Introduction
The incidence of type 2 diabetes (T2MD) is
increasing as people's living standards increase, population ageing
increases, and life structure changes. It is expected that T2MD
will become the seventh largest cause of death in the world by 2030
(1,2). The major cause of death in patients
with T2MD is major vascular complications (3). According to reports from the National
Institutes of Health, 80% of patients with T2MD died of
macrovascular complications directly (4). Therefore, how to treat T2MD
macrovascular complications has become a global public health
problem.
Atherosclerosis (AS) is the basis of T2MD
macrovascular complications. It is prone to occur early in patients
with T2MD, and the progression of AS is rapid and the prognosis of
patients is poor. At present, the pathogenesis of AS in T2MD
patients is not yet fully determined (5,6). Many
researchers have found that the damage and dysfunction of the
vascular endothelium, long-term chronic inflammatory response
mediated by inflammatory factors and other reasons are important
mechanisms for the occurrence and development of T2MD AS. Among
them, vascular endothelial growth factor (VEGF) and transforming
growth factor-β1 (TGF-β1) are the hot topics discussed in recent
years. VEGF and TGF-β1 are abnormally expressed in patients with
T2MD AS (5,7). Simvastatin is a competitive inhibitor
of HMG-CoA reductase, and can block cholesterol synthesis (8). Studies have reported that simvastatin,
in addition to regulating blood lipids, also has the effects of
inhibiting proliferation of vascular smooth muscle cells,
stabilizing AS plaques, resisting oxidative stress, preventing
platelet aggregation, and improving endothelial function (9,10).
However, its specific mechanism of action has not yet been studied
clearly.
In this study, a rat model of T2MD was established
to observe the effect of simvastatin on the expression of VEGF and
TGF-β1 in rats and to investigate the mechanism of simvastatin.
Materials and methods
Research objects
A total of 40 clean grade mature Sprague Dawley (SD)
rats were purchased from Animal Center of Xinjiang Medical
University [production license SCXK (new) 2003-0001] and were fed
with normal dry feed (Beijing Zhecheng Technology Co., Ltd.,
Beijing, China). SD rats were ~3 weeks old with an average age of
21.3±1.5 days, weight 150–160 g, average body weight 154.1±12.2 g,
rearing temperature was 20–27°C, relative humidity 70±10%, separate
feeding in the terrarium, changing the litter every morning and
evening, environmental noise below 85 dB, ammonia concentration not
exceeding 20 ppm, ventilation 8–12 times per hour, fluorescent lamp
light every 12 h, free food intake, free drinking water, feeding
box was replaced 1–2 times a week. The humidity in the feeding box
did not exceed 10%. Τhe water bottle was changed 1–2 times a week.
Ten random-number table methods were used to select 10 rats as
normal control groups, and the remaining 30 were used to establish
T2MDAS rat models. After successful modeling, they were randomly
divided into model and treatment group. The study was approved by
the Ethics Committee of Liaocheng People's Hospital (Liaocheng,
China).
Rat model establishment
Thirty rats were used to establish T2MD AS rat
model: High-fat diet (Keaoxieli Co., Ltd., Beijing, China) for 8
weeks, immediately after 8 weeks of feeding, fasted for 12 h, and
given a single intraperitoneal injection of Streptozocin solution
(Shanghai Baoman Biotechnology Co., Ltd., Shanghai, China) 45
mg/kg, after 72 h measured tail vein blood glucose, fasting blood
glucose ≥7.0 mmol/l, and postprandial blood glucose ≥11.1 mmol/l
was set as the evaluation criteria for successful T2MD model.
Establishment of AS rat model: T2MD successfully modeled rats were
intragastrically administered with vitamin D3 injection (SFDA
approval no. 31021259; Wuhan Dongkangyuan Technology Co., Ltd.,
Wuhan, China), and were administered at a total dose of 500,000
U/kg for 3 days. The normal control group was fed with normal dry
feed.
Treatment method
After simvastatin tablets (Shenzhen Roark Standards
Technology Co., Ltd., SFDA Approval no. H20123114) was ground into
a powder, 0.9% physiological saline was formulated into a 1 mg/ml
suspension, and the treatment group was given a 10 mg/kg/day
simvastatin suspension intragastrically for drug intervention. The
normal control and model group were given 0.9% physiological saline
5 mg/kg/day gavage as a control. Treatment was performed for 8
weeks. The normal control group was fed with normal dry feed, and
the model and treatment group were still fed with high-fat
diet.
ELISA
Serum VEGF and TGF-β1 were detected by ELISA. The
procedure was with reference to the instructions of the kit. VEGF
and TGF-β1 kits were purchased from Shanghai Jing Kang Biological
Engineering Co., Ltd. (Shanghai, China).
Observation indicators
The changes of VEGF and TGF-β1 levels before and
after modeling (before intervention), 1, 2, 4, and 8 weeks after
intervention were analyzed by tail vein blood sampling. The
relationship between VEGF, TGF-β1 levels and treatment time was
analyzed.
Statistical analysis
SPSS 19.0 (SPSS, Inc., Chicago, IL, USA) was
adopted. Enumeration data are expressed as rate, and rates were
compared by using the χ2 test. The measurement data are
expressed as mean ± SD. Analysis of variance was used for
comparison among multiple groups, LSD tests were used for pairwise
comparison, and repeated-variance measurement experiments or paired
t-tests were used as comparison at different times within the
group. The correlation between VEGF and TGF-β1 levels was analyzed
by using Pearsons correlation. P<0.05 was statistically
significant.
Results
Modeling results
T2MD rat models were first constructed in 30 rats. A
total of 28 rats were successfully modeled. The success rate of
modeling was 93.33%. Then AS rat model was established. Finally, 26
rats were successfully modeled. The success rate of modeling was
92.86%. There was no difference in body weight, fasting blood
glucose, VEGF and TGF-β1 between the three groups before modeling
(P>0.05). After successful modeling/before intervention, fasting
blood glucose, VEGF, and TGF-β1 in the normal group were not
different from those before modeling. Body weight, fasting blood
glucose, VEGF, and TGF-β1 were increased in the model group and the
treatment group at different degrees (P<0.05). The weight of
rats before intervention in the normal group was higher than that
before modeling (P<0.05). The vital signs before and after
modeling in the three groups of rats are shown in Table I.
 | Table I.Vital signs before and after modeling
in three groups of rats. |
Table I.
Vital signs before and after modeling
in three groups of rats.
|
| Groups |
|
|
|---|
|
|
|
|
|
|---|
| Items | Normal control | Model | Treatment | Statistics | P-value |
|---|
| Quantity | 10 | 13 | 13 |
|
|
| Sex |
|
|
| 0.020 | 0.981 |
| Male | 5 | 7 | 7 |
|
|
|
Female | 5 | 6 | 6 |
|
|
| Weight before
modeling (g) | 153.7±19.9 | 155.5±12.1 | 153.1±12.4 | 0.092 | 0.912 |
| Weight before
intervention (g) | 261.8±34.5 |
385.3±56.4a |
379.5±54.3a | 23.66 | <0.001 |
| Fasting blood glucose
before modeling (mmol/l) | 4.26±1.02 | 4.28±1.01 | 4.24±1.03 | 0.005 | 0.995 |
| Fasting blood glucose
before intervention (mmol/l) | 4.18±0.68 |
12.63±4.32a |
12.56±4.27a | 18.88 | <0.001 |
| Pre-modeling VEGF
(µg/l) | 76.03±12.14 | 77.12±11.49 | 76.59±12.34 | 0.023 | 0.977 |
|
| 77.47±11.69 |
329.14±68.47a |
332.29±67.56a | 68.09 | <0.001 |
| Pre-intervention VEGF
(µg/l) | 3.27±0.79 | 3.29±0.78 | 3.31±0.80 | 0.007 | 0.993 |
|
| 3.38±0.87 |
9.22±2.13a |
9.26±2.12a | 35.53 | <0.001 |
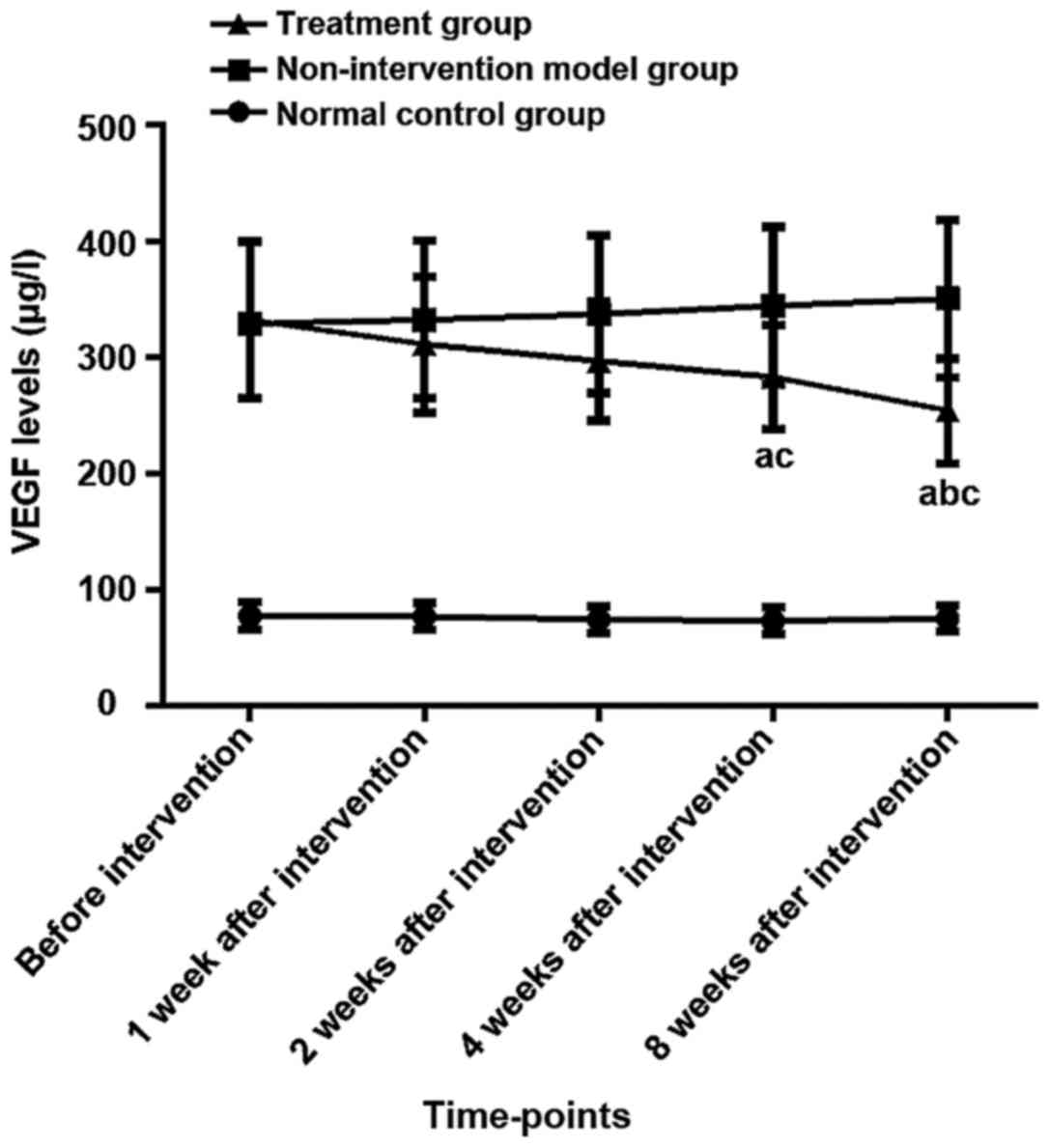
Changes of serum VEGF levels in three
groups before and after intervention
There was no difference in serum VEGF levels between
the three groups before modeling (P>0.05). Αnalysis of variance
showed there were differences in the expression of VEGF among three
groups of rats before intervention, 1, 2, 4, and 8 weeks after
intervention (P<0.05). The LSD test results showed that the VEGF
expression levels in the model and the treatment group at the five
time-points were higher than those in the normal control group
(P<0.05). The expression level of VEGF in the treatment group
was lower than the model group after 4 weeks and 8 weeks after the
intervention (P<0.05), and there was no difference at other
time-points (P>0.05). The results of repeated analysis of
variance at different time-points within the group showed that the
expression levels of VEGF in the normal control group and model
group had no significant changes before and after the intervention
(P>0.05). Τhe expression level of VEGF in the treatment group
after 4 and 8 weeks of intervention was lower than that before the
intervention (P<0.05). Τhe expression level of VEGF in the
treatment group after 8 weeks of intervention was lower than that
after 1 week (P<0.05), and there was no difference between any
of the other time-points (P>0.05) (Table II and Fig. 1).
 | Table II.Changes of serum VEGF levels in rats
before and after intervention (µg/l). |
Table II.
Changes of serum VEGF levels in rats
before and after intervention (µg/l).
|
| Groups |
|
|
|---|
|
|
|
|
|
|---|
| Items | Normal control | Model | Treatment | Statistics | P-value |
|---|
| Quantity | 10 | 13 | 13 |
|
|
| Before
intervention | 77.47±11.69 |
329.14±68.47c |
332.29±67.56c | 136.10 | <0.001 |
| 1 week after
intervention | 77.06±11.32 |
332.44±67.58c |
311.33±58.42c | 74.18 | <0.001 |
| 2 weeks after
intervention | 74.27±11.48 |
337.27±67.69c |
296.69±51.04c | 82.31 | <0.001 |
| 4 weeks after
intervention | 73.29±11.56 |
344.55±67.98c |
283.27±44.53a,c,d | 90.76 | <0.001 |
| 8 weeks after
intervention | 75.33±11.23 |
350.59±67.84c |
254.28±45.16a–d | 88.29 | <0.001 |
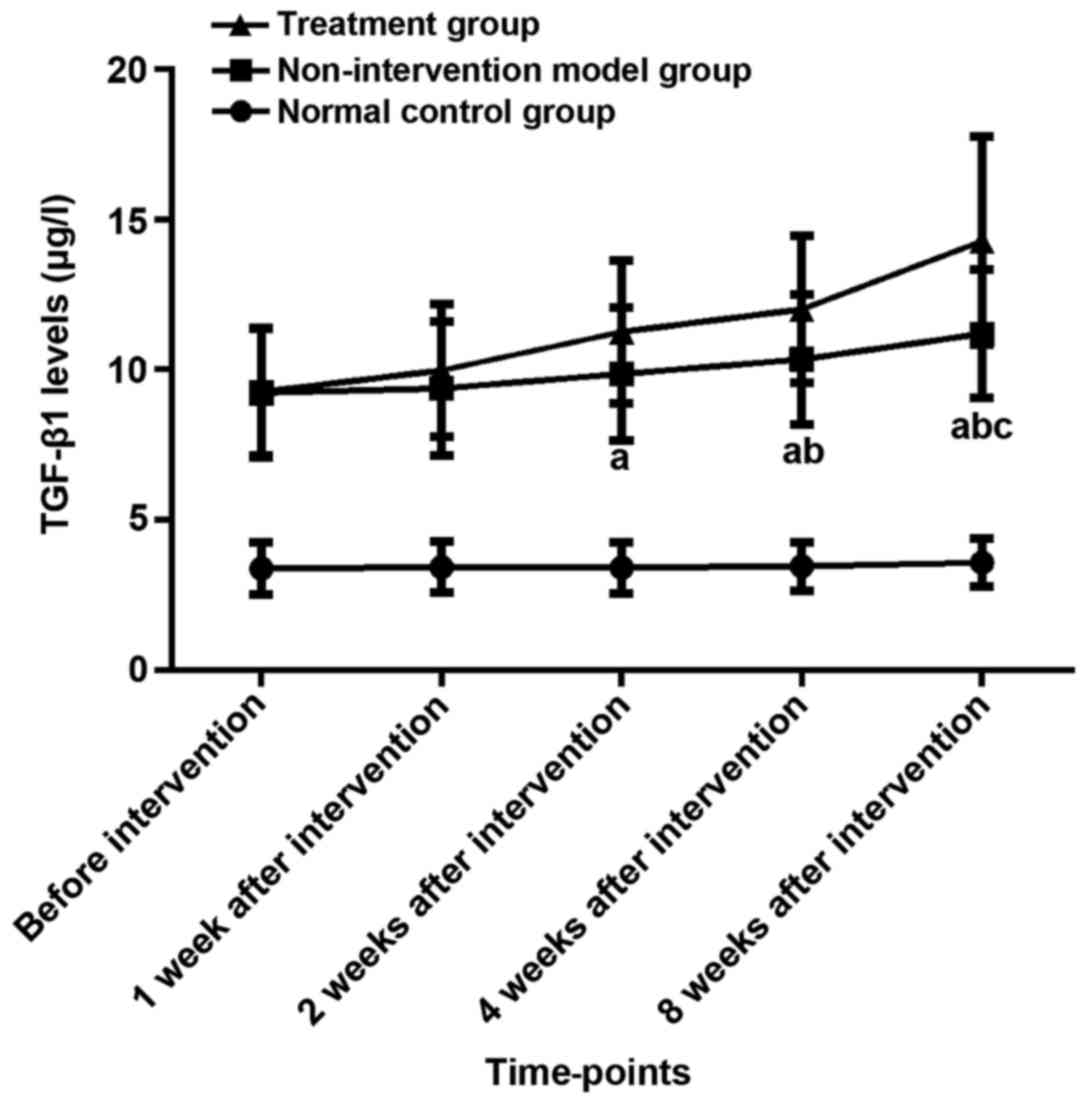
Changes of serum TGF-β1 levels in
three groups before and after intervention
There was no difference in the levels of serum
TGF-β1 between the three groups before modeling (P>0.05). The
analysis of variance showed that the expression levels of TGF-β1 of
the three groups of rats were different before the intervention, 1,
2, 4, and 8 weeks after the intervention (P<0.05). The results
of LSD showed that the expression levels of TGF-β1 in the model and
the treatment group at the five time-points were higher than those
in the normal control group (P<0.05). The expression level of
TGF-β1 in the treatment was higher than that in the model group
after 8 weeks of intervention (P<0.05), and there was no
difference at other time-points (P>0.05). The results of
repeated analysis of variance at different time-points within the
group showed that the expression level of TGF-β1 in normal control
rats did not change significantly before and after intervention
(P>0.05). Τhe expression level of TGF-β1 in the model group 8
weeks after intervention was higher than that before intervention
and 1 week after intervention (P<0.05), and there was no
difference in the expression level of TGF-β1 at other time-points
in the model group (P>0.05). The expression level of TGF-β1 in
the treatment group after 2, 4 and 8 weeks after intervention was
higher than before intervention (P<0.05). After 4 and 8 weeks of
intervention, the expression level of TGF-β1 was higher than that
after 1 week of intervention (P<0.05). There was no difference
between the other time-points in the treatment group (P>0.05)
(Table III and Fig. 2).
 | Table III.Changes of serum TGF-β1 levels in
rats before and after intervention (µg/l). |
Table III.
Changes of serum TGF-β1 levels in
rats before and after intervention (µg/l).
|
| Groups |
|
|
|---|
|
|
|
|
|
|---|
| Items | Normal control | Model | Treatment | Statistics | P-value |
|---|
| Quantity | 10 | 13 | 13 |
|
|
| Before
intervention | 3.38±0.87 |
9.22±2.13c |
9.26±2.12c | 35.53 | <0.001 |
| 1 week after
intervention | 3.42±0.85 |
9.37±2.22c |
9.97±2.22c | 37.61 | <0.001 |
| 2 weeks after
intervention | 3.40±0.86 |
9.85±2.21c |
11.25±2.38a,c | 47.30 | <0.001 |
| 4 weeks after
intervention | 3.45±0.81 |
10.34±2.16c |
12.01±2.44a–c | 55.58 | <0.001 |
| 8 weeks after
intervention | 3.58±0.80 |
11.20±2.14a–c |
14.28±3.46a–d | 46.00 | <0.001 |
Correlation analysis of VEGF and
TGF-β1
Pearsons correlation analysis showed that the
expression of VEGF was negatively correlated with TGF-β1 expression
in the treatment group (r=−0.712, P=0.015). Νegative correlation
was found between VEGF and treatment time (r=−0.769, P=0.003).
There was a positive correlation between TGF-β1 and treatment time
(r=0.812, P=0.001). (Data not shown).
Discussion
Studies have reported that simvastatin can mediate
CD40-CD40L signaling pathway in diabetic rats, reduce inflammation
in arterial walls of diabetic rats, and improve vascular
endothelium and its function in rats (11). However, there are many factors that
cause T2MD AS, in addition to CD40-CD40L-related factors, whether
VEGF and TGF-β1 play an important role in the treatment of diabetic
rats AS with simvastatin is not yet clear and was the focus of this
study.
A T2MD AS rat model was established in 2 steps.
Clean SD rats were used to establish a T2MD rat model by feeding of
high-fat diet for 8 weeks and using Streptozocin solution to
destroy rat islet B cells. High-dose vitamin D disrupted the
integrity of rat artery wall and replicated AS pathological model.
After successful modeling, body weight and fasting blood glucose
were significantly increased in rats, and VEGF and TGF-β1 were also
significantly elevated. Therefore, we initially speculated that
VEGF and TGF-β1 is involved in the development of rat T2MD AS. Then
we used low-dose simvastatin to treat 13 T2MD AS rats in the
treatment group. With the increase of treatment time, VEGF showed a
decreasing trend. Until 4 weeks after treatment, the VEGF levels in
the treatment group rats showed a significant difference compared
with those before the intervention, and there was also a
statistically significant difference in VEGF levels between the
treatment and the model group, indicating that simvastatin can
improve VEGF levels in T2MD rats. The occurrence of AS in humans or
rats causes insufficient blood supply to the arteries, resulting in
endothelial cells secreting higher VEGF (8,12). Some
studies have reported (13) that
VEGF can activate the VEGFR2/KDR/flk1 signaling pathway, increase
vascular permeability, promote adhesion of inflammatory cells in
the blood vessel wall, and infiltration of inflammatory cells,
promote neovascularization, and aggravate AS conditions. VEGF plays
an important role in the occurrence of T2MD AS. In the present
study, the results of TGF-β1 detection found that with the increase
of treatment time, TGF-β1 showed an upward trend. After 2 weeks of
treatment, serum TGF-β1 levels were statistically different from
those before the intervention, indicating that simvastatin can
increase TGF-β1 levels. Correlation analysis showed that VEGF was
negatively correlated with treatment time, and TGF-β1 was
positively correlated with treatment time. The inflammatory
response is an important cause of plaque instability in AS, so
improving the excessive inflammatory response is an important
process for the treatment of AS (14,15).
TGF-β1 has a strong non-specific anti-inflammatory effect (16). It was reported (17) that TGF-β1 can control the local
inflammatory response due to AS by downregulating E-selectin and
inhibiting the activity of CD4+ T cells. The
proliferation and migration of endothelial cells and the activation
of the coagulation system due to endothelial cell damage are also
important causes of AS (18,19). Some reports (20) indicated that TGF-β1 can activate the
TGF-β1/ALK5/Smad3 signaling pathway and promote the expression of
endothelin-1, thus hindering the proliferation and migration of
endothelial cells. It has also been reported (21) that TGF-β1 can promote endothelial
cells to express more prostacyclin, inhibit platelet aggregation,
and prevent thrombosis. Therefore, TGF-β1 also plays an important
role in the development of T2MD AS. Simvastatin can improve AS by
improving the levels of VEGF and TGF-β1 in serum of T2MD rats.
However, the trial of simvastatin in the treatment of diabetes
generally lasted for more than 2 years. Since the animal model was
used as the subject for analysis in this experiment, the analysis
results only represented short-term therapeutic effects, and the
long-term treatment effect could not be evaluated, and whether it
has the same effect in human still needs to be studied.
In conclusion, VEGF, TGF-β1 may be involved in the
occurrence of T2MD AS, and simvastatin may play a therapeutic role
in T2MD AS by downregulating VEGF and upregulating TGF-β1
expression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW wrote the manuscript. HW, JL and XF constructed
T2MD rat models. YL and QX were responsible for ELISA. LS helped
with statistical analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Liaocheng People's Hospital (Liaocheng, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Diabetes Association, . Standards
of medical care in diabetes-2015 abridged for primary care
providers. Clin Diabetes. 33:97–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrannini E, Baldi S, Frascerra S,
Astiarraga B, Heise T, Bizzotto R, Mari A, Pieber TR and Muscelli
E: Shift to fatty substrate utilization in response to
sodium-glucose cotransporter 2 inhibition in subjects without
diabetes and patients with type 2 diabetes. Diabetes. 65:1190–1195.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nolan CJ, Ruderman NB, Kahn SE, Pedersen O
and Prentki M: Insulin resistance as a physiological defense
against metabolic stress: Implications for the management of
subsets of type 2 diabetes. Diabetes. 64:673–686. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
American Diabetes Association, . Standards
of medical care in diabetes — 2014. Diabetes Care. 37 Suppl
1:S14–S80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Catapano AL, Graham I, De Backer G,
Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser
U, Pedersen TR, et al: Authors/Task Force Members: 2016 ESC/EAS
guidelines for the management of dyslipidaemias: The task force for
the management of dyslipidaemias of the European Society of
Cardiology (ESC) and European Atherosclerosis Society (EAS)
Developed with the special contribution of the European
Assocciation for Cardiovascular Prevention & Rehabilitation
(EACPR). Atherosclerosis. 253:281–344. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li H, Horke S and Förstermann U: Vascular
oxidative stress, nitric oxide and atherosclerosis.
Atherosclerosis. 237:208–219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alexopoulos N, Katritsis D and Raggi P:
Visceral adipose tissue as a source of inflammation and promoter of
atherosclerosis. Atherosclerosis. 233:104–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bhakdi S: Pathogenesis of atherosclerosis:
Infectious versus immune pathogenesis. A new concept. Herz.
