Introduction
Glioma is the tumor originating from neuroderm.
Glioma shows invasive growth in the brain, and is not well-defined
with normal brain tissue (1).
Therefore, it can hardly be completely removed surgically. Glioma
is not sensitive to chemotherapy or radiotherapy, and is extremely
likely to relapse (2). Glioma
growing in vital brain sites such as brain stem can hardly be
removed surgically (2). On the other
hand, drugs like chemical drugs and antitumor Chinese medicine have
unsatisfying effect due to the influence of blood brain barrier
(3). As a result, glioma remains one
of the systemic tumors with the poorest prognosis. New glioma cases
in USA from 2009 to 2013 have accounted for ~26.9% of all primary
intracranial tumors and ~84% of all malignant intracranial tumors.
Treatment for glioma remains the difficulty in Neurosurgery
(4). In addition, the genesis and
development mechanism of glioma remains unclear so far (4). Therefore, research on the genesis and
development mechanism of glioma, as well as glioma treatment at
molecular level, has become the hotspot of research in Neurosurgery
field currently (1).
Matrix metalloproteinases (MMPs) are a class of zinc
finger metalloprotein family for degrading extracellular matrix
proteins. MMPs can change the microenvironment of cell, thus
affecting various biological functions of cell (5). They include development, trauma repair,
inflammatory response, angiogenesis and pathological processes like
periodontitis, arthritis, tumor cell invasion and metastasis.
MMP-28 is a new member of the MMP family. It belongs to the MMP-19
subfamily in terms of structure (5).
It is first discovered in keratinocyte and testis. In rodent,
MMP-28 is expressed in many normal tissues, such as testis, small
intestine, skin and lung (6). This
indicates that it plays a key role in tissue homeostasis.
Researchers find that MMP-28 protein is highly expressed in some
tumors compared with that in normal tissue. However, other studies
suggest that MMP-28 protein expression is upregulated in malignant
tumor and cancer cell lines (6).
Marchenko and Strongin (7) showed
that MMP-28 is a new human MMP with an unusual cysteine-switch
sequence and widely expressed in tumors. However, MMP-28 protein
expression is widely expressed in glioma cell, and it is
unknown.
Transforming growth factor (TGF)-β is highly
expressed in multiple tumor tissues, which plays a key role during
tumor angiogenesis. Importantly, it is remarkably correlated with
tumor genesis, development and metastasis (8). Serum TGF-β concentration is notably
reduced in patients with glioma after treatment, and tumor invasion
capacity is declined (8). Therefore,
serum TGF-β is regarded to be partially correlated with the
prognosis for glioma patients (9).
Thus, serum TGF-β concentration is partially correlated with the
malignant grade of tumor (8).
Monitoring serum TGF-β can evaluate the clinical efficacy, thus
displaying certain clinical value for prognosis evaluation
(8). This study aims to investigate
the expression status of MMP-28 and its molecular mechanisms in
glioma cell.
Materials and methods
Clinical specimens
Serum samples from patients with glioma and healthy
volunteers who underwent surgical resection were obtained from the
Affiliated Hospital of Beihua University (Jilin, China) between
February 2010 and December 2014 (Table
I). Serum was stored at −80°C until analysis. The present study
was approved by the Ethics Committee of Affiliated Hospital of
Beihua University. The study was performed in accordance with the
regulations of the Institutional Review Board of Affiliated
Hospital of Beihua University. Written informed consent was
obtained prior to surgery from all enrolled patients.
 | Table I.Characteristics of glioma patients and
healthy volunteers. |
Table I.
Characteristics of glioma patients and
healthy volunteers.
| Variables | Patients (n=82) | Healthy volunteers
(n=42) |
|---|
| Age (years) |
|
|
| ≤55 | 40 | 19 |
|
>55 | 42 | 23 |
| Sex |
|
|
|
Female | 35 | 17 |
| Male | 47 | 25 |
| Tumor size (cm) |
|
|
| ≤3.0 | 15 | n/a |
|
>3.0 | 67 | n/a |
| Edmondson grade |
|
|
| I | 7 | n/a |
| II | 13 | n/a |
|
III–IV | 62 | n/a |
RNA extraction and microRNA (miRNA)
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
Total RNAs from both serum samples and cells were
extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific Inc., Waltham, MA, USA). Total RNAs was synthesize
complementary DNA using SuperScript II Reverse Transcriptase
(Invitrogen; Thermo Fisher Scientific Inc.). RT-qPCR was performed
with StepOne Real-Time PCR System (Applied Biosystems, Foster City,
CA, USA) and SYBR Premix Ex Taqä (Takara Biotechnology Co., Ltd.,
Dalian, China). The reaction conditions were pre-denaturation at
95°C for 10 min, followed by 40 cycles of denaturation at 95°C for
5 min, annealing at 60°C for 30 sec and elongation at 72°C for 30
sec. The relative expression levels were calculated using the
2−∆∆Cq method (10).
