Introduction
The association between assisted reproductive
technology (ART) and the risk of birth defects has previously been
re reported (1–4). Patients undergoing conventional in
vitro fertilization fresh-embryo transfer (IVF-ET) cycles often
do not achieve fertilization (5).
Total fertilization failure (TFF) increases the financial costs of
an already stressful and expensive treatment plan and may result in
further emotional strain on such patients (5). With frozen-thawed embryo transfer
(FET), it is unclear whether the cryoprotectant, freezing or
thawing procedures will have an adverse effect on the embryos and
thereby increase the risk of major congenital anomalies (CAs)
compared with ET procedures (6).
Despite recent improvements in ART, it has been reported that TFF
or near-TFF occurs in 15–20% of patients undergoing conventional
IVF-ET cycles (7).
The number of children conceived through ART is now
>5 million, and so determining whether there is an association
between ART and birth defects is of great importance (8). It has previously been reported that
maternal factors associated with infertility may in increase the
risk of birth defects (9,10), or that an inherent defect responsible
for infertility may also cause birth defects in the conceived child
(11).
The aim of the present study was to retrospectively
analyse ART data from 2004 to 2014 to determine whether CA rates
are increased in infants conceived by infertile women via ART
compared with those conceived by spontaneous conception (SC).
Materials and methods
Patients
The present study is a register-based cohort study.
Women who underwent ART treatment, including IVF-ET,
intracytoplasmic sperm injection fresh embryo transfer (ICSI) and
FET, resulting in live birth between 2004 and 2014 were recruited
from the Reproductive Medicine Centre of Tianjin Central Hospital
of Obstetrics and Gynecology Tianjin, China). Inclusion criteria
included patients exhibited a normal karyotype, normal levels
endocrine hormone. Mean age of the patients was 31.56±4.34. All the
recruited patients were contacted by telephone. If patients could
not be reached by telephone, they were excluded from the study. A
team of professional training nurses assessed the course and the
outcome of pregnancies via a telephone questionnaire and direct
contact at one week post-partum. Direct consultations with
obstetricians, paediatricians or ultrasound technicians were sought
in all cases where an anomaly was suspected during conception. In
total, 9,101 births after embryo transfer were assessed in the
present study, including 4,186 births resulting from FET and 4,915
births resulting from IVF/ICSI-ET. All patients provided written
informed consent and provided information regarding TFF and
follow-up, including neonatal outcomes. The present study was
approved by the Institutional Review Board of Tianjin Central
Hospital of Obstetrics and Gynecology.
Clinical procedures and embryo
transfer
All patients participating in this study underwent
controlled ovarian stimulation according to routine long or short
gonadotropin-releasing hormone (GnRH) agonist protocols (12). Pituitary suppression was achieved by
daily subcutaneous injections of triptorelin acetate (100 µg;
Ferring Pharmaceuticals Ltd., West Drayton, United Kingdom)
initiated at the mid-luteal phase of the preceding cycle. The
treating physician opted to use the GnRH agonist (3,978 patients)
or antagonist (937 patients) protocol on the basis of patient
characteristics or ovarian response during previous IVF cycles. The
ovarian response during treatment was monitored by measuring serum
E2 concentration and follicular growth (using vaginal ultrasound).
Dosages of follicle-stimulating hormone and human menopausal
gonadotropin were adjusted accordingly between 75 IU/day and 150
IU/day. Recombinant human chorionic gonadotropin (hCG; 5,000-10,000
IU/day; Lizhu Pharmacy, Zhuhai, China) was administered to trigger
ovulation when two leading follicles reached a mean diameter of 18
mm measured by vaginal ultrasound. Oocyte retrieval was performed
transvaginally 34–36 h following hCG administration. Oocytes were
fertilized using IVF-ET or ICSI according to sperm quality. Sperm
preparation, IVF-ET, ICSI and embryo culture were performed as
previously described (7,12). Semen samples were collected by
masturbation following 2–7 days of sexual abstinence. Samples were
stored at 37°C for 30 min for liquefaction, following which they
were analysed for sperm count, motility and morphology according to
World Health Organization criteria (13). During IVF-ET cycles, each oocyte was
inseminated with 10,000 motile spermatozoa 3–4 h following
retrieval. Patients whose partners were identified as having severe
oligospermia or azoospermia in previous IVF-ET cycles received
ICSI. During ICSI cycles, cumulus cells and the corona radiata of
the oocytes were removed by exposure to HYASE (Vitrolife AB,
Goteborg, Sweden) containing hyaluronidase for 10–15 sec at 2 h
following retrieval. ICSI was performed on metaphase II oocytes as
determined by observation under an inverted microscope
(magnification, ×200). The presence of two pronuclei was defined as
normal fertilization and fertilized oocytes. All cells were grown
in an incubator with a constant temperature of 37°C and 5%
CO2, and continuously cultured in G1 medium (Vitrolife
AB) for 2 days. All embryos from IVF-ET and ICSI were examined on
the morning of day 3 following oocyte retrieval (~69 h after
initial insemination). Every embryo was graded on the basis of the
regularity of blastomeres and degree of DNA fragmentation: Grade 1,
the sizes of the blastomeres were uniform, with no DNA
fragmentation; grade 2, the blastomere sizes were slightly uneven
with <20% DNA fragmentation; grade 3, the blastomere sizes were
heterogeneous or DNA fragmentation was 20–50%; and grade 4, >50%
DNA fragmentation. Good-quality embryos were defined as embryos
with a grade of 1 or 2 (14). Only
good quality embryos and ordinary quality embryos were selected for
transfer (15). Typically, the two
best-quality embryos were chosen for transfer on day 3, while
surplus embryos of good or fair quality were cryopreserved or
extensively cultured to the blastocyst stage for possible
cryopreservation according to the protocol developed by Chinese
legislation (16). From 2008
onwards, vitrification was used for embryo cryopreservation at this
centre. Briefly, the embryos were equilibrated in equilibration
medium [basal medium with 7.5% (v/v) ethylene glycol and 7.5% (v/v)
dimethylsulphoxide (DMSO)] at room temperature for 10 min. The
embryos were then transferred into the vitrification medium [basal
medium with 15% (v/v) ethylene glycol, 15% (v/v) DMSO and 0.5 mol/l
sucrose] at room temperature for 60 sec. The cryoprotectant-treated
embryos were placed onto a fine Cryotop® (Kitazato
BioPharma Co., Fuji, Japan) and then submerged immediately into
liquid nitrogen ready for storage.
FET cycles were initiated during natural cycles
following spontaneous ovulation as well as hormone replacement
treatment (HRT) cycles. For the natural cycles, transvaginal
ultrasound scans were performed on cycle days 10–12 to assess
endometrial thickness, follicle growth and ovulation. FET was
planned for 3 days after ovulation. Progesterone administration was
initiated for luteal support 1 day after ovulation. For the HRT
cycles, oral oestradiol (Progynova; Bayer AG, Leverkusen, Germany)
was administered at a dosage of 2 mg/day on cycle days 1–4, 4
mg/day on cycle days 5–8 and 6 mg/day on cycle days 9–12.
Transvaginal ultrasounds were performed to assess endometrial
thickness and ovulation at day 13 and the oestradiol dosage was
adjusted accordingly. When the endometrium reached ≥8 mm thickness,
40 mg of progesterone (Zhejiang Xianju Pharmaceutical Co., Ltd.,
Zhejiang, China) was administered intramuscularly. For the next 3
days, 60, 80 and 80 mg/day of progesterone was intramuscularly
administered, respectively, as performed previously (17). Embryo transfer was performed on day
4.
Outcome measures
Serum human chorionic gonadotropin (hCG) was used to
detect pregnancy 2 weeks after embryo transfer or 10 days after
blastocyst transfer and was subsequently tested serially to monitor
rising titres. Clinical pregnancy was defined as the presence of a
gestational sac with foetal heart activity on ultrasound
examination 5 weeks following oocyte retrieval. The implantation
rate was defined as the number of gestational sacs per total number
of transferred embryos (18).
Neonatal outcome data were obtained by telephone interviews with
parents following delivery. A questionnaire was used to obtain
information of 9,013 clinical pregnancy cycles on gestational
weeks, birth weight, sex and CAs. CAs were defined as all
structural, functional and genetic anomalies diagnosed in aborted
foetuses, at birth or during the neonatal period (19). CAs were classified and coded
according to an extended version of the International
Classification of Diseases (ICD-10) (20). Only cases with major CAs were
included in the analysis and were categorized by organ system
classification according to the ICD-10. Each organ system involved
was recorded once per infant, however infants with multiple major
anomalies may appear in several different groups depending on the
affected organ systems. In addition, a concern with FET is often
whether the cryoprotectants, freezing or thawing procedures have an
adverse effect on the embryos and whether, thereby,
cryopreservation processes increase the risk of major CAs (6). Therefore, only certain associated
factors were analyzed during the FET process and certain
characteristics of patients were not included.
Statistical analysis
Data were analysed using SPSS version 19.0 (IBM
Corp., Armonk, NY, USA). Parental reproductive and ART treatment
parameters, maternal characteristics and pregnancy and birth
outcomes in IVF-ET and FET groups were compared using Student's t
tests for continuous variables and χ2 tests for
categorical variables. Statistical analysis using univariate
logistic regression was used to evaluate the association between
maternal age, infertility diagnosis, plurality and the risk of CAs
in the FET group. P<0.05 was considered to indicate a
statistically significant difference.
Results
Pregnancy outcomes
The final sample population included 9,013 clinical
pregnancy cycles resulting in 9,101 live births (IVF-ET, n=2,919;
ICSI, n=1,996; FET, n=4,186). A total of 105 infants were born with
CAs (Table I). In the ART subgroups,
birth defects occurred in 37 infants conceived through IVF-ET
(1.27%), 22 infants conceived through ICSI (1.10%) and 46 infants
conceived through FET (1.10%). The birth defect rate was slightly
higher in the IVF-ET subgroup compared with the other sub groups,
however no significant difference was observed (Table I). For multiple births, the birth
defect rate was slightly lower in the FET subgroup compared with
the IVF-ET subgroup. For all subgroups, the birth defect rate in
infants conceived by mothers aged >35 years was slightly but not
significantly higher compared with those with mothers aged ≤35
years. The organ system distribution of birth defects is presented
in Table II.
 | Table I.CA rates for live-born infants. |
Table I.
CA rates for live-born infants.
|
| Babies born with CAs
(% of total in group) |
|
|
|---|
|
|
|
|
|
|---|
| Parameter | IVF-ET | ICSI | FET | χ2 | P-value |
|---|
| Total | 37 (1.27) | 22 (1.10) | 46 (1.10) | 0.488 | 0.783 |
| Sex |
|
|
|
|
|
| Male | 20 (1.27) | 15 (1.51) | 25 (1.14) | 0.731 | 0.694 |
|
Female | 17 (1.26) | 7 (0.70) | 21 (1.05) | 1.804 | 0.406 |
| Plurality |
|
|
|
|
|
|
Single | 20 (1.20) | 16 (1.34) | 34 (1.58) | 1.025 | 0.599 |
|
Multiple | 17 (1.36) | 6 (0.75) | 12 (0.59) | 5.502 | 0.064 |
| Maternal age |
|
|
|
|
|
| >35
years | 13 (2.60) | 4 (1.33) | 15 (2.97) | 2.182 | 0.336 |
| <35
years | 24 (0.99) | 18 (1.06) | 31 (0.84) | 0.717 | 0.699 |
 | Table II.Types of congenital abnormalities
among live-born infants. |
Table II.
Types of congenital abnormalities
among live-born infants.
