Introduction
Breast cancer is one of the most common malignancies
and the leading cause of cancer-associated mortality in females
worldwide (1). Considering that
breast cancer has a high heterogeneity (2), various specific biomarkers, including
estrogen receptor (ER) and human epidermal growth factor-2 (HER2)
(3–5), have been discovered to classify breast
cancer into four subtypes: Basal-like (BL), luminal A, luminal B
and HER2-enriched (6), which allows
for the application of more individualized therapies for breast
cancer patients. However, the crude mortality due to breast cancer
in China in 2010 was 8.65 per 100,000 individuals, and was
accountable for 7.90% of all cancer-associated mortalities in women
(7). Therefore, to identify a novel
marker consistent with existing ones but able to further refine
breast cancer diagnostics has significance for improving the
efficacy of breast cancer therapy.
The role of insulin-like growth factor-1 receptor
(IGF-1R) in breast carcinogenesis has attracted increasing
attention over the last two decades (8,9). IGF-1R
is a tyrosine kinase cell surface receptor, which has mitogenic,
proliferative and anti-apoptotic effects in cells and was
identified to have an increased expression in various types of
malignant tumor tissue, including breast cancer (10–13).
Several studies have been performed to identify the role and
expression of IGF-1R in various subtypes of breast cancer (14). In addition, the overexpression of
IGF-1R may be correlated with disease development, aggressive
phenotypes, clinical outcomes and the therapy resistance (15). However, the predictive and prognostic
significance of IGF-1R expression in breast cancer lesions remains
controversial. For instance, certain studies report IGF-1R as a
favorable prognostic indicator in breast cancer (16), while others indicate that IGF-1R
overexpression is associated with an increased probability of
metastasis, a poor response to treatment and a decreased survival
rate (17).
Considering IGF-1R phosphorylation as an essential
step for the function of IGF-1R (18), the present study assessed the levels
of phosphorylated (p)-IGF-1R in breast cancer tissue, and their
correlation with clinicopathological features and clinical
outcomes. The present study also investigated whether p-IGF-1R is a
predictive and prognostic biomarker by focusing on its association
with the expression of other outcome-associated biomarkers, overall
survival (OS) and response to neoadjuvant chemotherapy. It was
investigated whether p-IGF-1R has the potential to refine the
existing breast cancer classification and prognostication by
markers routinely used in the clinic.
Materials and methods
Patients
A total of 348 female patients admitted to the
Department of Breast Surgery of the Second Hospital of Shandong
University (Jinan, China) from January 2010 to December 2014 were
enrolled in the present study. All patients included in the final
analysis were newly diagnosed and histologically confirmed to have
breast cancer. The complete clinical data of each patient were
recorded, and their breast cancer tissue samples were collected.
All records were collected on the basis of patient written informed
consent for the use of clinical data, their enrollment in the
present study and for the analysis of their tissues. None of them
had received any prior cancer treatment, including surgery,
chemotherapy, radiotherapy and endocrine therapy. After enrollment,
all treatments given by our department were based on NCCN guideline
(19). All procedures were in
accordance with the guidelines set by the Declaration of Helsinki
and approved by the Clinical Research Ethics Committee of the
Second Hospital of Shandong University (Jinan, China).
Data collection
The clinical information of all patients was
retrieved from electronic and paper-based medical records available
from the Second Hospital of Shandong University (Jinan, China),
which had been obtained from patients or their family members upon
admission. All records were collected on the basis of complete
informed consent. The patient data comprised of seven aspects,
including i) demographic characteristics: Gender, age, ethnicity,
marital status, occupation, height and weight; ii) gynecological
details: Age of menarche, duration of menstruation, menstrual cycle
and menopausal status; iii) fertility conditions: Number of
children, age at first birth and breastfeeding conditions; iv)
personal medical history (diagnosed by doctors prior to enrollment
in the present study): Hypertension, diabetes mellitus, heart
disease, viral hepatitis, benign breast disorders, uterine fibroids
and ovarian cysts; v) family history: Breast cancer, other cancer,
all types of cancer; vi) physical breast examination: Size,
position, texture, mobility and smoothness of tumor and palpation
of lymph nodes; vii) treatment-associated information: Time and
methods of operation, details regarding lymph node dissection,
regimen of chemotherapy, neoadjuvant chemotherapy and endocrine
therapy; and viii) pathological characteristics: Pathological
types, tumor stage, ER, progesterone receptor (PR), HER2, Ki-67 and
lymph node metastasis status.
Immunohistochemistry
In total, immunohistochemical analysis of the
expression of p-IGF-1R was performed in 388 breast cancer tissue
sections as previously reported (20,21). In
brief, all specimens were formalin-fixed and paraffin-embedded.
Tissue sections (3 µm) were dewaxed with xylene and dehydrated with
a graded ethanol series. Antigen retrieval was performed by boiling
in citrate buffer (Beyotime Institute of Biotechnology, Haimen,
China) and the slides were allowed to cool at room temperature. The
slides were then washed with PBS three times for two minutes each
time. Next, 50 µl of 3% H2O2 solution was
added to each slide to inhibit the endogenous peroxidase. Slides
were incubated with anti-p-IGF-1R polyclonal antibody (cat. no.
39398; 1:200 dilution; Abcam, Cambridge, MA, USA) for 1 h at 37°C,
followed by washing with PBS three times as above. Secondary
antibody (cat. no. DV9000; 1:1 dilution; OriGene Technologies,
Inc., Rockville, MD, USA) was added to each slide, followed by
incubation at room temperature for 30 min. After washing with PBS,
freshly prepared diaminobenzidine solution was added, followed by
incubation for 2 min at room temperature. All slides were washed
with tap water and counterstained with hematoxylin for 30 sec.
Gradient alcohol dehydration and clearing with xylene were
performed, and all slides were mounted with neutral balata (OriGene
Technologies, Inc., Rockville, MD, USA).
Imaging analysis
Either membranous or cytoplasm staining for p-IGF-1R
was defined as positive. The results were assessed by an
experienced pathologist using the immunoreactive scoring (IRS)
criteria (22,23). A total of 10 randomly selected
high-power microscopic fields for each section were observed and
100 cells in each field were counted. IRS=SI (staining intensity)
xPP (percentage of positive cells). The staining intensity
(22) was rated as 0 (absent), 1
(weak), 2 (moderate) and 3 (strong). The percentage of positive
cells was defined as follows: 1, 1–10; 2, 11–50; 3, 51–80; and 4,
81–100%. For the semi-quantitative analysis, samples with a score
of 0–2, 3/4, 5–8 and 9–12 were labeled as -, +, ++ and +++,
respectively (23). The sections
scoring 0 were considered as negative for p-IGF-1R, and the
remaining ones were considered as positive (24).
End-points
OS was defined as the time from diagnosis (date of
biopsy) until the time-point of mortality or the last follow-up for
patients. Disease-free survival (DFS) was defined as the time from
diagnosis (date of biopsy) to events, including local relapse or
distant metastases, the occurrence of a new primary tumor or death
without evidence of cancer.
Statistical analysis
SPSS version 18.0 (SPSS, Inc., Chicago, IL, USA) was
used for statistical analysis of the data. For descriptive
analysis, the values of continuous variables were expressed as the
mean ± the standard deviation, and those of categorical variables
were presented as the frequency. To describe the distribution of
p-IGF-1R and to test whether p-IGF-1R was correlated with any
clinicopathological parameters, Pearson's Chi-Square tests were
performed for categorical variables. The Wilcoxon rank sum test was
performed for comparing p-IGF-1R alterations during neoadjuvant
chemotherapy (NAC). Logistic regression was performed to identify
factors associated with p-IGF-1R levels. Correlations between
p-IGF-1R and OS were analyzed by Kaplan-Meier survival analysis and
multivariable Cox regression, and differences between subgroups
were calculated using the log-rank test. All tests were two-sided,
and P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
Of the 348 female breast cancer patients enrolled in
the present study, 80 received NAC. Among those 80 patients,
paraffin-embedded tumor tissue sections from the pre-NAC and
post-NAC time-points were available for 40 patients. These tumor
tissues had been collected by biopsy and resection, respectively.
In total, 388 tumor tissue sections were analyzed by
immunohistochemistry to detect p-IGF-1R. The demographic
characteristics and medical history of the patients are summarized
in Table I.
 | Table I.Basic demographic characteristics and
medical history of the breast cancer patients stratified by
p-IGF-1R status. |
Table I.
Basic demographic characteristics and
medical history of the breast cancer patients stratified by
p-IGF-1R status.
|
|
| p-IGF-1R |
|
|
|---|
|
|
|
|
|
|
|---|
| Variables | N (%) | Negative | Positive | χ2 | P-value |
|---|
| Age (years; range,
22–84) |
|
|
| 2.893 | 0.411 |
|
20–35 | 24 (6.9) | 19 (8.0) | 5 (4.5) |
|
|
|
36–50 | 151 (43.4) | 99 (41.6) | 52 (47.3) |
|
|
|
51–65 | 132 (37.9) | 89 (37.4) | 43 (39.1) |
|
|
|
>65 | 41 (11.8) | 31 (13.0) | 10 (9.1) |
|
|
| Occupation |
|
|
| 17.101 | 0.004 |
|
Farmer | 85 (24.4) | 53 (22.3) | 32 (29.1) |
|
|
|
Workera | 85 (24.4) | 67 (28.2) | 18 (16.4) |
|
|
|
Retiree | 62 (17.8) | 43 (18.1) | 19 (17.3) |
|
|
|
Unemployed | 51 (14.7) | 39 (16.4) | 12 (10.9) |
|
|
|
Teacher, public servant,
accountant or medical staff | 17 (4.9) | 13 (5.5) | 4 (3.6) |
|
|
|
Other | 48 (13.8) | 23 (9.7) | 25 (22.7) |
|
|
| Ethnicity |
|
|
| 0.775 | 0.681 |
|
Han | 345 (99.1) | 236
(99.2) | 109 (99.1) |
|
|
|
Hui | 2 (0.6) | 1 (0.4) | 1 (0.9) |
|
|
|
Manchu | 1 (0.3) | 1 (0.4) | 0 (0) |
|
|
| Marital status |
|
|
| 0.028 | 1.000 |
|
Married | 339 (97.4) | 232 (97.5) | 107 (97.3) |
|
|
|
Single | 7 (2.0) | 5 (2.1) | 2 (1.8) |
|
|
|
Unknown | 2 (0.6) | 1 (0.4) | 1 (0.9) |
|
|
| BMI
(kg/m2) |
|
|
| 1.982 | 0.555 |
|
<18.5 | 5 (1.4) | 3 (1.3) | 2 (1.8) |
|
|
|
18.5–23.9 | 139 (39.9) | 101 (42.4) | 38 (34.5) |
|
|
|
24.0–27.9 | 138 (39.7) | 90 (37.8) | 48 (43.6) |
|
|
|
≥28 | 53 (15.2) | 36 (15.1) | 17 (15.5) |
|
|
|
Unknown | 13 (3.7) | 8 (3.4) | 5 (4.5) |
|
|
| Family history of
breast cancerb |
|
|
| 0.530 | 0.467 |
|
Yes | 31 (8.9) | 23 (9.7) | 8 (7.3) |
|
|
| No | 317 (91.1) | 215 (90.3) | 102 (92.7) |
|
|
| Family history of
other cancerb type |
|
|
| 2.178 | 0.140 |
|
Yes | 52 (14.9) | 31 (13.0) | 21 (19.1) |
|
|
| No | 296 (85.1) | 207 (87.0) | 89 (80.9) |
|
|
| Family history of
cancerb,c |
|
|
| 0.319 | 0.572 |
|
Yes | 82 (23.6) | 54 (22.7) | 28 (25.5) |
|
|
| No | 266 (76.4) | 184 (77.3) | 82 (74.5) |
|
|
|
Hypertensiond |
|
|
| 2.099 | 0.147 |
|
Yes | 80 (23.0) | 60 (25.2) | 20 (18.2) |
|
|
| No | 268 (77.0) | 178 (74.8) | 90 (81.8) |
|
|
| Diabetes
mellitusd |
|
|
| 0.915 | 0.339 |
|
Yes | 33 (9.5) | 25 (10.5) | 8 (7.3) |
|
|
| No | 315 (90.5) | 213 (89.5) | 102 (92.7) |
|
|
| Heart
diseased |
|
|
| 2.117 | 0.146 |
|
Yes | 34 (9.8) | 27 (11.3) | 7 (6.4) |
|
|
| No | 314 (90.2) | 211 (88.7) | 103 (93.6) |
|
|
| Breast benign
tumord |
|
|
| 1.322 | 0.361 |
|
Yes | 13 (3.7) | 7 (2.9) | 6 (5.5) |
|
|
| No | 335 (96.3) | 231 (97.1) | 104 (94.5) |
|
|
| Uterine
fibroidd |
|
|
| 6.150 | 0.013 |
|
Yes | 28 (8.0) | 25 (10.5) | 3 (2.7) |
|
|
| No | 320 (92.0) | 213 (89.5) | 107 (97.3) |
|
|
| Ovarian
cystc |
|
|
| 0.131 | 0.711 |
|
Yes | 8 (2.3) | 5 (2.1) | 3 (2.7) |
|
|
| No | 340 (97.7) | 233 (97.9) | 107 (97.3) |
|
|
The age of these 348 patients ranged from 22 to 84
years, and the average age was 51.73±11.85 years. The cohort
included 144 underweight or normal weight patients (BMI<24
kg/m2, accounting for 41.3%) and 191 overweight or obese
patients (BMI≥24 kg/m2, accounting for 54.9%), while BMI
data were missing for 13 patients.
p-IGF-1R levels
Baseline p-IGF-1R was determined by
immunohistochemical analysis of p-IGF-1R in tissue collected by
resection for patients who did not receive NAC and in the biopsy
specimens for patients who were to receive NAC. The baseline
expression of p-IGF-1R was assessed in all 348 cases of breast
cancer. The results demonstrated that 238 cases (68.4%) had a
p-IGF-1R negative status, while 110 cases (31.6%) had a positive
status, among whom weak (+), moderate (++) and strong (+++)
p-IGF-1R staining was observed in 74 (21.3%), 32 (9.2%) and 4
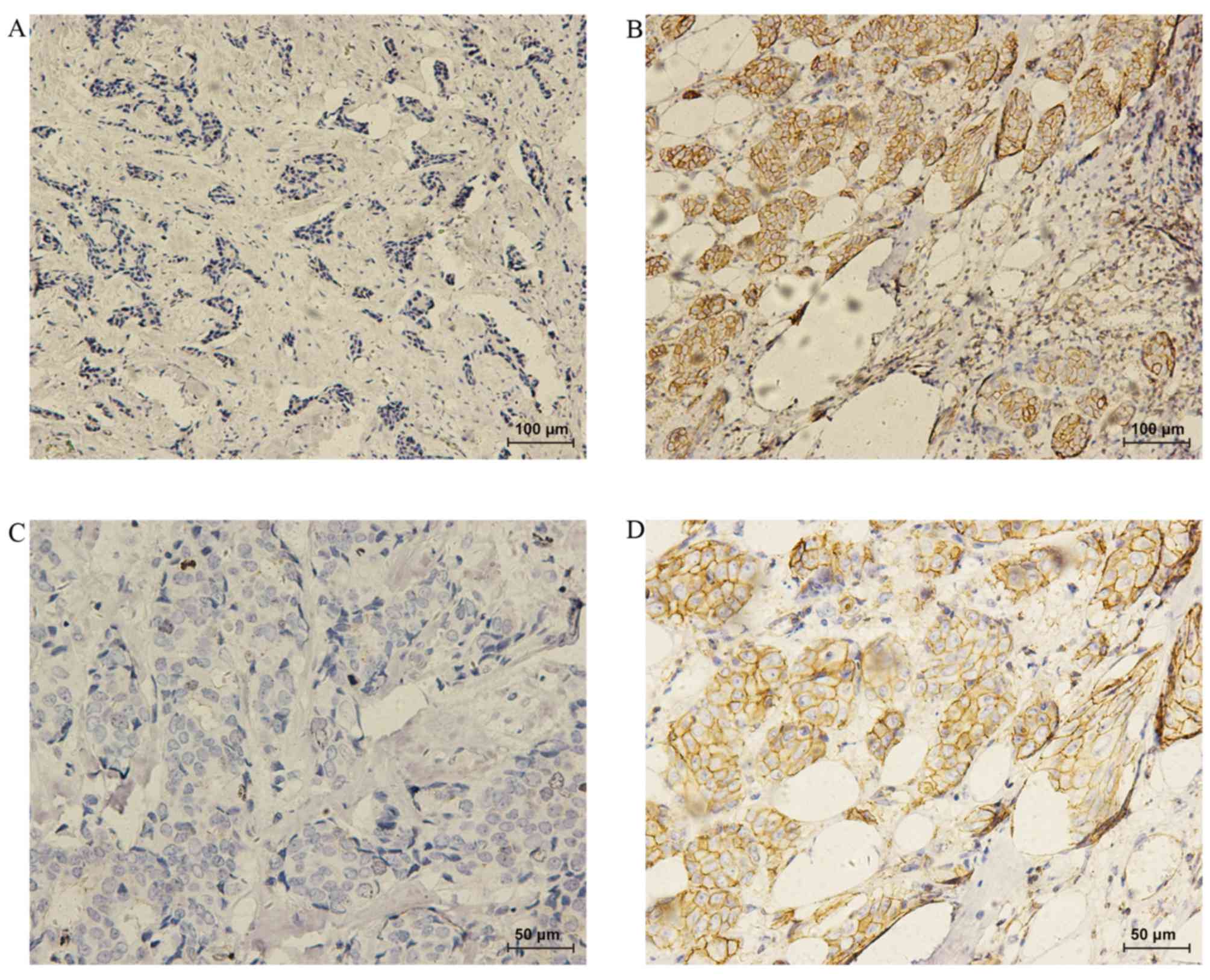
(1.1%) cases, respectively. Representative immunohistochemical
images are displayed in Fig. 1. The
results indicated that compared with the p-IGF-1R-negative cases,
less p-IGF-1R positive cases had a history of uterine fibroids (2.7
vs. 10.5%; P=0.013; Table I). The
distribution of p-IGF-1R among occupations were significantly
different (P=0.004). However, the age, ethnicity, personal medical
history regarding hypertension, diabetes, heart disease, breast
cancer and benign tumors, and even family history of breast cancer
had no significant association with the p-IGF-1R status (P>0.05;
Table I).
Clinicopathological characteristics
associated with p-IGF-1R status
To investigate whether p-IGF-1R may affect the
clinicopathological characteristics of breast cancer patients, the
association between the p-IGF-1R status and clinicopathological
features was assessed (Table II).
While 296 patients (85.1% of the total) were diagnosed with
invasive ductal carcinoma, 29 (8.3%), 5 (1.4%) and 17 (4.9%)
patients were diagnosed with ductal carcinoma in situ,
invasive lobular carcinoma and other types of breast cancer,
respectively. The results of the statistical analysis indicated
that the p-IGF-1R status of breast cancer tissue was neither
associated with the pathological type and tumor-nodes-metastasis
stage, nor with the status of ER, PR, Ki-67 and lymph node
metastasis (P>0.05). However, the level of p-IGF-1R in the
tumors differed significantly between the groups with and without
NAC treatment, and the groups with and without HER2 expression in
the tissues. Compared with the p-IGF-1R-negative cases, more
p-IGF-1R-positive cases had received NAC (30.0 vs. 19.7%; P=0.035).
p-IGF-1R-positive cases had a higher rate of positivity for HER2
compared with the p-IGF-1R-negative cases (30.0 vs. 14.3%)
(P<0.001). The difference also reached statistical significance
among the groups with different molecular subtypes. More luminal A,
luminal B and triple negative breast cancer (TNBC) tumors had a
p-IGF-1R-negative status (P=0.022). These results suggest that the
phosphorylation of IGF-1R may be associated with HER2
signaling.
 | Table II.Association of p-IGF-1R status with
various clinicopathological characteristics. |
Table II.
Association of p-IGF-1R status with
various clinicopathological characteristics.
|
|
| p-IGF-1R |
|
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | N (%) | Negative | Positive | χ2 | P-value |
|---|
| NAC |
|
|
| 4.466 | 0.035 |
|
Yes | 80 (23.0) | 47 (19.7) | 33 (30.0) |
|
|
| No | 268 (77.0) | 191 (80.3) | 77 (70.0) |
|
|
| Pathological
type |
|
|
| 0.424 | 0.935 |
|
IDC | 296 (85.1) | 202 (84.9) | 94 (85.5) |
|
|
|
DCIS | 29 (8.3) | 20 (8.4) | 9 (8.2) |
|
|
|
ILC | 5 (1.4) | 4 (1.7) | 1 (0.9) |
|
|
| Other
type | 17 (4.9) | 11 (4.6) | 6 (5.5) |
|
|
|
Unknown | 1 (0.3) | 1 (0.4) | 0 (0) |
|
|
| pT
stagea |
|
|
| 5.195 | 0.073 |
| T1 | 131 (37.6) | 93 (39.1) | 38 (34.5) |
|
|
| T2 | 99 (28.4) | 61 (25.6) | 38 (34.5) |
|
|
| T3 | 6 (1.7) | 6 (2.5) | 0 (0) |
|
|
|
Unknown | 112 (32.2) | 78 (32.8) | 34 (30.9) |
|
|
| pTNM
stageb |
|
|
| 0.142 | 0.931 |
| I | 112 (32.2) | 77 (32.4) | 35 (31.8) |
|
|
| II | 140 (40.2) | 99 (41.6) | 41 (37.3) |
|
|
|
III | 64 (18.4) | 44 (18.5) | 20 (18.2) |
|
|
|
Unknown | 32 (9.2) | 18 (7.6) | 14 (12.7) |
|
|
| Molecular
subtype |
|
|
| 9.598 | 0.022 |
| Luminal
A | 60 (17.4) | 47 (19.9) | 13 (11.9) |
|
|
| Luminal
B | 204 (59.1) | 132 (55.9) | 72 (66.1) |
|
|
|
HER2-enriched | 17 (4.9) | 8 (3.4) | 9 (8.3) |
|
|
|
TNBC | 23 (6.7) | 19 (8.1) | 4 (3.7) |
|
|
|
Unknown | 41 (11.9) | 30 (12.7) | 11 (10.1) |
|
|
| ER status |
|
|
| 0.005 | 0.945 |
|
Positive | 288 (82.8) | 197 (82.8) | 91 (82.7) |
|
|
|
Negative | 53 (15.2) | 36 (15.1) | 17 (15.5) |
|
|
|
Unknown | 7 (2.0) | 5 (2.1) | 2 (1.8) |
|
|
| PR status |
|
|
| 0.753 | 0.385 |
|
Positive | 232 (66.7) | 162 (68.1) | 70 (63.6) |
|
|
|
Negative | 106 (30.5) | 69 (29.0) | 37 (33.6) |
|
|
|
Unknown | 10 (2.9) | 7 (2.9) | 3 (2.7) |
|
|
| HER2 status |
|
|
| 15.173 | <0.001 |
|
Positive | 67 (19.3) | 34 (14.3) | 33 (30.0) |
|
|
|
Negative | 211 (60.6) | 160 (67.2) | 51 (46.4) |
|
|
|
Unknown | 70 (20.1) | 44 (18.5) | 26 (23.6) |
|
|
| Ki-67 status |
|
|
| 1.074 | 0.300 |
|
0–14% | 102 (29.3) | 73 (30.7) | 29 (26.4) |
|
|
|
>14 | 228 (65.5) | 150 (63.0) | 78 (70.9) |
|
|
|
Unknown | 18 (5.2) | 15 (6.3) | 3 (2.7) |
|
|
| Lymph node
statusc |
|
|
| 6.364 | 0.095 |
| 0 | 207 (59.5) | 140 (58.8) | 67 (60.9) |
|
|
|
1–3 | 61 (17.5) | 48 (20.2) | 13 (11.8) |
|
|
|
4–9 | 24 (6.9) | 13 (5.5) | 11 (10.0) |
|
|
|
>9 | 31 (8.9) | 24 (10.1) | 7 (6.4) |
|
|
|
Unknown | 25 (7.2) | 13 (5.5) | 12 (10.9) |
|
|
Downregulation of p-IGF-1R expression
during NAC
To examine whether the level of p-IGF-1R changes
during NAC, p-IGF-1R was measured in breast cancer patients prior
to and after NAC (Table III). The
level of p-IGF-1R was significantly different between the biopsy
(pre-NAC) and resection (post-NAC) samples (P=0.005), and p-IGF-1R
displayed a trend towards a downregulation after NAC.
 | Table III.Changes in p-IGF-1R levels in
patients receiving NAC. |
Table III.
Changes in p-IGF-1R levels in
patients receiving NAC.
|
|
| p-IGF-1R level |
|
|
|---|
|
|
|
|
|
|
|---|
| Group | N (%) | 0 | + | ++ | +++ | W | P-value |
|---|
| Biopsy
(pre-NAC) | 40 (100) | 22 (55.0) | 12 (30.0) | 4 (10.0) | 2 (5.0) | 1,386.000 | 0.005 |
| Resection
(post-NAC) | 40 (100) | 34 (85.0) | 3 (7.5) | 3 (7.5) | 0 (0) |
|
|
Factors associated with p-IGF-1R
expression
To examine the association between multiple factors
and p-IGF-1R expression in breast cancer, a univariate analysis was
performed. The results are presented in Table IV. An analysis of the full dataset
indicated that NAC [odds ratio (OR), 1.742; 95% confidence interval
(CI), 1.038–2.923; P=0.036] and HER2 status (OR, 3.045; 95% CI,
1.716–5.402; P<0.001) were significantly associated with the
p-IGF-1R levels in breast cancer tissues. Compared to patients with
luminal A tumors, patients with the molecular subtype luminal B
(OR, 4.067; 95% CI, 1.001–3.885; P=0.050) and HER2-enriched subtype
(OR, 4.067; 95% CI, 1.310–12.632; P=0.015) had a higher probability
of positivity for p-IGF-1R on immunohistochemistry. According to
the multivariate logistic regression (Table V), NAC was significantly associated
with p-IGF-1R (OR, 2.326; 95% CI, 1.018–5.318; P=0.045). However,
the HER2 status was not independently associated with p-IGF-1R (OR,
2.093; 95% CI, 0.982–4.462; P=0.056).
 | Table IV.Association of clinicopathological
characteristics of breast cancer patients with p-IGF-1R positivity
determined by univariate logistic regression. |
Table IV.
Association of clinicopathological
characteristics of breast cancer patients with p-IGF-1R positivity
determined by univariate logistic regression.
|
| Overall
survival |
|---|
|
|
|
|---|
| Variables | OR | 95%CI | P-value |
|---|
| NAC (yes vs.
no) | 1.742 | 1.038–2.923 | 0.036 |
| pT stage (pT2 and
pT3 vs. pT1) | 1.156 | 0.704–1.897 | 0.567 |
|
pT1 | 1.000 |
|
|
|
pT2 | 1.525 | 0.876–2.652 | 0.135 |
|
pT3 | 0.000 | 0.000 | 0.999 |
| pTNM stage (III/IV
vs. I/II) | 0.988 | 0.712–1.372 | 0.944 |
| Molecular subtype
(Luminal B, HER2-enriched and TNBC vs. Luminal A) | 1.085 | 0.788–1.492 | 0.618 |
| Luminal
A | 1.000 |
| 0.027 |
| Luminal
B | 1.972 | 1.001–3.885 | 0.050 |
|
HER2-enriched | 4.067 | 1.310–12.632 | 0.015 |
|
TNBC | 0.761 | 0.220–2.632 | 0.666 |
| ER (positive vs.
negative) | 0.978 | 0.522–1.833 | 0.945 |
| PR (positive vs.
negative) | 0.806 | 0.495–1.313 | 0.386 |
| HER2 (positive vs.
negative) | 3.045 | 1.716–5.402 | <0.001 |
| Ki-67 (≥14 vs.
<14%) | 1.309 | 0.786–2.179 | 0.301 |
 | Table V.Association of the NAC and HER2
status of breast cancer patients with p-IGF-1R positivity
determined by multivariate logistic regression. |
Table V.
Association of the NAC and HER2
status of breast cancer patients with p-IGF-1R positivity
determined by multivariate logistic regression.
|
| Overall
survival |
|---|
|
|
|
|---|
| Variables | OR | 95%CI | P-value |
|---|
| NAC (yes vs.
no) | 2.326 | 1.018–5.318 | 0.045 |
| HER2 (yes vs.
no) | 2.093 | 0.982–4.462 | 0.056 |
Survival analysis
The follow-up survival data were available for 276
patients. A total of 259 patients survived at the last time-point
of follow-up and the mean duration of follow-up was 3 years,
ranging from 1–6 years. The mean OS time was 39.8±11.6 months
(range, 10.1–74.7 months). The mean OS time for patients with
p-IGF-1R-negative and -positive tumors was 42.0±10.8 months and
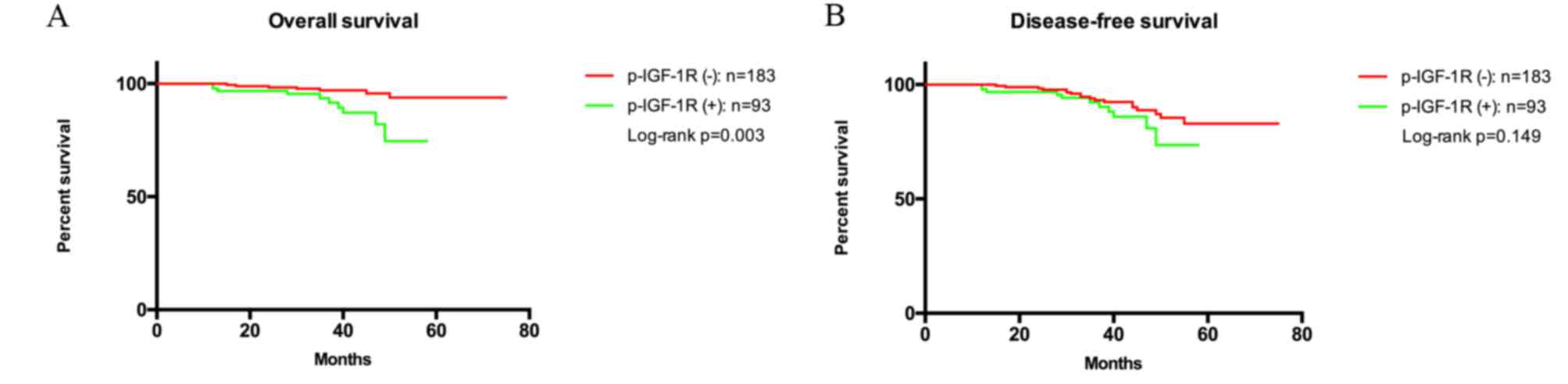
35.6±12.0 months, respectively. The p-IGF-1R levels at baseline
were significantly associated with OS (log-rank test, P=0.003;
Fig. 2A), but not associated with
DFS (log-rank test, P=0.149; Fig.
2B). Further Kaplan-Meier analyses were performed, where
patients were stratified according to the expression of routinely
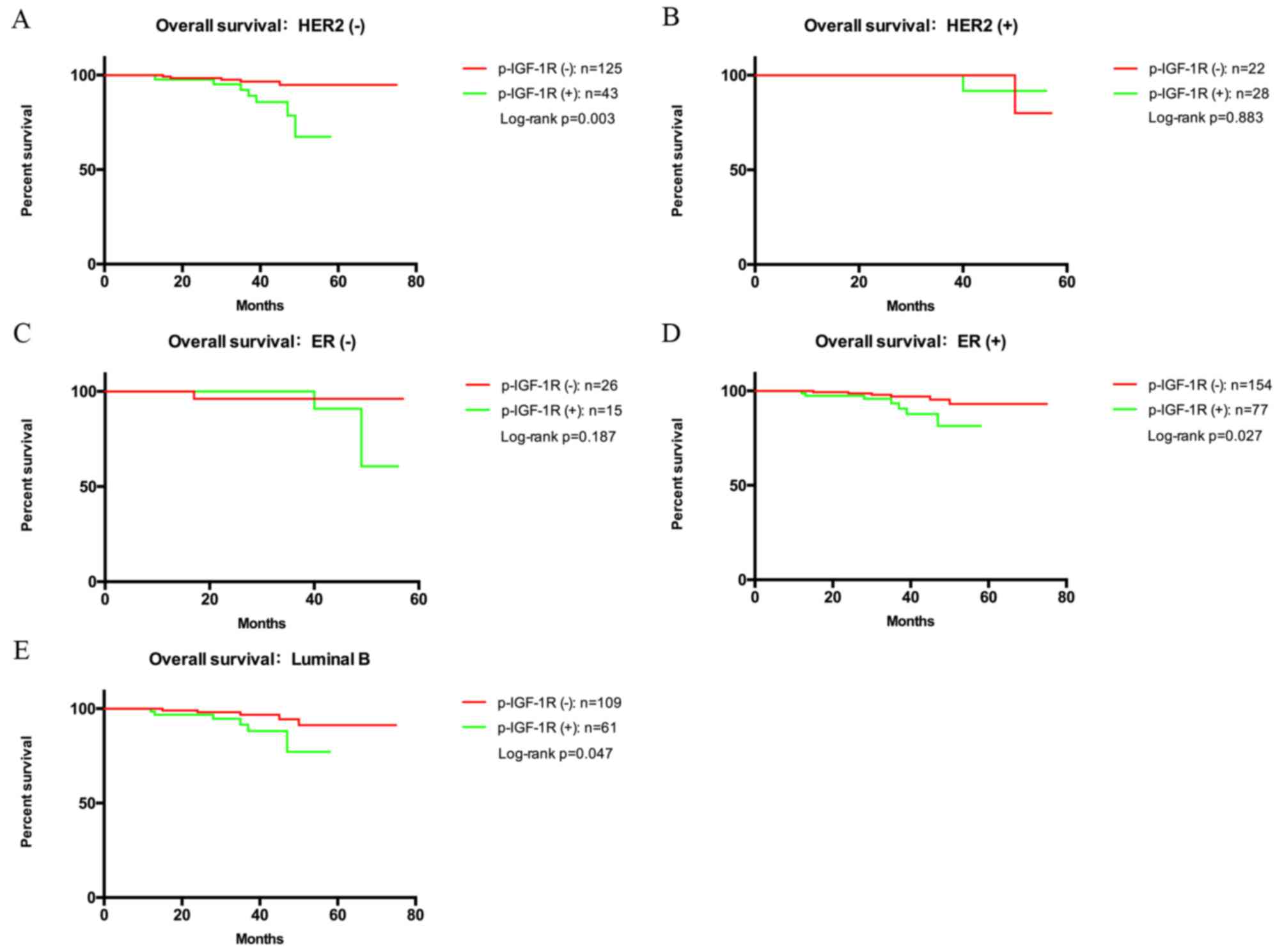
used biomarkers (Fig. 3). In the
subgroups with HER2-negative status (P=0.003), ER-positive status
(P=0.027) and luminal B (P=0.047), patients with p-IGF-1R-positive
tumors had a shorter OS (Fig. 3A, D and
E, respectively). However, the p-IGF-1R status had no
significant impact on OS in HER2-positive (P=0.883) and ER-negative
patients (P=0.187; Fig. 3B and C,
respectively). Cox regression analysis indicated that p-IGF-1R was
an independent prognostic factor with OS as the endpoint (OROR,
3.640; 95% CI, 1.246–10.630; P=0.018; Table VI).
 | Table VI.Association between p-IGF-1R status
and overall survival, as determined by Cox regression analysis. |
Table VI.
Association between p-IGF-1R status
and overall survival, as determined by Cox regression analysis.
|
| Overall
survival |
|---|
|
|
|
|---|
| Variable | OR | 95%CI | P-value |
|---|
| IGF-1R (positive
vs. negative) | 3.640 | 1.246–10.630 | 0.018 |
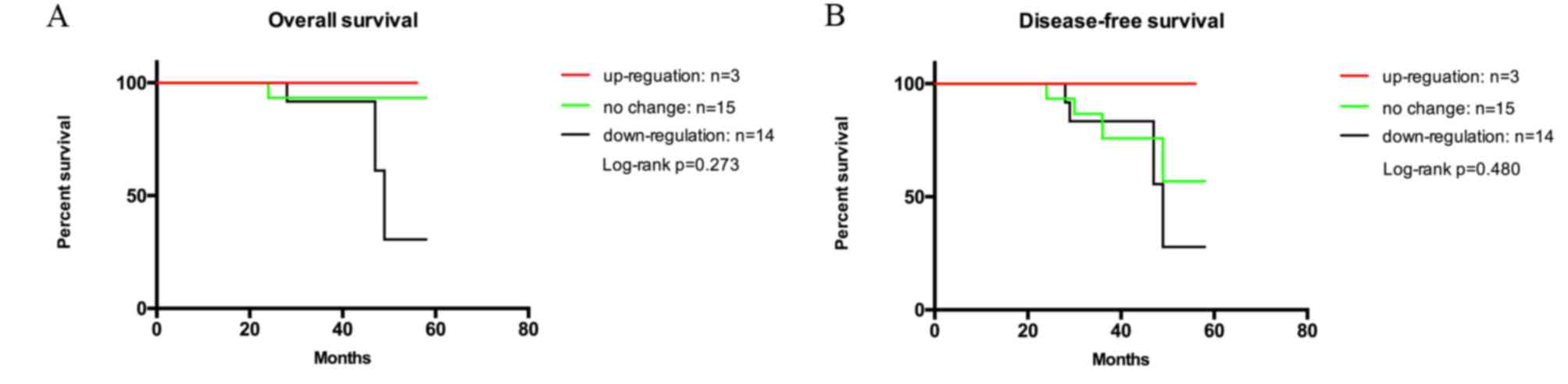
Considering the alteration of p-IGF-1R during NAC
mentioned above, the present study further assessed the association
of alterations of p-IGF-1R levels with OS and DFS (Fig. 4A and B). Among the 40 patients who
received NAC with biopsy and resection data, the follow-up results
of only 32 patients were available. The mean follow-up time of the
patients was 38.4±11.9 months (range, 10.1–58.0 months). The mean
OS time for patients in which p-IGF-1R was upregulated, not
affected and downregulated after NAC was 40.8±16.1, 40.2±10.9 and
35.9±12.5 months, respectively. This may be due to a small sample
size, as OS and DFS were almost the same. Kaplan-Meier analysis did
not indicate a statistically significant association between the
change of p-IGF-1R after NAC and OS or DFS (log-rank test, P=0.273
or 0.480, respectively).
Discussion
In the present study, factors reported by others to
be associated with the baseline IGF-1R expression were assessed
(20,21). The previously reported induction of
p-IGF-1R during NAC and its potential association with OS was also
analyzed (25).
IGF-1R is a heterotetrameric transmembrane receptor
tyrosine kinase, which is widely expressed in normal human tissues
and is frequently upregulated in breast cancer (15). It is well established that IGF-1R is
involved in the regulation of numerous biological processes of
cells, including differentiation, proliferation, transformation and
survival (26), particularly the
progression and development of various cancer types (26). The potential mechanism may be that
after ligand binding and subsequent phosphorylation, IGF-1R
signaling subsequently activates the anti-apoptotic pathways of
phosphatidylinositol-3-kinase/AKT and proliferation-driving
pathways of Ras/Raf/the mitogen-activated protein kinases (MAPK)
extracellular signal-regulated kinases (27). Considering IGF-1R phosphorylation as
an essential step for the function of IGF-1R (18), the present study assessed p-IGF-1R in
breast cancer tissues.
In the present study, an association between
baseline p-IGF-1R expression and a wide range of factors was
identified. To date, several studies have investigated the
association of clinicopathological features with p-IGF-1R
expression in breast cancer (20,21). The
present study confirmed that baseline p-IGF-1R levels were
significantly associated with NAC, molecular subtypes and the HER2
status according to the univariate analysis, which was in
accordance with what was expected. However, the association between
p-IGF-1R and HER2 did not reach statistical difference (P=0.056)
based on multivariate logistic regression. It may be speculated
that this is due to patients with HER2 (2+) status, which were not
further identified by fluorescence in situ hybridization.
Furthermore, an insufficient sample size for defining the HER2
status may have led to different statistical results between
univariate and multivariate analysis. Even though the multivariate
logistic regression result for the HER2 status indicated no
statistical significance, integrated univariate analysis indicated
that HER2-positive tumors were more likely to express p-IGF-1R than
HER2-negative tumors. Although the underlying mechanisms remain
elusive, there are three possible explanations for this phenomenon.
First, HER2 may promote aromatase activity by phosphorylating AKT
and MAPK (28). Increased aromatase
activity leads to elevated estrogen levels, which in turn
contributes to the upregulation of IGF-1R (29). Furthermore, elevated IGF-1R levels
have been reported to be correlated with trastuzumab resistance in
breast cancer (14). Previous
studies also demonstrated that the levels of IGF-1R inhibitors,
miR-630 and miR-375, are downregulated in trastuzumab-resistant
breast cancer, leading to an upregulation of IGF-1R expression
(30,31). Of note, >60% of HER2-enriched
breast cancer patients with metastases have been reported to
present with trastuzumab resistance (32), indicating that HER2-overexpressing
tumors are more likely accompanied by IGF-1R positivity. Finally, a
heterodimer formed by IGF-1R and HER2 facilitates the process of
IGF-1R phosphorylating HER2 and promoting the invasion of
tratuzumab-resistant cells (33).
Therefore, treatments co-targeting IGF-IR and HER2 have higher
antitumor activity than those only targeting either receptor alone
(34).
Breast cancer is classified into four major subtypes
based on its ER, PR, HER2 and Ki-67 status: Luminal A, luminal B,
HER2-enriched and TNBC. One study has demonstrated that high IGF-1R
expression levels were more frequently seen in luminal A (52%),
luminal B (57.5%) and luminal/HER2 (44.8%) patients, whereas the
HER2-enriched (90.3%) and BL (77.5%) tumors had lower IGF-1R
expression (21). In the present
study, it was indicated that the patterns of p-IGF-1R were notably
different among several of the above-mentioned subtypes, which
means that it may be reasonable to treat breast cancer in different
subtypes specifically. Among luminal A, luminal B and HER2-enriched
subgroups, the probability of p-IGF-1R positivity in luminal B
cases is almost twice as high as that in luminal A cases, while the
probability in HER2-enriched cases is >4 times of that in
luminal A cases. TNBC accounted for ~6.7% of all breast cancer
samples assessed in the present study, which is lower than the
previously reported rate (10–20%) (35). More samples are therefore required
for future investigation to clarify this discrepancy. Unlike other
subtypes, TNBC is a cluster of diseases, with heterogeneity in its
genetic locus, intrasubgroup, prognosis and sensitivity to therapy
(36), including basal-like 1 (BL1),
basal-like 2 (BL2), immunomodulatory (IM), mesenchymal (M),
mesenchymal stem-like (MSL), luminal androgen receptor (LAR) and
unsTable (UNS), with different characters. BL1 and BL2 are the
primary components (~80%) of TNBC, characterized by an enrichment
of genes, which regulate the cell cycle (36). Higher level of basal cytokeratin can
be found in BL1, BL2, UNS and M subtypes while LAR tends to express
a high level of luminal cytokeratin. In addition, Masuda et
al (37) highlighted that TNBC
have different pathological complete response rate (pCR) to
standard neoadjuvant chemotherapy. The complexity of
pathophenotypes may also be a cause for the undefined association
of p-IGF-1R expression with TNBC in the present study.
At present, the prognostic significance of IGF-1R is
controversial. One study reported that IGF-1R expression is not
associated with any clinical outcomes (38), while others suggested IGF-1R as a
favorable prognostic marker by elucidating that IGF-1R positivity
in tumor cells was correlated with DFS, breast-cancer specific
survival (BCCS) and OS (21). By
contrast, Law et al (39)
performed a study including 438 patients with early breast
carcinoma and revealed that a high level of p-IGF-1R was
significantly correlated with a shorter BCCS. Similarly, the
present study indicated that p-IGF-1R positivity predicts a shorter
OS, while there was no significance regarding its predictive value
for DFS. This suggests that anti-p-IGF-1R targeted therapies may
improve the outcome for patients with breast cancer.
In order to further explore the prognostic efficacy
of p-IGF-1R in different clinicopathological variables, subgroup
analyses were performed. In the subgroups of HER2-negative,
ER-positive and luminal B tumors, patients with a p-IGF-1R-positive
status had a shorter OS. As is known, unlike that of luminal types,
which may be treated with endocrine therapy, the prognosis of TNBC
patients is almost always poor due to the lack of molecular
treatment targets (21). Patients
with TNBC have a high probability of distant metastasis instead of
locoregional relapse compared with other types (40). Based on the present results, p-IGF-1R
positivity may be regarded as an unfavorable prognostic indicator
in patients with HER2-negative tumors, and p-IGF-1R may be an
attractive therapeutic target, particularly for TNBC.
To date, only a few clinical studies have indicated
that chemotherapy may induce alterations in IGF-1R. Heskamp et
al (25) demonstrated that
upregulation of IGF-1R expression during neoadjuvant therapy
predicted poor outcomes in breast cancer patients. These results
imply a possible role for IGF-1R in therapy resistance. The
association between IGF-1R expression and therapy resistance has
been previously observed in several types of cancer. In the present
study, changes of p-IGF-1R expression were induced by NAC. However,
this alteration was neither associated with OS nor with DFS, which
may be due to the small sample size.
In conclusion, the presence of p-IGF-1R determined
by immunohistochemistry was indicated to be associated with several
clinical and pathological variables, and is paralleled with certain
biomarkers linked with poor outcome, including HER2. Based on the
Cox regression result, p-IGF-1R is an independent prognostic
factor, which indicates that p-IGF-1R may be a promising indicator
for predicting clinical outcomes and an attractive target for
improving the efficiency of anti-tumor therapy, particularly for
patients with HER2-negative, ER-positive and luminal B tumors.
Further studies with larger sample sizes of patients receiving NAC
will elucidate whether the changes of p-IGF-1R expression caused by
NAC may be associated with survival.
Acknowledgements
The authors would like to thank Dr Alejandro
Fernandez-Escobar from the Faculty of Medicine of CES University
(Medellin, Colombia). for his contribution in revising the
manuscript, all patients involved in the present study for their
participation and the Department of Pathology (The Second Hospital
of Shandong University, Shandong, China) for their collaboration
and cooperation.
Funding
The authors are grateful for the support from the
Key Project of the Natural Science Foundation of Shandong Province,
China (grant no. ZR2014HZ004), the Key Research and Development
Program of Shandong Province (grant no. 2016GSF201130) and the
Youth Fund of the Second Hospital of Shandong University (grant no.
Y2015010026).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZY and LiL conceived and designed the experiments;
SL, LYL, ZM, MF, CY, WZ, YW, LuL, FW, LY, FZ, YX, SH, QF, QZ and DG
performed the experiments; SL, LYL and ZM analyzed the data; SL and
LYL wrote and revised the manuscript; ZY supplied suggestions on
study design and prepared the manuscript. The final version of the
manuscript has been read and approved by all authors, and each
author believes that the manuscript represents honest work.
Ethical approval and consent to
participate
All procedures were in accordance with the
guidelines set by the Declaration of Helsinki and approved by the
Clinical Research Ethics Committee of the Second Hospital of
Shandong University (Jinan, China). All records were collected on
the basis of patient written informed consent for the use of
clinical data, their enrollment in the present study and for the
analysis of their tissues
Patient consent for publication
Patients consent for publication of the histology
images.
Competing interests
The authors declare that there are no competing
interests regarding the publication of this article.
References
|
1
|
Bray F, Ren JS, Masuyer E and Ferlay J:
Global estimates of cancer prevalence for 27 sites in the adult
population in 2008. Int J Cancer. 132:1133–1145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garcia-Closas M, Hall P, Nevanlinna H,
Pooley K, Morrison J, Richesson DA, Bojesen SE, Nordestgaard BG,
Axelsson CK, Arias JI, et al: Heterogeneity of breast cancer
associations with five susceptibility loci by clinical and
pathological characteristics. PLoS Genet. 4:e10000542008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gianni L, Dafni U, Gelber RD, Azambuja E,
Muehlbauer S, Goldhirsch A, Untch M, Smith I, Baselga J, Jackisch
C, et al: Treatment with trastuzumab for 1 year after adjuvant
chemotherapy in patients with HER2-positive early breast cancer: A
4-year follow-up of a randomised controlled trial. Lancet Oncol.
12:236–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu DG, Selth LA, Tarulli GA, Meech R,
Wijayakumara D, Chanawong A, Russell R, Caldas C, Robinson JL,
Carroll JS, et al: Androgen and estrogen receptors in breast cancer
coregulate human UDP-glucuronosyltransferases 2B15 and 2B17. Cancer
Res. 76:5881–5893. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weigel MT and Dowsett M: Current and
emerging biomarkers in breast cancer: Prognosis and prediction.
Endocr Relat Cancer. 17:R245–R262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cancer Genome Atlas Network, .
Comprehensive molecular portraits of human breast tumours. Nature.
490:61–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zeng H, Zheng R, Zhang S, Zou X and Chen
W: Female breast cancer statistics of 2010 in China: Estimates
based on data from 145 population-based cancer registries. J Thorac
Dis. 6:466–470. 2014.PubMed/NCBI
|
|
8
|
Becker MA, Ibrahim YH, Cui X, Lee AV and
Yee D: The IGF pathway regulates ERα through a S6K1-dependent
mechanism in breast cancer cells. Mol Endocrinol. 25:516–528. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sarfstein R, Pasmanik-Chor M, Yeheskel A,
Edry L, Shomron N, Warman N, Wertheimer E, Maor S, Shochat L and
Werner H: Insulin-like growth factor-I receptor (IGF-IR)
translocates to nucleus and autoregulates IGF-IR gene expression in
breast cancer cells. J Biol Chem. 287:2766–2776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Giovannucci E: Insulin-like growth
factor-I and binding protein-3 and risk of cancer. Horm Res. 51
Suppl 3:S34–S41. 1999.
|
|
11
|
Gong Y, Yao E, Shen R, Goel A, Arcila M,
Teruya-Feldstein J, Zakowski MF, Frankel S, Peifer M, Thomas RK, et
al: High expression levels of total IGF-1R and sensitivity of NSCLC
cells in vitro to an anti-IGF-1R antibody (R1507). PLoS One.
4:e72732009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kucab JE and Dunn SE: Role of IGF-1R in
mediating breast cancer invasion and metastasis. Breast Dis.
17:41–47. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Valsecchi ME, McDonald M, Brody JR, Hyslop
T, Freydin B, Yeo CJ, Solomides C, Peiper SC and Witkiewicz AK:
Epidermal growth factor receptor and insulinlike growth factor 1
receptor expression predict poor survival in pancreatic ductal
adenocarcinoma. Cancer. 118:3484–3493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Farabaugh SM, Boone DN and Lee AV: Role of
IGF1R in breast cancer subtypes, stemness, and lineage
differentiation. Front Endocrinol (Lausanne). 6:592015.PubMed/NCBI
|
|
15
|
Christopoulos PF, Msaouel P and
Koutsilieris M: The role of the insulin-like growth factor-1 system
in breast cancer. Mol Cancer. 14:432015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Papa V, Gliozzo B, Clark GM, McGuire WL,
Moore D, Fujita-Yamaguchi Y, