGaA).
Liver tissues fixed in formalin were embedded in
paraffin and serial sections (5 µm thickness) were cut from each
block. Sections were stained with hematoxylin and eosin and images
were captured using an Olympus BX51WI microscope (magnification,
×100; Olympus Corporation, Tokyo, Japan) and then used for
histological feature analysis in a blind manner by two pathologists
(21). Features examined included
steatosis, inflammation and hepatocellular ballooning and were
evaluated with the previously described scoring systems (27,28).
Steatosis and lobular inflammation was scored from 0 to 3,
respectively. Hepatocyte ballooning was scored from 0 to 2. NAFLD
activity score (NAS) was the sum of steatosis, inflammation and
ballooning scores and ranged from 0 to 8 as previously described
(29). Oil Red O staining was
performed as previously described and images were captured with an
Olympus BX51W1 light microscope at magnification, ×100 (21,29).
Reverse transcription-quantitative
polymerase chain reaction (qPCR)
Total RNA was isolated from liver tissues using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and was reverse transcribed using a Prime Script
RT Reagent kit (Takara Bio, Inc., Otsu, Japan). qPCR was conducted
using LightCycler® 480 SYBR Green I Master kit (Roche
Diagnostics, Mannheim, Germany) with the LightCycler 480II
Real-Time PCR system (Roche Diagnostics) under the following
conditions: Denature at 95°C for 30 sec; 40 cycles of 95°C for 5
sec and 60°C for 34 sec. Relative expression levels of tested genes
were calculated and normalized to GAPDH using the 2−ΔΔCq
method (21). Primers used for qPCR
were as follows: SIRT1, forward, 5′-GATGATGCTGACAGACCGGA-3′ and
reverse, 5′-AGTTCCCAATGCTGGTGGAG-3′; AMPKα1, forward,
5′-GAGCCCTGAACTTGCTTTTACA-3′ and reverse,
5′-TGTCCGTTCTATGCGCTGG-3′; acetyl CoA carboxylase 1 (ACC1),
forward, 5′-GCGGCTCTGGAGGTATATGTT-3′ and reverse,
5′-TCATGCCGTAGTGGTTGAGG-3′; carnitine palmitoyltransferase 1
(CPT1), forward, 5′-GTCTGAGCCATGGAGGTTGT-3′ and reverse,
5′-GGAGACACCATAGCCGTCAT-3′; FAS, forward,
5′-GGTTCATTTGGCGGACTGTG-3′ and reverse, 5′-CACAGCCTTCTCCTCCTGTG-3′;
GAPDH, forward, 5′-TGATGGGTGTGAACCACGAG-3′ and reverse,
5′-ATCACGCCACAGCTTTCCAG-3′.
Western blot analysis
Lysates were extracted from liver tissues using a
radioimmunoprecipitation buffer (Cell Signaling Technology, Inc.,
Danvers, MA, USA) with a cOmplete® mini protease
inhibitor (Roche Diagnostics). Protein concentration was determined
using a BCA kit (Thermo Fisher Scientific, Inc.) and 40 µg of total
protein per lane was fractionated on SDS-PAGE, and transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). Membranes were then blocked with 5% bovine serum albumin
(cat. no. A9647) in Tris-buffered saline (pH 8.0) with 0.1%
Tween-20 (all Sigma-Aldrich; Merck KGaA) for 1 h at 20°C. Primary
antibodies targeting, pAMPKα (Thr-172; cat. no. 2531, 1:1,000),
AMPKα (cat. no. 2532, 1:1,000), ACC1 (cat. no. 4190, 1:1,000),
pACC1 (Ser-79) (cat. no. 3661, 1:1,000), FAS (cat. no. 3189,
1:1,000) (all Cell Signaling Technology, Inc., Danvers, MA, USA),
CPT1A (cat. no. ab83862, 1:1,000, Abcam, Cambridge, MA, USA), SIRT1
(cat. no. sc-15404, 1:500) and GAPDH (cat. no. sc-47724, 1:10,000)
(both Santa Cruz Biotechnology, Inc., Dallas, TX, USA) were used
for immunoblotting at 4°C for 16 h. Blots were then incubated with
goat anti-rabbit immunoglobulin G (IgG)-horse radish peroxidase
(HRP) (cat. no. sc-2004) or goat anti-mouse IgG-HRP (cat. no.