| ICD10 code | Total, n (%) | IVF-ET, n | ICSI, n | FET, n |
|---|
| Q00-Q07 central
nervous system | 7 (6.1) | 4 | 1 | 2 |
| Q10-Q18 eye, ear,
face and neck | 2 (1.7) | 2 | 0 | 0 |
| Q20-Q28
cardiovascular and circulation | 46 (40.1) | 17 | 9 | 20 |
| Q30-Q34
respiratory | 1 (0.8) | 1 | 0 | 0 |
| Q35-Q37
cheilopalatognathus | 12 (10.2) | 1 | 3 | 8 |
| Q38-Q45
gastrointestinal | 14 (12.1) | 6 | 2 | 6 |
| Q50-Q64
genitourinary system | 7 (6.1) | 3 | 1 | 3 |
| Q65-Q79
musculoskeletal | 11 (9.3) | 5 | 3 | 3 |
| Q80-Q89 other | 4 (3.4) | 0 | 2 | 2 |
| Q90-Q99
chromosomal | 13 (11.2) | 1 | 3 | 9 |
| Total no. birth
defectsa | 114 (100.0) | 39 | 24 | 51 |
| Total no. of babies
with birth defects | 105 (92.1) | 37 | 22 | 46 |
Parental factors
In the IVF-ET group, the number of birth defects was
significantly higher with maternal age >35, male factor
diagnoses and diminished ovarian reserve (P<0.05; Table III). In the FET group, an increased
risk of birth defects was significantly associated with multiple
births and maternal age >35 years (P<0.05; Table IV).
 | Table III.Characteristics of maternal and
treatment cycles of live birth in the IVF-ET group. |
Table III.
Characteristics of maternal and
treatment cycles of live birth in the IVF-ET group.
| Parameter | SC (n) | CA (n) | P-value |
|---|
| Sex |
|
| 0.275 |
|
Male | 2,534 | 35 |
|
|
Female | 2,322 | 24 |
|
| Plurality |
|
| 0.665 |
| Single
births | 2,827 | 36 |
|
|
Multiple births | 2,029 | 23 |
|
| ART |
|
| 0.601 |
|
IVF | 2,882 | 37 |
|
|
ICSI | 1,974 | 22 |
|
| Maternal age
(years) |
|
| 0.009 |
|
>35 |
783 | 17 |
|
|
≤35 | 4,073 | 42 |
|
| Abortion
history |
|
| 0.834 |
|
Yes | 2,156 | 27 |
|
| No | 2,700 | 32 |
|
| BMI |
|
| 0.263 |
|
<23.0 | 2,732 | 36 |
|
|
≥23.0–25.0 | 1,134 | 16 |
|
|
≥25.0 |
990 | 7 |
|
| Infertility
diagnosis |
|
| 0.000 |
| Male
factora | 1,842 | 14 | <0.001 |
| Tubal
factors | 2,404 | 30 | 0.053 |
| Diminished ovarian
reserve | 99 | 8 | 0.001 |
| Uterine
factors | 23 | 1 | 0.385 |
|
Endometriosis |
125 | 0 | 0.182 |
|
Ovulation disorders | 87 | 3 | 0.428 |
|
Unexplained |
246 | 3 | 0.487 |
| Other
factors | 30 | 0 | 0.545 |
| Duration of
infertility (years) |
4.16±3.04 |
4.56±3.33 | 0.32 |
| Number of retrieved
oocytes | 14.05±7.58 | 13.37±7.46 | 0.496 |
| Embryos
transferred |
2.10±0.55 |
2.15±0.15 | 0.443 |
 | Table IV.Characteristics of maternal and
treatment cycles of live birth in the FET group. |
Table IV.
Characteristics of maternal and
treatment cycles of live birth in the FET group.
| Parameter | SC (n) | CA (n) | P-value |
|---|
| Sex |
|
| 0.769 |
|
Male | 2,160 | 25 |
|
|
Female | 1,980 | 21 |
|
| Plurality |
|
| 0.002 |
| Single
births | 2,118 | 34 |
|
|
Multiple births | 2,020 | 12 |
|
| Maternal age
(years) |
|
| 0.000 |
|
>35 |
490 | 15 |
|
|
≤35 | 3,650 | 31 |
|
| Embryo
transfer | 2.56±0.604 | 2.65±0.604 | 0.347 |
Multivariate analysis
Multivariate analysis was performed to determine
independent predictors of CAs in the IVF-ET and FET groups. In the
IVF-ET group, CAs were not significantly correlated with maternal
age or infertility diagnosis (Table
V). However, maternal age was an independent predictor of CAs
in the FET group (P<0.05; Table
IV).
 | Table V.Multivariate analysis to determine
independent predictors of congenital anomalies in the IVF-ET and
FET groups. |
Table V.
Multivariate analysis to determine
independent predictors of congenital anomalies in the IVF-ET and
FET groups.
| Parameter | Odds ratio | 95% confidence
interval | P-value |
|---|
| IVF-ET group |
|
|
|
|
Maternal age | 1.623 | 0.341–1.114 | 0.109 |
|
Infertility diagnosis | 1.156 | 0.803–1.665 | 0.435 |
| FET group |
|
|
|
|
Plurality | 1.134 | 0.737–1.744 | 0.567 |
|
Maternal age | 1.799 | 1.313–2.466 | <0.001 |
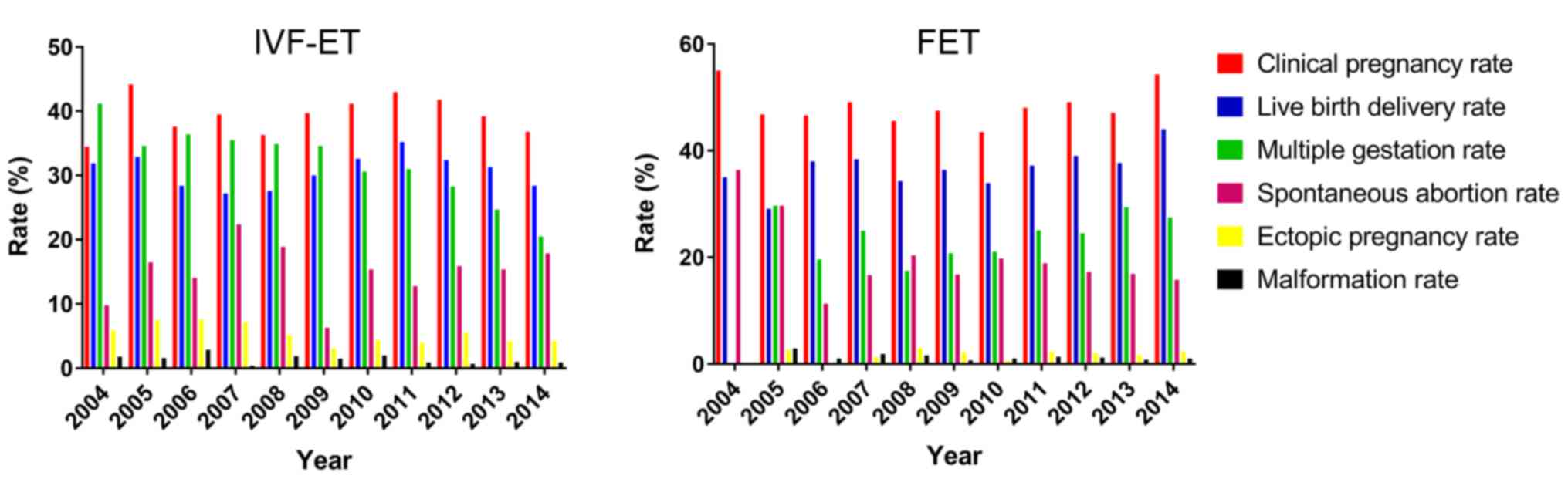
Although there were fluctuations in clinical
pregnancy rates during the study period, there was an overall
increase in the clinical pregnancy rate in the IVF-ET group between
2004 and 2014 (Fig. 1). In the FET
group, a substantial decline in the clinical pregnancy rate was
observed from 2004 to 2005, followed by a period of no obvious
change over the next 9 years (Fig.
1). Similarly, no significant differences in temporal trends
for live birth delivery rates were observed between the groups. The
spontaneous abortion rate in the IVF-ET group increased throughout
the study period, reaching nearly 50% in 2014. Conversely, the
spontaneous abortion rate declined in the FET group from 2004–2006
until 2006 and then plateaued until 2014 (Fig. 1). Ectopic pregnancy rates in the
IVF-ET group decreased throughout the study period, however they
remained higher compared with the FET group (Fig. 1). The multiple gestation rate
decreased gradually in the IVF-ET group, whereas In the FET group a
decline occurred from 2005 to 2008 followed by an increase from
2009 to 2014 (Fig. 1). Throughout
the study period, the overall malformation rate remained relatively
stable in the IVF-ET and FET groups (Fig. 1). None of the identified trends were
statistically significant.
Discussion
The aim of the present study was to evaluate the
risk of CAs in infants conceived through ART treatments, including
IVF-ET and FET, relative to infants born after SC in infertile
women; additionally, the impact of IVF-ET and ICSI on the risk of
CAs was evaluated. The rate of CAs in infants conceived through ART
infants ranged from 1.10–1.20%. Yin et al (21) previously reported the rate of major
CAs to be 2.22% in ART infants in China. A meta-analysis published
by Wen et al (22) reviewed
46 studies including 124,468 infants conceived through ART and
reported a pooled risk estimation of 1.37. Another recent paper
analysed the outcomes for infants born after ART treatments
(23). The current study
demonstrated that the rate of CAs (3.75%) was higher compared with
that reported by the European Surveillance of Congenital Anomalies
(EUROCAT; 2.0%) (23). However, the
rate of CAs in infants conceived via ART was not significantly
different compared with those conceived via SC within the infertile
population.
In the present study, the prevalence of major
anomalies and the distribution of anomalies in infants conceived
through ART was similar to the data reported by the Chinese Center
for Disease Control and Prevention (CDC) (24). The most frequent anomalies were heart
defects, followed by gastrointestinal anomalies and anomalies of
cheilopalatognathus. The underlying mechanisms responsible for the
association between ART and CAs are uncertain and warrant further
research. An excess risk of CAs in infants conceived through ART is
associated with multiple factors (1). Factors associated with treatment that
may increase the risk of birth defects include the age of infertile
couples, underlying causes of infertility and medications used to
induce ovulation or maintain the pregnancy in the early stages,
which in turn may have effects on endometrial and cervical tissues
and placentation or may impair embryo-endometrial synchronization.
Furthermore, factors associated with ART procedures, including the
culture media composition, length of culture, freezing and thawing
of embryos, potential for polyspermic fertilization, delayed
fertilization of the oocyte, altered hormonal environment at the
time of implantation and manipulation of gametes and embryos may
affect the risk of Cas (25,26). Older females (≥35 years) may explain
the results of the current study as demonstrated by a previous
study (27). Older females
undergoing ART have an increased risk of producing abnormal
gametes, resulting in poor obstetrical and perinatal outcomes
(27). In the present study,
maternal age parameters in the FET group were demonstrated to be
correlated with CAs.
High rates of multiple births were observed in the
present study. Previous studies have reported that ART leads to
more multiple pregnancies compared with SC, the majority of which
are twin pregnancies. Additionally, CAs are more frequent in twins
compared with than single births (28–30). The
results of the present study also suggest that the risk of CAs in
infants conceived via ART may be associated with male factors. It
has previously been reported that abnormal sperm morphology and
subfertility in fathers is associated with hypospadias in offspring
(31). The concern is that bypassing
the natural protective barriers to poor quality sperm fertilization
may be associated with an increased risk of future health problems
in offspring (32). However, the
risk of CAs in infants conceived via ICSI compared with those in
the IVF-ET and FET groups was not significantly different.
The present study has a number of limitations. The
most obvious is the reliance upon retrospective data, which may
result in recall bias. Another disadvantage is that pregnancy
outcomes in patients undergoing ART were not compared with
naturally conceived infants at birth. Data were collected during
the hospitalization at birth, and thus an evaluation of the delayed
or long-term effects of ART was not attempted and would require
extended follow-up. Although the present study was adjusted for
maternal age uncontrolled or unmeasured risk factors could
potentially produce biases.
To the best of our knowledge, this is the first
study systematically designed to compare the risk of CAs with
various ART methods. In summary, in the present study of 9,101
infants conceived via ART offspring, no significant increase in CAs
was observed compared with those conceived via SC. This is
consistent with a previous Chinese multicentre study (1.35%)
(33). Although the majority of
infants conceived via ART were free of birth defects, it is unclear
whether other factors contributed to or could explain the observed
associations. The results of the present study may be used to
provide guidance when counselling patients who are considering
treatment for infertility in China.
Acknowledgements
The authors would like to thank all of the doctors,
nurses and embryologists in the Reproductive Medicine Center of
Tianjin Central Hospital of Obstetrics and Gynecology for their
help in collecting data.
Funding
The present study was supported by the Tianjin Key
Technologies Research and Development Program (grant no.
05YFGZGX09900).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YH, HL and YZ contributed to project development and
data collection. YH was responsible for writing the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Tianjin Central Hospital of Obstetrics and
Gynecology. Informed consent was obtained from all participants
included in this study.
Consent for publication
All data was anonymized, however written informed
consent for publication of clinical data and clinical images was
obtained from the patients.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Qin J, Sheng X, Wang H, Liang D, Tan H and
Xia J: Assisted reproductive technology and risk of congenital
malformations: A meta-analysis based on cohort studies. Arch
Gynecol Obstet. 292:777–798. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boulet SL, Kirby RS, Reefhuis J, Zhang Y,
Sunderam S, Cohen B, Bernson D, Copeland G, Bailey MA, Jamieson DJ,
et al: Assisted reproductive technology and birth defects among
liveborn infants in florida, massachusetts, and michigan,
2000–2010. JAMA Pediatr. 170:e1549342016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liberman RF, Getz KD, Heinke D, Luke B,
Stern JE, Declercq ER, Chen X, Lin AE and Anderka M: Assisted
reproductive technology and birth defects: Effects of subfertility
and multiple births. Birth Defects Res. 109:1144–1153. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barnhart KT: Assisted reproductive
technologies and perinatal morbidity: Interrogating the
association. Fertil Steril. 99:299–302. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang B, Qian K, Li Z, Yue J, Yang W, Zhu
G and Zhang H: Neonatal outcomes after early rescue
intracytoplasmic sperm injection: An analysis of a 5-year period.
Fertil Steril. 103(1432–1437): e12015.PubMed/NCBI
|
|
6
|
Pelkonen S, Hartikainen AL, Ritvanen A,
Koivunen R, Martikainen H, Gissler M and Tiitinen A: Major
congenital anomalies in children born after frozen embryo transfer:
A cohort study 1995–2006. Hum Reprod. 29:1552–1557. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang B, Li Z, Zhu L, Hu D, Liu Q, Zhu G
and Zhang H: Progesterone elevation on the day of HCG
administration may affect rescue ICSI. Reprod Biomed Online.
29:88–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shankaran S: Outcomes from infancy to
adulthood after assisted reproductive technology. Fertil Steril.
101:1217–1221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saunders DM, Mathews M and Lancaster PA:
The australian register: Current research and future role. A
preliminary report. Ann N Y Acad Sci. 541:7–21. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sutcliffe AG and Ludwig M: Outcome of
assisted reproduction. Lancet. 370:351–359. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yue MX, Fu XW, Zhou GB, Hou YP, Du M, Wang
L and Zhu SE: Abnormal DNA methylation in oocytes could be
associated with a decrease in reproductive potential in old mice. J
Assist Reprod Genet. 29:643–650. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang B, Hu D, Qian K, Ai J, Li Y, Jin L,
Zhu G and Zhang H: Is frozen embryo transfer cycle associated with
a significantly lower incidence of ectopic pregnancy? An analysis
of more than 30,000 cycles. Fertil Steril. 102:1345–1349. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Menkveld R: Clinical significance of the
low normal sperm morphology value as proposed in the fifth edition
of the WHO laboratory manual for the examination and processing of
human semen. Asian J Androl. 12:47–58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fang C, Huang R, Li TT, Jia L, Li LL and
Liang XY: Day-2 and day-3 sequential transfer improves pregnancy
rate in patients with repeated IVF-embryo transfer failure: A
retrospective case-control study. Reprod Biomed Online. 26:30–35.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alpha Scientists in Reproductive Medicine
and ESHRE Special Interest Group of Embryology: The Istanbul
consensus workshop on embryo assessment: Proceedings of an expert
meeting. Hum Reprod. 26:1270–1283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuwayama M: Highly efficient vitrification
for cryopreservation of human oocytes and embryos: The Cryotop
method. Theriogenology. 67:73–80. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang X, Dong X, Huang K, Wang L, Xiong T,
Ji L and Zhang H: The effect of accompanying dominant follicle
development/ovulation on the outcomes of frozen-thawed blastocyst
transfer in HRT cycle. Int J Clin Exp Pathol. 6:718–723.