Cell lines, culture and
transfection
The U251 human glioma cell line was purchased from
the Cell Bank of Type Culture Collection of Chinese Academy of
Sciences (Shanghai, China) and maintained in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1%
antibiotic-antimycotic solution at 37°C in a humidified 5%
CO2. miRNA-34a, anti-miRNA-34a and negative control
mimics were purchased from Shanghai GenePharma Co., Ltd. (Shanghai,
China). U251 cells were transfected with miRNA-34a, anti-miRNA-34a
and negative control mimics using Lipofectamine 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol.
Cell proliferation assay
The cells (1×104 /well) were placed in
96-well plates and transfected with Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific Inc.). MTT (20 µl) was added into each
well and incubated for 4 h at 37°C. 150 µl isopropanol added and
the cells incubated at room temperature in the dark for 20 min. The
absorbance was measured using a microplate spectrophotometer
(Bio-Tek Instruments Inc., Winosski, VT, USA) at 492 nm.
Transwell assay
Cells (2×104 cells) were seeded into the
upper chambers of Transwell chambers (Corning Inc., Corning, NY,
USA) and 500 µl DMEM supplemented with 10% FBS was added into the
lower wells as the chemo-attractant. After cultivation for 48 h,
the filters were stained with crystal violet.
Cell apoptosis assay
Cell was washed with PBS and harvested at 1,000 g
for 10 min at room temperature. Cell was stained with Annexin V
(allophycocyanin) and propidium iodide for 15 min at room
temperature in the dark. Apoptosis rate was acquired with a
fluorescence-activated cell sorting Canto II flow cytometer (BD
Biosciences, San Jose, CA, USA) and analyzed using Flowjo 7.6.1
(FlowJo, LLC, Ashland, OR, USA).
Western blot analysis
Cellular nuclear protein was extracted using PIRA
assay and the protein concentration was detected using a BCA kit
(Beyotime Institute of Biotechnology, Haimen, China). 50 µg of
protein were separated using 10% SDS-PAGE gel and transferred onto
PVDF membranes (EMD Millipore, Billerica, MA, USA). Membranes were
blocked with 5% skim milk in TBST for 2 h at room temperature, and
incubated with primary antibodies MMP-28, TGF-β and GAPDH overnight
at 4°C. Membranes were washed with TBST for 15 min and incubated
with corresponding horseradish peroxidase-conjugated secondary
antibodies (Beyotime Institute of Biotechnology; 1:1,000 dilution)
for 1 h at room temperature. Membranes were visualized using a
Millipore Enhanced Chemiluminescence system and analyzed using
Image Lab 3.0 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Immunofluorescence
Cells were washed with PBS and fixed in 4%
paraformaldehyde at 4°C for 15 min at room temperature. Cells were
blocked with 5% BSA and 0.25 Triton X-100 for 1 h. Cells were
incubated with TGF-β at 4°C overnight. Cells were washed with PBST,
555-secondary antibodies (HRP conjugated, PerkinElmer, Inc.,
Waltham, MA, USA) were used for 1 h at 37°C. Cells were stained
with DAPI assay for 15 min at darkness and washed with PBST for 15
min. Laser scanning confocal microscopy (Leica Microsystems GmbH,
Wetzlar, Germany) was used for cell observation.
Statistical analysis
The data were expressed as the mean ± standard
deviation, and were analyzed using SPSS 17.0 software (SPSS, Inc.,
Chicago, IL, USA). The differences between different groups were
compared using Student t-tests or one-way analysis of variance and
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
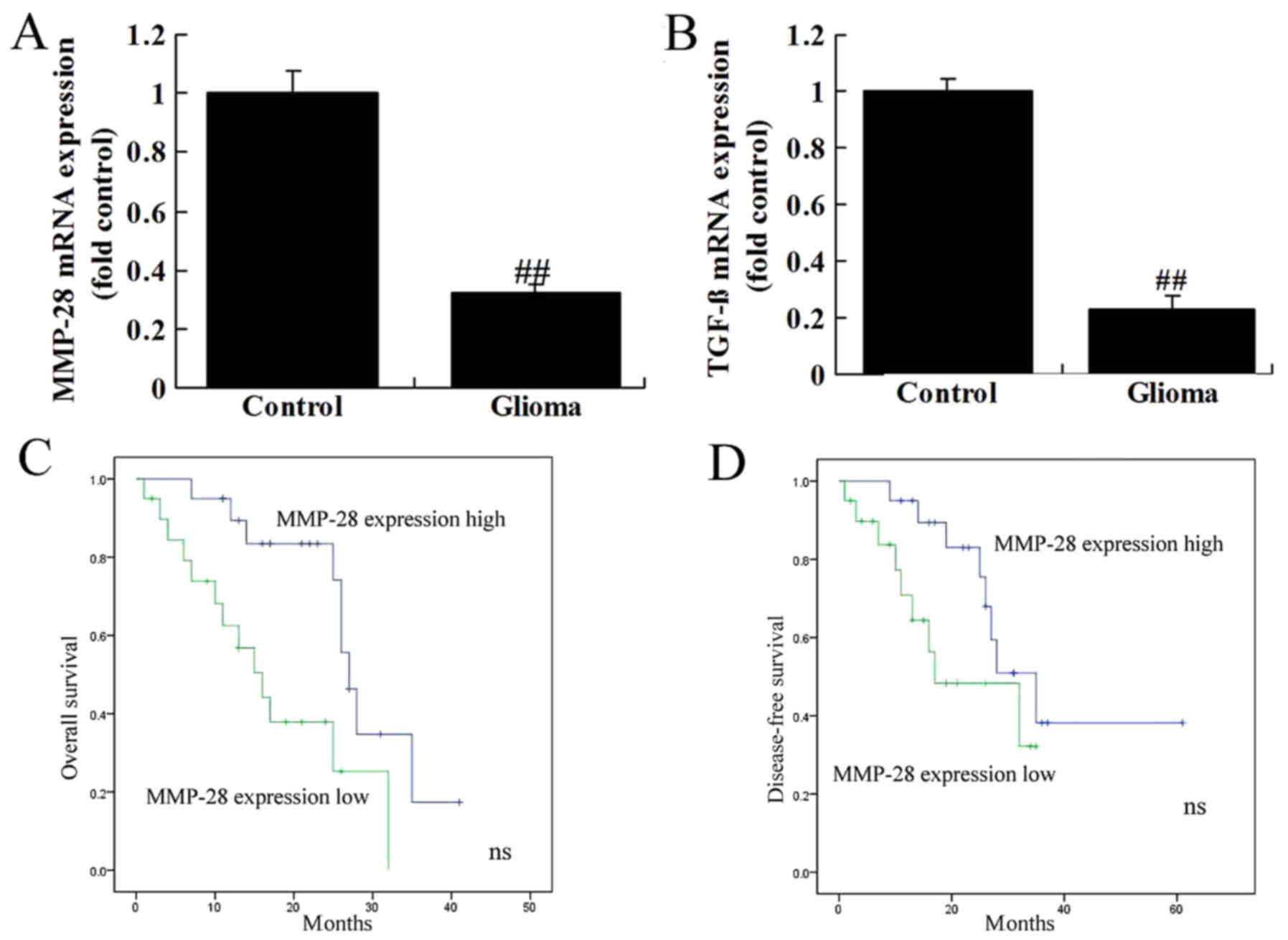
MMP-28 and TGF-β expression in glioma
patients
The mRNA expression of MMP-28 and TGF-β was reduced
in glioma patients, compared normal group (Fig. 1A and B). Overall survival (OS) and
disease-free survival (DFS) of patients with low MMP-28 expression
were lower than those with low MMP-28 expression (Fig. 1C and D).
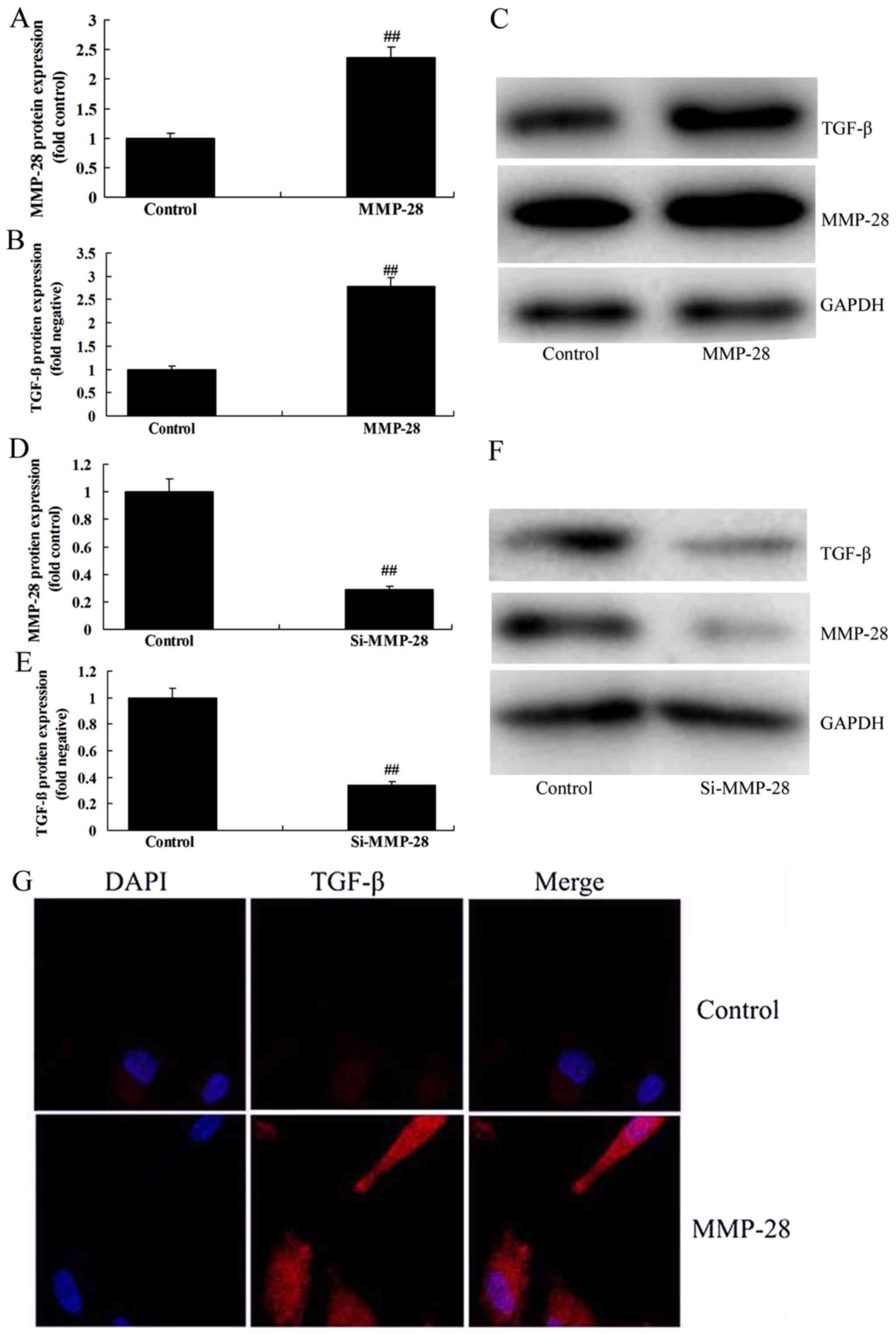
MMP-28 regulated TGF-β protein
expression in glioma cell
Further, we investigated whether MMP-28 regulated
TGF-β protein expression in glioma cell. As shown in Fig. 2A-F, over-expression of MMP-28 induced
TGF-β protein expression, while downregulation of MMP-28 suppressed
TGF-β protein expression in glioma cell, compared with control
group. In addition, immunofluorescence showed that over-expression
of MMP-28 induced TGF-β protein expression in glioma cell, in
comparison with control group (Fig.
2G).
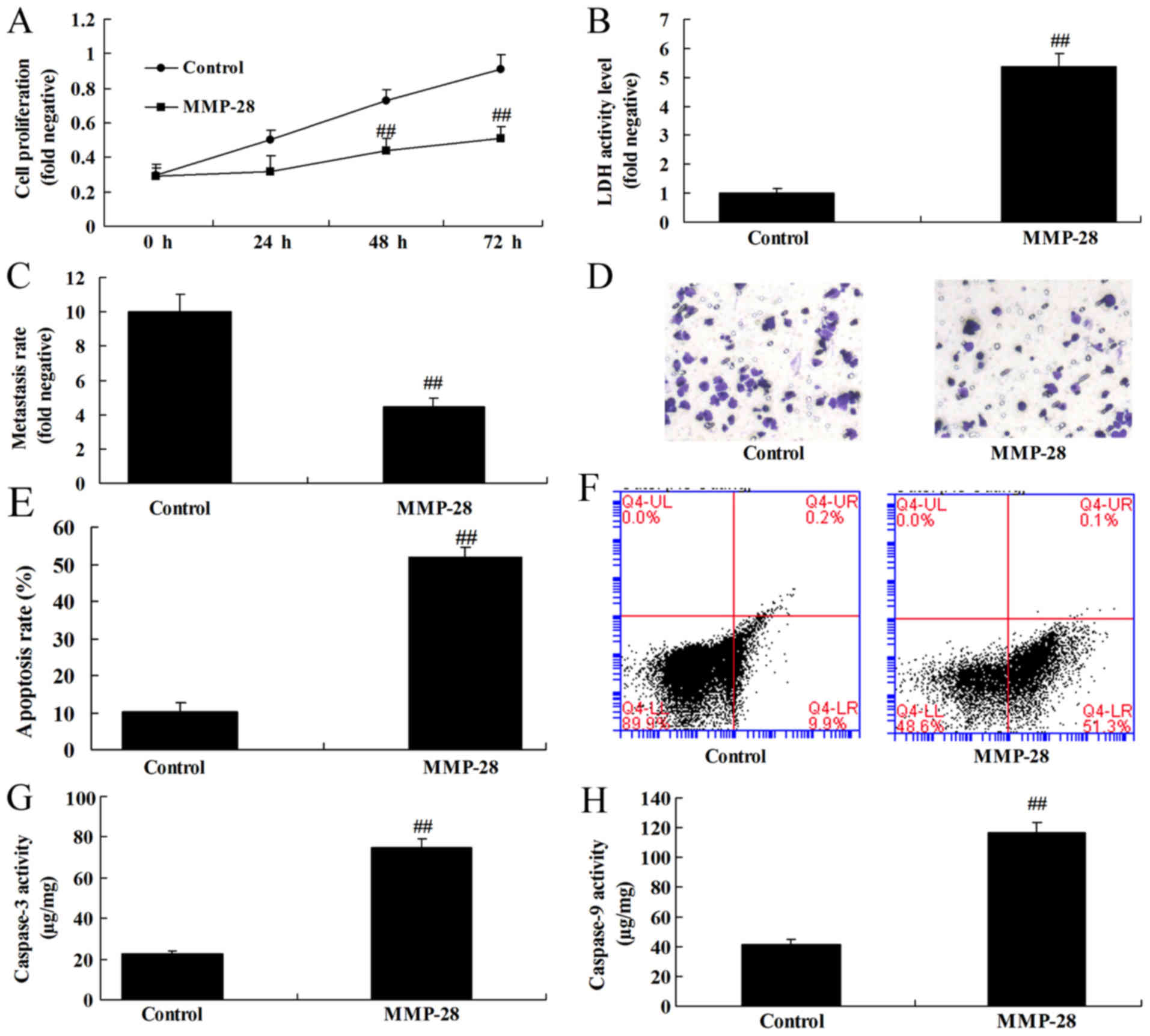
Upregulation of MMP-28 reduced cell
growth of glioma cell
To examine the function of MMP-28 on cell growth of
glioma cell, MMP-28 expression was upregulated in glioma cell.