25:84–86. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murphy SA, Cannon CP, Blazing MA,
Giugliano RP, White JA, Lokhnygina Y, Reist C, Im K, Bohula EA,
Isaza D, et al: Reduction in total cardiovascular events with
ezetimibe/simvastatin post-acute coronary syndrome: The IMPROVE-IT
Trial. J Am Coll Cardiol. 67:353–361. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Criner GJ, Connett JE, Aaron SD, Albert
RK, Bailey WC, Casaburi R, Cooper JA Jr, Curtis JL, Dransfield MT,
Han MK, et al: COPD Clinical Research Network; Canadian Institutes
of Health Research: Simvastatin for the prevention of exacerbations
in moderate-to-severe COPD. N Engl J Med. 370:2201–2210. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xue L, Zhu XH, Yang XF, Bao XC, Gao XQ,
Qiu YH, Wu Z, Ji XP and Li HW: Effect of pioglitazone combined with
simvastatin on the CD40-CD40 ligand system in rabbits with
atherosclerosis. Eur Rev Med Pharmacol Sci. 19:322–327.
2015.PubMed/NCBI
|
|
12
|
Al Rifai M, Silverman MG, Nasir K, Budoff
MJ, Blankstein R, Szklo M, Katz R, Blumenthal RS and Blaha MJ: The
association of nonalcoholic fatty liver disease, obesity, and
metabolic syndrome, with systemic inflammation and subclinical
atherosclerosis: The Multi-Ethnic Study of Atherosclerosis (MESA).
Atherosclerosis. 239:629–633. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim SH, Pei QM, Jiang P, Yang M, Qian XJ
and Liu JB: Effect of active vitamin D3 on VEGF-induced ADAM33
expression and proliferation in human airway smooth muscle cells:
Implications for asthma treatment. Respir Res. 18:72017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mangge H, Weghuber D, Prassl R, Haara A,
Schnedl W, Postolache TT and Fuchs D: The role of vitamin D in
atherosclerosis inflammation revisited: More a bystander than a
player? Curr Vasc Pharmacol. 13:392–398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lutgens E, Lievens D, Beckers L, Wijnands
E, Soehnlein O, Zernecke A, Seijkens T, Engel D, Cleutjens J,
Keller AM, et al: Deficient CD40-TRAF6 signaling in leukocytes
prevents atherosclerosis by skewing the immune response toward an
antiinflammatory profile. J Exp Med. 207:391–404. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kotlarz D, Marquardt B, Barøy T, Lee WS,
Konnikova L, Hollizeck S, Magg T, Lehle AS, Walz C, Borggraefe I,
et al: Human TGF-β1 deficiency causes severe inflammatory bowel
disease and encephalopathy. Nat Genet. 50:344–348. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Edwards JP, Hand TW, Morais da Fonseca D,
Glass DD, Belkaid Y and Shevach EM: The GARP/Latent TGF-β1 complex
on Treg cells modulates the induction of peripherally derived Treg
cells during oral tolerance. Eur J Immunol. 46:1480–1489. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gerdes N, Seijkens T, Lievens D, Kuijpers
MJ, Winkels H, Projahn D, Hartwig H, Beckers L, Megens RT, Boon L,
et al: Platelet CD40 exacerbates atherosclerosis by transcellular
activation of endothelial cells and leukocytes. Arterioscler Thromb
Vasc Biol. 36:482–490. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gimbrone MA Jr and García-Cardeña G:
Endothelial cell dysfunction and the pathobiology of
atherosclerosis. Circ Res. 118:620–636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Zou N, Wang J, Wang KW, Li FY, Chen
FX, Sun BY and Sun DJ: TGF-β1/Smad3 signaling pathway mediates T-2
toxin-induced decrease of type II collagen in cultured rat
chondrocytes. Toxins (Basel). 9:92017. View Article : Google Scholar
|
|
21
|
Hwangbo C, Tae N, Lee S, Kim O, Park OK,
Kim J, Kwon SH and Lee JH: Syntenin regulates TGF-β1-induced Smad
activation and the epithelial-to-mesenchymal transition by
inhibiting caveolin-mediated TGF-β type I receptor internalization.
Oncogene. 35:389–401. 2016. View Article : Google Scholar : PubMed/NCBI
|