sc-2005) (both Santa Cruz Biotechnology) at 1:10,000 dilution at
20°C for 2 h and detected with SuperSignal West Pico Substrate
(Thermo Fisher Scientific, Inc.) and exposed to X-ray films (Thermo
Fisher Scientific, Inc.). Densitometry analysis of target genes was
performed using Image Pro Plus 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Statistical comparisons were performed with one-way
analysis of variance and Tukey's repeated measures test. Graphpad
Prism version 5 software was used (GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Sitagliptin improves HFD-induced
abnormal lipid accumulation in blood and liver
To generate a rat model of NAFLD, rats were fed with
HFD for 20 weeks, which mimicked the long-term ingestion of HF
content in obese individuals. Compared with the NC group, HFD-fed
rats had significantly increased wet liver weight and higher
liver/body weight ratio, although there was no significant
difference in their body weights (Table
I). Serological analysis demonstrated that HFD induced
significantly higher levels of glucose, insulin, TG and FFA
compared with a normal diet. HOMA-IR analysis suggested that HFD
significantly induced IR in rats. Significantly higher levels of
serum ALT and AST activity in the HF group compared with the NC
group indicated abnormal liver function was induced by HFD. These
results suggest that HFD successfully induced NAFLD-like symptoms,
including abnormal lipid accumulation in the serum and liver
dysfunction.
 | Table I.Effect of sitagliptin on
characteristics of the high fat diet-induced non-alcoholic fatty
liver disease model. |
Table I.
Effect of sitagliptin on
characteristics of the high fat diet-induced non-alcoholic fatty
liver disease model.
| Characteristic | NC (n=10) | HF (n=10) | HF + XI (n=10) |
|---|
| Body weight
(g) | 597.4±56.47 | 608.9±40.32 | 593.20±48.94 |
| Liver weight
(g) |
16.33±3.79a | 22.77±4.39 | 20.67±3.34 |
| Liver/body weight
ratio |
2.74±0.67a | 3.56±1.87 | 3.27±1.39 |
| FBG (mmol/l) |
6.03±0.41a | 7.12±0.63 | 6.92±0.55 |
| Insulin (µU/l) |
26.13±8.48a | 33.35±9.41 |
28.61±7.56a |
| HOMA-IR |
6.98±1.92a | 7.69±3.27 |
7.26±2.45a |
| TG (mmol/l) |
0.38±0.13a | 0.72±0.24 |
0.42±0.54a |
| TC (mmol/l) | 2.34±0.94 | 2.67±0.40 | 2.58±0.78 |
| ALT (U/l) |
36.93±8.62a | 49.24±10.04 | 45.63±9.34 |
| AST (U/l) |
45.23±9.34a | 56.65±12.31 | 52.22±13.42 |
| FFA (mmol/l) |
9.76±2.21a | 11.88±3.36 |
8.54±2.76a |
| Liver TG
(mmol/l) |
11.86±4.61a | 15.32±2.24 |
9.67±2.66a,b |
Furthermore, it was investigated whether sitagliptin
could affect these physiological and biochemical parameters and
improve IR induced by HFD in the rat NAFLD model (Table I). Notably, sitagliptin significantly
suppressed the increase of insulin induced by HFD without affecting
the FBG level. Sitagliptin also significantly suppressed serum TG
and FFA induced by HFD, resulting in improved IR, as demonstrated
by significantly decreased HOMA-IR in the HF + XI group compared
with the HF group. Sitagliptin treatment also exhibited mild
effects on other abnormal alterations induced by HFD, but these
were not observed to be significant. These findings indicate that
sitagliptin improves NAFLD-like symptoms induced by HFD in a rat
model.
Sitagliptin suppresses HFD-induced
pathological changes in the rat liver
To determine whether sitagliptin could directly
affect fat accumulation at liver, TG level was determined in the
rat liver (Table I). It was
identified that rats in the HF group exhibited a significantly
higher level of liver TG compared with the NC group. The HF + XI
group exhibited a significantly lower liver TG level compared with
the NC group or the HF group. These findings suggest that
sitagliptin may block the accumulation of TG in the rat liver.
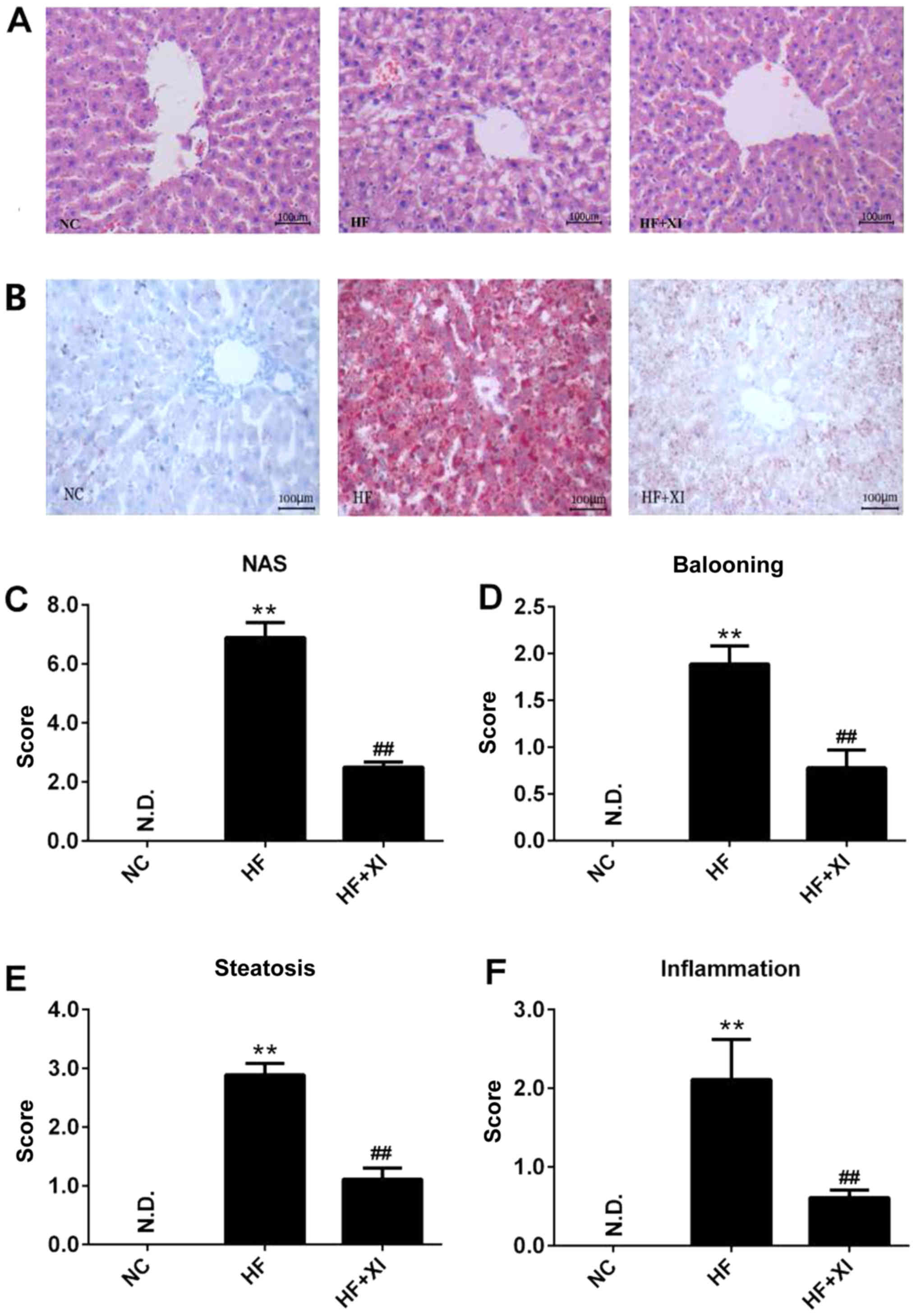
Furthermore, the pathological development of fatty
liver was evaluated (Fig. 1A). Fatty
infiltration was observed in <5% of rat liver tissues, and
ballooning and inflammation were not observed in rat liver tissues
from the NC group. Sitagliptin treatment greatly suppressed the
accumulation of fatty acid in the liver induced by HFD, which was
determined by Oil Red O staining of liver sections (Fig. 1B). In the HF group, significantly
increased fatty infiltration was observed in the midlobular region
of the liver, resulting in high NAFLD activity score (Fig. 1C). Sitagliptin treatment (HF + XI
group) significantly reduced ballooning (Fig. 1D), microvesicular steatosis (Fig. 1E), cell swelling, local inflammation
(Fig. 1F), and blocked the progress
of NAFLD-like symptoms.
Sitagliptin reactivates the
HFD-suppressed SIRT1/AMPK pathway
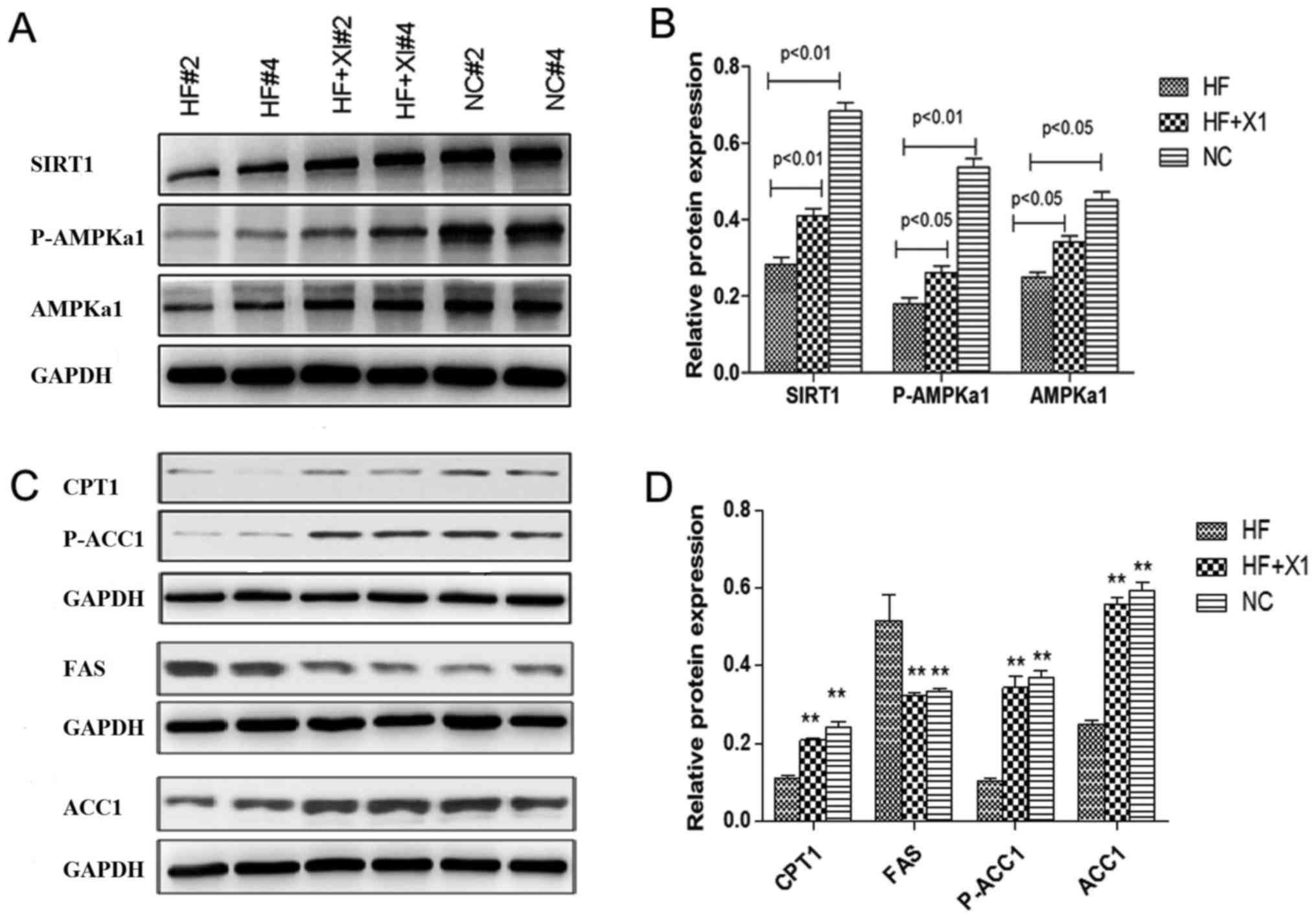
To explore the underlying mechanism of the effect of
sitagliptin, the expression levels of proteins in the SIRT1/AMPK
pathway were evaluated in rat liver lysates (Fig. 2). HFD significantly suppressed the
protein level of SIRT1 and AMPKα1, and significantly reduced the
phosphorylation of AMPKα1 at Thr-172 and of ACC at Ser-79.
Sitagliptin treatment rescued the expression of SIRT1 and total
AMPKα1, and also enhanced the phosphorylation of AMPKα1 (Fig. 2A and B).
The expression levels of FAS, ACC1 and CPT1
proteins, downstream targets of the SIRT1/AMPK pathway, were also
evaluated. HFD induced significantly increased levels of FAS
protein and significantly decreased expression of CPT1 and ACC1
phosphorylation (p-ACC1Ser79) in rat liver. Sitagliptin treatment
significantly reduced the expression of FAS protein to untreated
control level and rescued the expression of CPT1 and ACC1
phosphorylation (p-ACC1Ser79) in rat liver (Fig. 2C and D). These results demonstrated
that sitagliptin reactivates AMPK pathway downstream targets and
restores fatty acid oxidation in the rat liver.
Sitagliptin restores AMPK pathway
activity suppressed by HFD
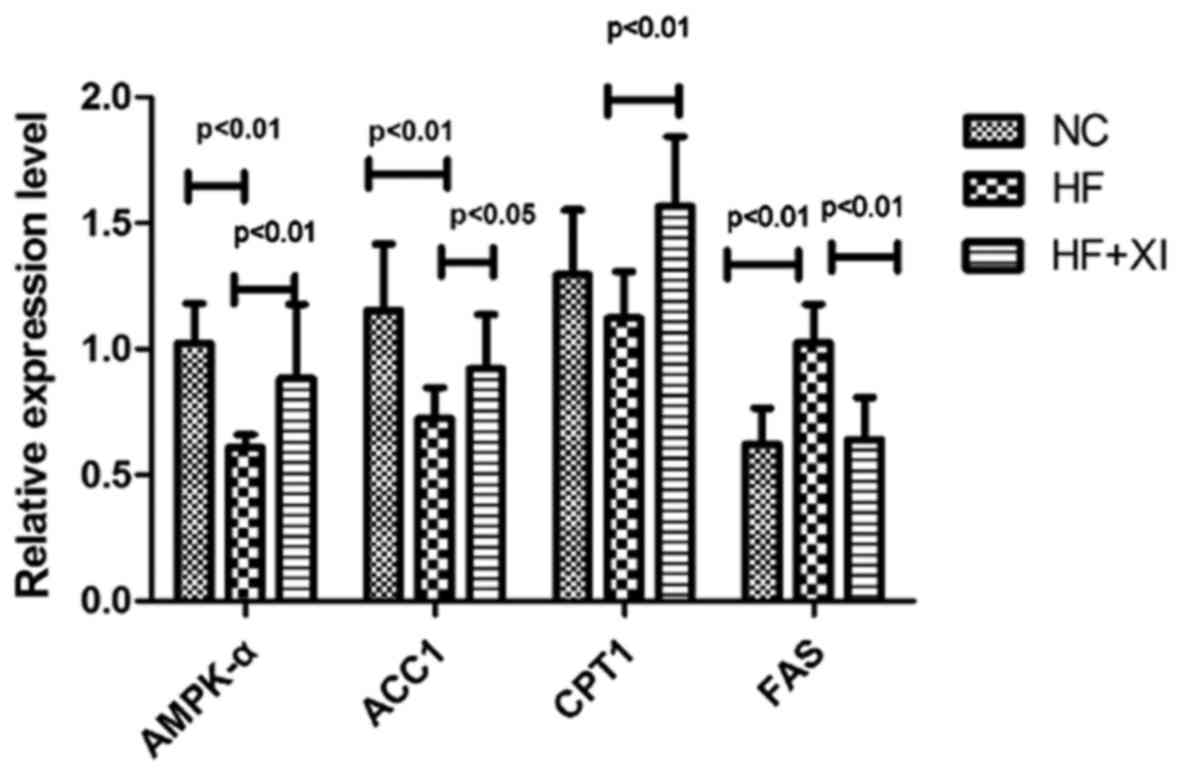
To further explore activity of the AMPK pathway,
mRNA expression of AMPK pathway genes in rat livers was evaluated
(Fig. 3). HFD significantly
suppressed the mRNA level of AMPKα1 and ACC1 and significantly
upregulated the mRNA level of FAS. Sitagliptin treatment restored
the mRNA levels of AMPKα1, ACC1, CPT1 and FAS. These results were
consistent with the alterations observed in the expression level of
ACC1, CPT1 and FAS proteins. These findings indicate that
sitagliptin restores the AMPK/CPT pathway to enhance fatty acid
oxidation, suppress fatty acid synthesis and block TG accumulation
in the rat liver.
Discussion
NAFLD is the most common cause of liver disease in
western countries, presenting in >30% of the general population
(30,31). The prevalence in Asian populations
ranges from 6 to 25% (3,22). The prevalence rate of NAFLD has
doubled in Chinese cities in the past two decades (22). Previous results have indicated that
IR serves a pathogenic function in T2MD and NAFLD (32). The pathogenesis of NAFLD was
originally described by the ‘two-hit hypothesis’, and subsequently
modified as the ‘multi-hit hypothesis’, which considers multiple
injuries acting together to induce NAFLD and provides a more
accurate explanation of NAFLD pathogenesis (33,34). IR
serves a central function in hepatic injury as a result of
dysregulation of fatty acid metabolism, leading to steatosis
(26). High levels of fat content in
the diet also contribute to lipid deposition in the liver (5,17,18,21,35).
Approximately 80% of FFA, originating from the diet, TG
decomposition of adipose tissue or liver fatty acid de novo
synthesis, is primarily transported to the liver and causes
intracellular accumulation of lipid metabolites and hepatic TG
deposition (3,5,32,36,37).
Therefore, improving IR and decreasing the level of FFA are
essential for arresting the development of NAFLD.
IR has been recognized as the primary pathogenic
mechanism of NAFLD and diabetes (32). Antidiabetic drugs, particularly
insulin sensitizer agents, have been indicated to improve NAFLD
and/or slow down its progression (6,8,19,32,37,38).
Metformin treatment improves the sensitivity of insulin response in
NAFLD patients, but it has no certain effects on liver histology
(6). Pioglitazone, a
thiazolidinedione derivative has been tested for the treatment of
NAFLD in clinical trials, however it has safety issues for
long-term treatment and a disadvantage of increasing body weight
(38). Sitagliptin, as a DPP-4
inhibitor, has been widely used in the treatment of T2DM (39,40). It
also indirectly inhibits hepatic fat accumulation and hepatic
steatosis in mice and humans (39,40). A
single-arm, open-label study revealed that long-term treatment with
sitagliptin at 100 mg/day could reduce the body mass index and ALT
levels of patients (40). Another
DPP-4 inhibitor, des-fluoro-sitagliptin, reduced the accumulation
of TG in the liver and blocked hepatic steatosis in mice fed with
linoleic acid and sucrose diet (41). These findings reveal that sitagliptin
has potential activity to improve IR and hepatic lipid
dysregulation in NAFLD.
In the present study, the effect of sitagliptin on
lipid dysregulation induced by HFD was evaluated in a rat model.
HFD treatment induced an increase of liver weight, blood/liver TG
level, FBG and insulin level, resulting in higher HOMA-IR compared
with the control group. A previous study reported that HFD also
causes peripheral IR and dysregulation of glucose and lipid
metabolism in a mouse model (41).
Sitagliptin functions as a DPP-4 inhibitor to block the degradation
of GLP-1 and gastric inhibitory polypeptide (GIP) secreted by
intestinal L cells, which promote the release of insulin and
suppress the secretion of glucagon in order to reduce the blood
glucose level (42). This blockade
relies on the intestinal glucose level. In the present study, HF
diet caused a slight increase of FBG, while sitagliptin reduced,
instead of enhanced, the production of insulin. These results
indicate that dysfunction of glucose metabolism in the tested model
was not sufficient to enhance GLP-1 and GIP secretion and stimulate
the generation of endogenous insulin. Furthermore, sitagliptin
significantly reduced hepatic TG level in the HF + XI group and
reversed lipid accumulation in the liver. Based on the combination
of reduced insulin level and HOMA-IR, sitagliptin induced a notable
improvement in HFD-induced hyperinsulinemia and IR.
In the present study, it was identified that
sitagliptin reactivated the SIRT1/AMPK pathway, which was
suppressed by HFD treatment. SIRT1, a mammalian sirtuin, is an
NAD+-dependent protein deacetylase that serves a key
function in regulating energy homeostasis in response to nutrient
availability (10). SIRT1 can
enhance insulin sensitivity, regulate liver fat metabolism,
suppress oxidative stress and reduce inflammatory response in NAFLD
(21,35,43).
AMPK serves a central function in controlling lipid metabolism
through modulating the phosphorylation of ACC and regulating the
activity of CPT1 (11,18,44,45). In
the present study, the presence of high levels of fatty acid
suppressed SIRT1 and AMPK activity, resulting in reduced fatty acid
utilization and abnormal lipid deposition in the liver. Sitagliptin
reactivated the expression of SIRT1 and AMPK proteins in the rat
liver.
Aside from its role in direct phosphorylation of
target proteins, AMPK functions as an important transcription
factor in regulating the response to stress and alterations in
metabolism (18,44,46).
AMPK can directly control the status of histone H2B
phosphorylation, which recruits transcription factors to bind with
DNA, and further regulates the transcription of AMPK pathway target
genes such as Acc1 and Cpt1c (46).
In the present study, it was identified that the reactivated
SIRT1/AMPK pathway upregulated the transcription level of
downstream target genes ACC1 and CPT1, while FAS mRNA level was
downregulated. These findings are consistent with the function of
sitagliptin in suppressing fatty acid synthesis and enhancing fatty
acid β-oxidation. Therefore, reactivation of SIRT1/AMPK pathway is
indicated to be one of the primary mechanisms of sitagliptin in the
prevention of NAFLD. The present study demonstrated that
sitagliptin is an effective agent in preventing the development of
hepatic lipid dysregulation and has potential as a clinical
therapeutic strategy for NAFLD.
Acknowledgements
Not applicable.
Funding
This study was supported by the Shanghai Municipal
Commission of Health and Family Planning Project (grant no.
2013Y079), the Backup Medical Expert Cultivation Plan of Putuo
Hospital Affiliated to Shanghai University of Traditional Chinese
Medicine (grant no. B-X-79) and Shanghai Putuo District Key
Clinical Specialty Project, Endocrinology (grant no.
2016PTZK05).
Availability of data and materials
The datasets generated during the current study are
not publicly available as they are currently being used for further
research, however they are available from the corresponding author
on reasonable request following the completion of this
research.
Authors' contributions
TS and TL conceived and designed the research. TS,
BLX, ZHN and CPZ performed the experiments and statistical
analysis. TS, CPZ and LC acquired and interpreted the data. TS and
LC drafted and revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Animal Care and Ethics
Committee of Putuo Hospital Affiliated to Shanghai University of
Traditional Chinese Medicine (Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Angulo P: Nonalcoholic fatty liver
disease. N Engl J Med. 346:1221–1231. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dowman JK, Tomlinson JW and Newsome PN:
Pathogenesis of non-alcoholic fatty liver disease. QJM. 103:71–83.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hassan K, Bhalla V, El Regal ME and
A-Kader HH: Nonalcoholic fatty liver disease: A comprehensive
review of a growing epidemic. World J Gastroenterol.
20:12082–12101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Higuera-de la Tijera F and Servín-Caamaño
AI: Pathophysiological mechanisms involved in non-alcoholic
steatohepatitis and novel potential therapeutic targets. World J
Hepatol. 7:1297–1301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hui E, Xu A, Yang Bo H and Lam KS: Obesity
as the common soil of non-alcoholic fatty liver disease and
diabetes: Role of adipokines. J Diabetes Investig. 4:413–425. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fruci B, Giuliano S, Mazza A, Malaguarnera
R and Belfiore A: Nonalcoholic Fatty liver: A possible new target
for type 2 diabetes prevention and treatment. Int J Mol Sci.
14:22933–22966. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leite NC, Villela-Nogueira CA, Cardoso CR
and Salles GF: Non-alcoholic fatty liver disease and diabetes: From
physiopathological interplay to diagnosis and treatment. World J
Gastroenterol. 20:8377–8392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Del Ben M, Polimeni L, Baratta F, Pastori
D, Loffredo L and Angelico F: Modern approach to the clinical
management of non-alcoholic fatty liver disease. World J
Gastroenterol. 20:8341–8350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dixon JB, Bhathal PS, Hughes NR and
O'Brien PE: Nonalcoholic fatty liver disease: Improvement in liver
histological analysis with weight loss. Hepatology. 39:1647–1654.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang HC and Guarente L: SIRT1 and other
sirtuins in metabolism. Trends Endocrinol Metab. 25:138–145. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hardie DG: AMP-activated/SNF1 protein
kinases: Conserved guardians of cellular energy. Nat Rev Mol Cell
Biol. 8:774–785. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hou X, Xu S, Maitland-Toolan KA, Sato K,
Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, et al:
SIRT1 regulates hepatocyte lipid metabolism through activating
AMP-activated protein kinase. J Biol Chem. 283:20015–20026. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Price NL, Gomes AP, Ling AJ, Duarte FV,
Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro
JS, et al: SIRT1 is required for AMPK activation and the beneficial
effects of resveratrol on mitochondrial function. Cell Metab.
15:675–690. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boschmann M, Engeli S, Dobberstein K,
Budziarek P, Strauss A, Boehnke J, Sweep FC, Luft FC, He Y, Foley
JE and Jordan J: Dipeptidyl-peptidase-IV inhibition augments
postprandial lipid mobilization and oxidation in type 2 diabetic
patients. J Clin Endocrinol Metab. 94:846–852. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Itou M, Kawaguchi T, Taniguchi E and Sata
M: Dipeptidyl peptidase-4: A key player in chronic liver disease.
World J Gastroenterol. 19:2298–2306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McIntosh CH, Demuth HU, Pospisilik JA and
Pederson R: Dipeptidyl peptidase IV inhibitors: How do they work as
new antidiabetic agents? Regul Pept. 128:159–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Y, Wei R and Hong TP: Potential roles
of glucagon-like peptide-1-based therapies in treating
non-alcoholic fatty liver disease. World J Gastroenterol.
20:9090–9097. 2014.PubMed/NCBI
|
|
18
|
Ideta T, Shirakami Y, Miyazaki T, Kochi T,
Sakai H, Moriwaki H and Shimizu M: The dipeptidyl peptidase-4
inhibitor teneligliptin attenuates hepatic lipogenesis via AMPK
activation in non-alcoholic fatty liver disease model mice. Int J
Mol Sci. 16:29207–29218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Blaslov K, Bulum T, Zibar K and Duvnjak L:
Incretin based therapies: A novel treatment approach for
non-alcoholic fatty liver disease. World J Gastroenterol.
20:7356–7365. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. The National Academies Press; Washington, DC: 2011
|
|
21
|
Xu F, Li Z, Zheng X, Liu H, Liang H, Xu H,
Chen Z, Zeng K and Weng J: SIRT1 mediates the effect of GLP-1
receptor agonist exenatide on ameliorating hepatic steatosis.
Diabetes. 63:3637–3646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan JG: An introduction of strategies for
the management of nonalcoholic fatty liver disease (NAFLD)
recommended by asia pacific working party on NAFLD. Zhonghua Gan
Zang Bing Za Zhi. 15:552–553. 2007.(In Chinese). PubMed/NCBI
|
|
23
|
Chiang YT, Ip W, Shao W, Song ZE, Chernoff
J and Jin T: Activation of cAMP signaling attenuates impaired
hepatic glucose disposal in aged male p21-activated protein
kinase-1 knockout mice. Endocrinology. 155:2122–2132. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gault VA, Lennox R and Flatt PR:
Sitagliptin, a dipeptidyl peptidase-4 inhibitor, improves
recognition memory, oxidative stress and hippocampal neurogenesis
and upregulates key genes involved in cognitive decline. Diabetes
Obes Metab. 17:403–413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Joo KW, Kim S, Ahn SY, Chin HJ, Chae DW,
Lee J, Han JS and Na KY: Dipeptidyl peptidase IV inhibitor
attenuates kidney injury in rat remnant kidney. BMC Nephrol.
14:982013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bell LN, Wang J, Muralidharan S, Chalasani
S, Fullenkamp AM, Wilson LA, Sanyal AJ, Kowdley KV,
Neuschwander-Tetri BA, Brunt EM, et al: Relationship between
adipose tissue insulin resistance and liver histology in
nonalcoholic steatohepatitis: A pioglitazone versus vitamin E
versus placebo for the treatment of nondiabetic patients with
nonalcoholic steatohepatitis trial follow-up study. Hepatology.
56:1311–1318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takahashi Y and Fukusato T: Histopathology
of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis.
World J Gastroenterol. 20:15539–15548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takahashi Y, Soejima Y and Fukusato T:
Animal models of nonalcoholic fatty liver disease/nonalcoholic
steatohepatitis. World J Gastroenterol. 18:2300–2308. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kleiner DE, Brunt EM, Van Natta M, Behling
C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS,
Unalp-Arida A, et al: Design and validation of a histological
scoring system for nonalcoholic fatty liver disease. Hepatology.
41:1313–1321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Browning JD, Szczepaniak LS, Dobbins R,
Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson
MS, Unalp-Arida A, et al: Prevalence of hepatic steatosis in an
urban population in the United States: Impact of ethnicity.
Hepatology. 40:1387–1395. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Loomba R and Sanyal AJ: The global NAFLD
epidemic. Nat Rev Gastroenterol Hepatol. 10:686–690. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Van Wagner LB and Rinella ME: The role of
insulin-sensitizing agents in the treatment of nonalcoholic
steatohepatitis. Therap Adv Gastroenterol. 4:249–263. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Day CP and James OF: Steatohepatitis: A
tale of two ‘hits’? Gastroenterology. 114:842–845. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Buzzetti E, Pinzani M and Tsochatzis EA:
The multiple-hit pathogenesis of non-alcoholic fatty liver disease
(NAFLD). Metabolism. 65:1038–1048. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park J, Jeon YD, Kim HL, Kim DS, Han YH,
Jung Y, Youn DH, Kang J, Yoon D, Jeong MY, et al: Veratri nigri
rhizoma et radix (Veratrum nigrum L.) and its constituent jervine
prevent adipogenesis via activation of the LKB1-AMPKα-ACC axis in
vivo and in vitro. Evid Based Complement Alternat Med.
2016:86743972016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baran B and Akyüz F: Non-alcoholic fatty
liver disease: What has changed in the treatment since the
beginning? World J Gastroenterol. 20:14219–14229. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Machado MV and Cortez-Pinto H:
Non-alcoholic fatty liver disease: What the clinician needs to
know. World J Gastroenterol. 20:12956–12980. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sanyal AJ, Chalasani N, Kowdley KV,
McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE,
Tonascia J, Unalp A, et al: Pioglitazone, vitamin E, or placebo for
nonalcoholic steatohepatitis. N Engl J Med. 362:1675–1685. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ohki T, Isogawa A, Iwamoto M, Ohsugi M,
Yoshida H, Toda N, Tagawa K, Omata M and Koike K: The effectiveness
of liraglutide in nonalcoholic fatty liver disease patients with
type 2 diabetes mellitus compared to sitagliptin and pioglitazone.
ScientificWorldJournal. 2012:4964532012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yilmaz Y, Yonal O, Deyneli O, Celikel CA,
Kalayci C and Duman DG: Effects of sitagliptin in diabetic patients
with nonalcoholic steatohepatitis. Acta Gastroenterol Belg.
75:240–244. 2012.PubMed/NCBI
|
|
41
|
Shirakawa J, Fujii H, Ohnuma K, Sato K,
Ito Y, Kaji M, Sakamoto E, Koganei M, Sasaki H, Nagashima Y, et al:
Diet-induced adipose tissue inflammation and liver steatosis are
prevented by DPP-4 inhibition in diabetic mice. Diabetes.
60:1246–1257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Singh AK: Dipeptidyl peptidase-4
inhibitors: Novel mechanism of actions. Indian J Endocrinol Metab.
18:753–759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee J, Hong SW, Chae SW, Kim DH, Choi JH,
Bae JC, Park SE, Rhee EJ, Park CY, Oh KW, et al: Exendin-4 improves
steatohepatitis by increasing Sirt1 expression in high-fat
diet-induced obese C57BL/6J mice. PLoS One. 7:e313942012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ix JH and Sharma K: Mechanisms linking
obesity, chronic kidney disease, and fatty liver disease: The roles
of fetuin-A, adiponectin, and AMPK. J Am Soc Nephrol. 21:406–412.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Viollet B, Mounier R, Leclerc J, Yazigi A,
Foretz M and Andreelli F: Targeting AMP-activated protein kinase as
a novel therapeutic approach for the treatment of metabolic
disorders. Diabetes Metab. 33:395–402. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bungard D, Fuerth BJ, Zeng PY, Faubert B,
Maas NL, Viollet B, Carling D, Thompson CB, Jones RG and Berger SL:
Signaling kinase AMPK activates stress-promoted transcription via
histone H2B phosphorylation. Science. 329:1201–1205. 2010.
View Article : Google Scholar : PubMed/NCBI
|