2013.PubMed/NCBI
|
|
18
|
Zhu L, Xi Q, Zhang H, Li Y, Ai J and Jin
L: Blastocyst culture and cryopreservation to optimize clinical
outcomes of warming cycles. Reprod Biomed Online. 27:154–160. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zegers-Hochschild F, Adamson GD, de Mouzon
J, Ishihara O, Mansour R, Nygren K, Sullivan E and van der Poel S:
International Committee for Monitoring Assisted Reproductive
Technology; World Health Organization: The international committee
for monitoring assisted reproductive technology (ICMART) and the
world health organization (WHO) revised glossary on ART
terminology, 2009. Hum Reprod. 24:2683–2687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
World Health Organization, . ICD-10:
International statistical classification of diseases and related
health problems: Tenth revision. 2nd edition. Beijing: People's Med
Publ House; 2004
|
|
21
|
Yin L, Hang F, Gu LJ, Xu B, Ma D and Zhu
GJ: Analysis of birth defects among children 3 years after
conception through assisted reproductive technology in China. Birth
Defects Res A Clin Mol Teratol. 97:744–749. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wen J, Jiang J, Ding C, Dai J, Liu Y, Xia
Y, Liu J and Hu Z: Birth defects in children conceived by in vitro
fertilization and intracytoplasmic sperm injection: A
meta-analysis. Fertil Steril. 97(1331–1337): e1–e4. 2012.PubMed/NCBI
|
|
23
|
Setti Levi PE, Moioli M, Smeraldi A,
Cesaratto E, Menduni F, Livio S, Morenghi E and Patrizio P:
Obstetric outcome and incidence of congenital anomalies in 2351
IVF/ICSI babies. J Assist Reprod Genet. 33:711–717. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ministry of Health of the People's
Republic of China. Beijing: China Birth Defects Prev Rep; 2012
|
|
25
|
Hansen M, Kurinczuk JJ, Bower C and Webb
S: The risk of major birth defects after intracytoplasmic sperm
injection and in vitro fertilization. N Engl J Med. 346:725–730.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hansen M, Kurinczuk JJ, Milne E, de Klerk
N and Bower C: Assisted reproductive technology and birth defects:
A systematic review and meta-analysis. Hum Reprod Update.
19:330–353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lean SC, Derricott H, Jones RL and Heazell
AEP: Advanced maternal age and adverse pregnancy outcomes: A
systematic review and meta-analysis. PLoS One. 12:e01862872017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Centers for Disease Control and Prevention
(CDC): Contribution of assisted reproductive technology and
ovulation-inducing drugs to triplet and higher-order multiple
births-United States, 1980–1997. MMWR Morb Mortal Wkly Rep.
49:535–538. 2000.PubMed/NCBI
|
|
29
|
Reynolds MA, Schieve LA, Martin JA, Jeng G
and Macaluso M: Trends in multiple births conceived using assisted
reproductive technology, United States, 1997–2000. Pediatrics.
111:1159–1162. 2003.PubMed/NCBI
|
|
30
|
Moini A, Shiva M, Arabipoor A, Hosseini R,
Chehrazi M and Sadeghi M: Obstetric and neonatal outcomes of twin
pregnancies conceived by assisted reproductive technology compared
with twin pregnancies conceived spontaneously: A prospective
follow-up study. Eur J Obstet Gynecol Reprod Biol. 165:29–32. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fritz G and Czeizel AE: Abnormal sperm
morphology and function in the fathers of hypospadiacs. J Reprod
Fertil. 106:63–66. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simon L, Murphy K, Shamsi MB, Liu L, Emery
B, Aston KI, Hotaling J and Carrell DT: Paternal influence of sperm
DNA integrity on early embryonic development. Hum Reprod.
29:2402–2412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yan J, Huang G, Sun Y, Zhao X, Chen S, Zou
S, Hao C, Quan S and Chen ZJ: Birth defects after assisted
reproductive technologies in China: Analysis of 15,405 offspring in
seven centers (2004 to 2008). Fertil Steril. 95:458–460. 2011.
View Article : Google Scholar : PubMed/NCBI
|