Consequently, upregulation of MMP-28 induced cell growth and
metastasis, and reduced apoptosis and caspase-3/9 activity level in
glioma cell, compared with control group (Fig. 3).
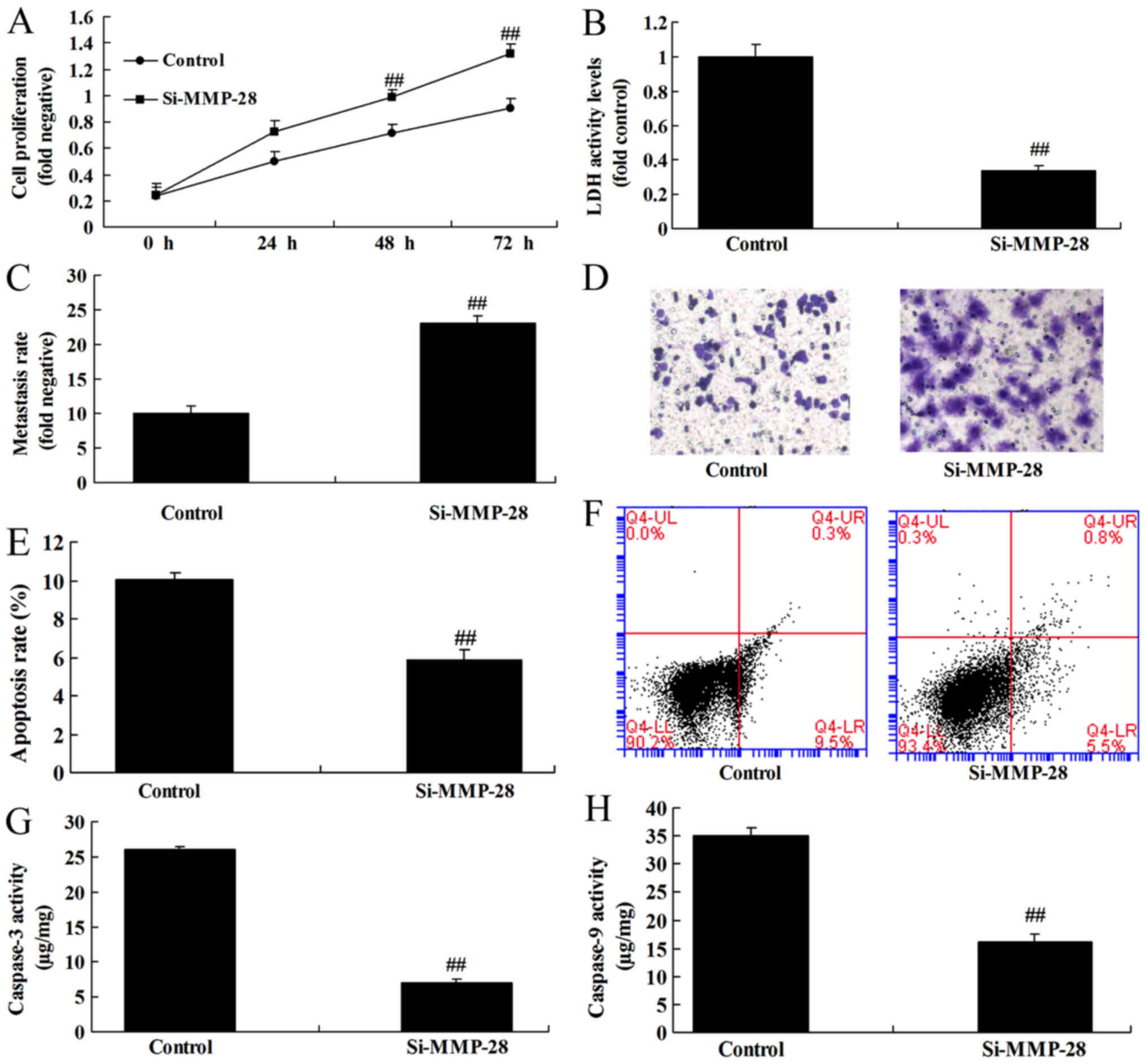
Downregulation of MMP-28 induced cell
growth of glioma cell
To further determine the function of MMP-28 on cell
growth of glioma cell, si-MMP-28 was used to transfect glioma cell.
As a result, downregulation of MMP-28 reduced cell growth and
metastasis, and induced apoptosis and caspase-3/9 activity level in
glioma cell, in comparison with control group (Fig. 4).
TGF-β inhibitor attenuated the effects
of MMP-28 in glioma cell
To confirm the function of TGF-β in the effects of
MMP-28 in glioma cell, 0.5 µM of ITD-1, inhibitor of TGF-β, was
used in this study. As shown in Fig. 5A
and B, TGF-β inhibitor suppressed TGF-β protein expression in
glioma cell by MMP-28, compared with MMP-28 group. In addition,
TGF-β inhibitor attenuated the effects of MMP-28 on cell growth and
metastasis, and apoptosis and caspase-3/9 activity level in glioma
cell (Fig. 5C-J).
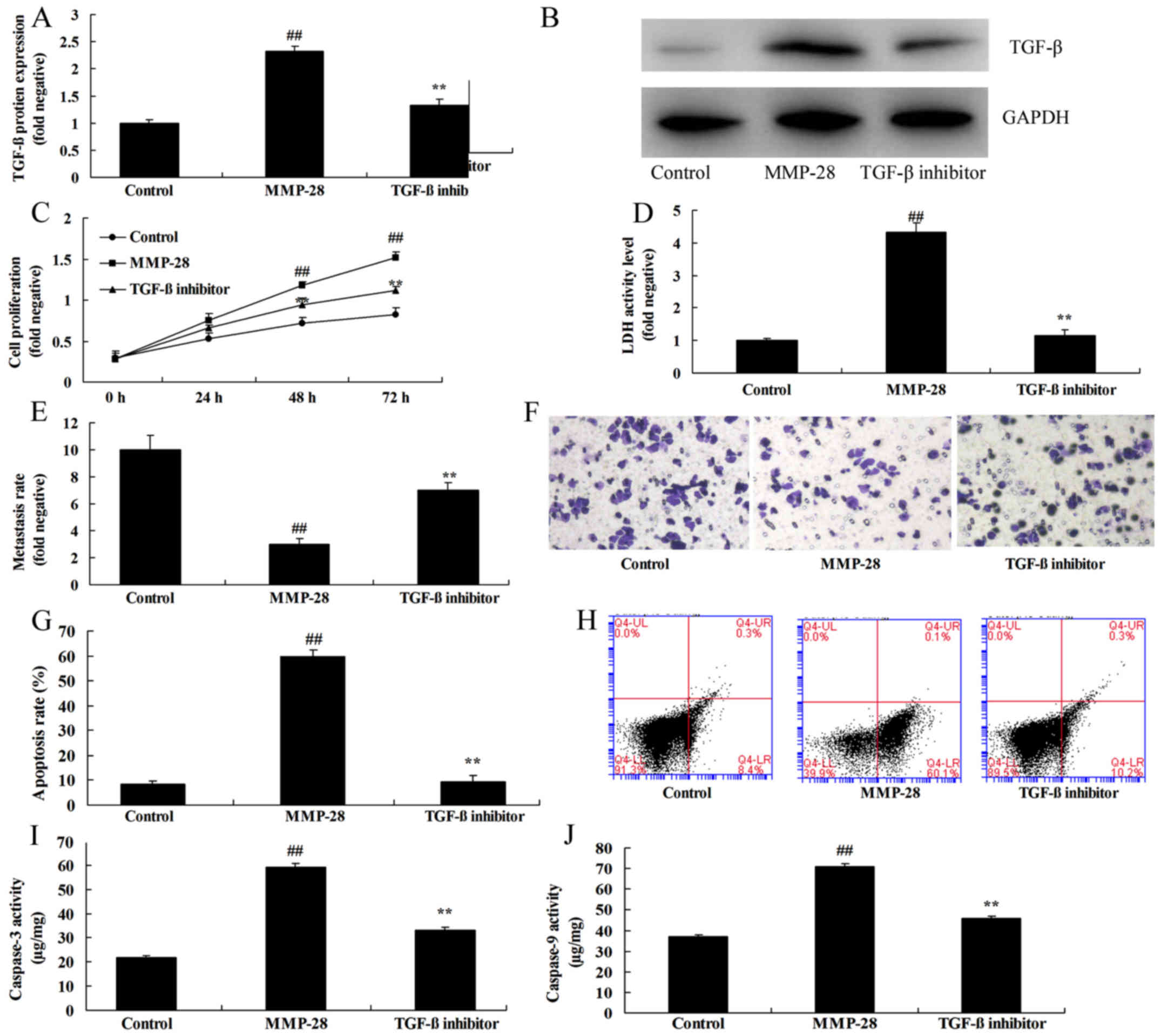 | Figure 5.TGF-β inhibitor inhibits the effects
of MMP-28 in glioma cells. (A) TGF-β protein expression was
determined using (B) western blot analysis. (C) Cell growth, (D)
LDH activity level, (E and F) migration rate (magnification, ×10),
(G) apoptotic rate, which was determined using (H) flow cytometry,
and (I) caspase-3 and (J) caspase-9 activity were also measured.
##P<0.01 vs. control group; **P<0.01 vs. MMP-28
group. Control, negative control group; MMP, matrix
metalloproteinase; MMP-28, overexpression of MMP-28 group; TGF-β,
transforming growth factor-β; TGF-β inhibitor, overexpression of
MMP-28 and TGF-β inhibitor group. |
Discussion
Glioma is characteristic of high invasion, high
recurrence rate and high mortality. The major cause of high
glioma-induced mortality can be attributed to its high invasion.
Glioma is invasive and ill-defined with the normal brain tissue
(3). Therefore, it can hardly be
completely removed surgically. Moreover, glioma growing in vital
sites like brain stem cannot even be treated surgically (3). Glioma is not sensitive to radiotherapy
or chemotherapy, and is extremely likely to relapse. Therefore,
glioma patients are associated with low survival rate (3). The results demonstrated that MMP-28 and
TGF-β mRNA expression were reduced in glioma patients. OS and DFS
of MMP-28 expression low were lower than those of MMP-28 expression
low group. These results showed that MMP-28 participated in the
genesis and development of glioma patients. In this study, we only
used one cell line U251 cell, which is astrocytoma line and an
insufficient present study, and we will use more cell model in
further study.
The MMPs family is constituted by 23 structurally
correlated enzymes, which can reconstruct and degrade the
extracellular matrix (5). At the
same time, they can act on the non-extracellular matrix components
to promote the release of soluble factors, such as growth factor
and extracellular matrix cytokines (7). Numerous studies suggest that, under
physiological conditions, MMP-28 plays a vital role in
embryogenesis and trauma healing (7). Pathological conditions, such as tumor
growth and metastasis, destruction of arthritic cartilage and
cardiovascular diseases, are also related to MMPs (11).
MMP-28 is highly expressed in skin basal cell and
keratinocyte in upper basement, as well as in the developing
spermatogonium in testis and lung (7). MMP-28 expression is relatively high in
lung, heart, rectum, small intestine and brain at protein level. In
the injured skin, MMP-28 expression in mitotic cell at the edge of
wound is upregulated (11). However,
in keratinocyte with migration capacity, MMP-28 expression is not
upregulated (11). MMP-28 is also
related to the immune function because it is expressed in normal
circulatory T cell. Moreover, its expression quantity is increased
in cartilage in the case of osteoarthritis. MMP-28 has different
expression profiles in different tumors (12). Typically, MMP-28 expression is
downregulated during the formation of colorectal cancer (13). This indicates that MMP-28 plays a key
role in maintaining homeostasis of cell. In addition, research
finds that, in oral squamous cell carcinoma, MMP-28 knockdown will
delay cell growth (12). In lung
adenocarcinoma, high MMP-28 expression can activate the potential
TGF β pathway. Thus, it can induce the epithelial-mesenchymal
transition (EMT) of epithelial cell (14). These results demonstrated that
over-expression of MMP-28 induced TGF-β protein expression in
glioma cell. Illman et al (14) suggested that MMP-28 regulates TGF-β
in malignant cells. These results were similar to our results, and
indicated that MMP-28 regulates TGF-β in glioma cell and malignant
cells.
The role of MMP-28 in regulating the effect of
β-catenin on the TGF-β1-induced EMT of human lung adenocarcinoma
cell line A549 is observed. Such irreversible transition manifests
in the following aspects (15).
Firstly, expression, mutation and deletion of cell surface
E-cadherin are reduced. Secondly, TGF-β complex protein is
depolymerized (16). Thirdly, active
TGF-β level is increased. The TGF-β activity in the above cascade
events inducing the initiation of EMT can be neutralized by MMPs
inhibitor GM6001 or its antibody (17). However, the blocking effect cannot
reverse the cell phenotype once the EMT begins. Experiment
indicates that, MMP-28 can induce EMT genesis and cell invasion
through the TGF-β-dependent mechanism (16). This reveals that such enzyme
participates in regulating epithelial cell function and mediating
tumor genesis (15). This study
indicated that TGF-β inhibitor inhibited the effects of MMP-28 in
glioma cell. Illman et al (15) reported that MMP-28 induces TGF-β
mediated epithelial to mesenchymal transition in lung carcinoma
cells.
In conclusion, our study firstly demonstrated that
MMP-28 and TGF-β expression were inhibited in glioma patients.
Moreover, our results showed that the function of MMP-28 in human
glioma cell to induces TGF-β, which may provide a novel insight
into tumorigenesis and the basis for the development of
MMP-28/TGF-β-targeting therapies against glioma.
Acknowledgements
Not applicable.
Funding
The present study was supported by funds from the
Education Department of Jilin Province (grant no.
JJKH20170056KJ).
Availability of data and materials
The analyzed datasets generated during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JP designed the experiments. XW, XC, LS, XB, HH and
LC performed the experiments. JP and XW analyzed the data, and JP
wrote the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Beihua University. The
study was performed in accordance with the regulations of the
Institutional Review Board of Affiliated Hospital of Beihua
University. Written informed consent was obtained prior to surgery
from all enrolled patients.
Patient consent for publication
Written informed consent was obtained prior to
surgery from all enrolled patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Weller M, Butowski N, Tran DD, Recht LD,
Lim M, Hirte H, Ashby L, Mechtler L, Goldlust SA, Iwamoto F, et al:
Rindopepimut with temozolomide for patients with newly diagnosed,
EGFRvIII-expressing glioblastoma (ACT IV): A randomised,
double-blind, international phase 3 trial. Lancet Oncol.
18:1373–1385. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blumenthal DT, Rankin C, Stelzer KJ,
Spence AM, Sloan AE, Moore DF Jr, Padula GD, Schulman SB, Wade ML
and Rushing EJ: A Phase III study of radiation therapy (RT) and
O6-benzylguanine + BCNU versus RT and BCNU alone and
methylation status in newly diagnosed glioblastoma and gliosarcoma:
Southwest Oncology Group (SWOG) study S0001. Int J Clin Oncol.
20:650–658. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pollack IF, Jakacki RI, Butterfield LH,
Hamilton RL, Panigrahy A, Potter DM, Connelly AK, Dibridge SA,
Whiteside TL and Okada H: Antigen-specific immune responses and
clinical outcome after vaccination with glioma-associated antigen
peptides and polyinosinic-polycytidylic acid stabilized by lysine
and carboxymethylcellulose in children with newly diagnosed
malignant brainstem and nonbrainstem gliomas. J Clin Oncol.
32:2050–2058. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eisenstat DD, Pollack IF, Demers A, Sapp
MV, Lambert P, Weisfeld-Adams JD, Burger PC, Gilles F, Davis RL,
Packer R, et al: Impact of tumor location and pathological
discordance on survival of children with midline high-grade gliomas
treated on Children's Cancer Group high-grade glioma study CCG-945.
J Neurooncol. 121:573–581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yan H, Wang W, Dou C, Tian F and Qi S:
Securin promotes migration and invasion via matrix
metalloproteinases in glioma cells. Oncol Lett. 9:2895–2901. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bister VO, Salmela MT,
Karjalainen-Lindsberg ML, Uria J, Lohi J, Puolakkainen P,
Lopez-Otin C and Saarialho-Kere U: Differential expression of three
matrix metalloproteinases, MMP-19, MMP-26, and MMP-28, in normal
and inflamed intestine and colon cancer. Dig Dis Sci. 49:653–661.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marchenko GN and Strongin AY: MMP-28, a
new human matrix metalloproteinase with an unusual cysteine-switch
sequence is widely expressed in tumors. Gene. 265:87–93. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pan YB, Zhang CH, Wang SQ, Ai PH, Chen K,
Zhu L, Sun ZL and Feng DF: Transforming growth factor beta induced
(TGFBI) is a potential signature gene for mesenchymal subtype
high-grade glioma. J Neurooncol. 137:395–407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo Z, Li G, Bian E, Ma CC, Wan J and Zhao
B: TGF-β-mediated repression of MST1 by DNMT1 promotes glioma
malignancy. Biomed Pharmacother. 94:774–780. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suomela S, Koljonen V, Skoog T, Kukko H,
Böhling T and Saarialho-Kere U: Expression of MMP-10, MMP-21,
MMP-26, and MMP-28 in Merkel cell carcinoma. Virchows Arch.
455:495–503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gencer S, Cebeci A and Irmak-Yazicioglu
MB: Matrix metalloproteinase gene expressions might be oxidative
stress targets in gastric cancer cell lines. Chin J Cancer Res.
25:322–333. 2013.PubMed/NCBI
|
|
13
|
Manicone AM, Gharib SA, Gong KQ, Eddy WE,
Long ME, Frevert CW, Altemeier WA, Parks WC and Houghton AM: Matrix
metalloproteinase-28 is a key contributor to emphysema
pathogenesis. Am J Pathol. 187:1288–1300. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Illman SA, Lohi J and Keski-Oja J:
Epilysin (MMP-28)-structure, expression and potential functions.
Exp Dermatol. 17:897–907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Illman SA, Lehti K, Keski-Oja J and Lohi
J: Epilysin (MMP-28) induces TGF-beta mediated epithelial to
mesenchymal transition in lung carcinoma cells. J Cell Sci.
119:3856–3865. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rao S, Zaidi S, Banerjee J, Jogunoori W,
Sebastian R, Mishra B, Nguyen BN, Wu RC, White J, Deng C, et al:
Transforming growth factor-β in liver cancer stem cells and
regeneration. Hepatol Commun. 1:477–493. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Åström P, Juurikka K, Hadler-Olsen ES,
Svineng G, Cervigne NK, Coletta RD, Risteli J, Kauppila JH, Skarp
S, Kuttner S, et al: The interplay of matrix metalloproteinase-8,
transforming growth factor-β1 and vascular endothelial growth
factor-C cooperatively contributes to the aggressiveness of oral
tongue squamous cell carcinoma. Br J Cancer. 117:1007–1016. 2017.
View Article : Google Scholar : PubMed/NCBI
|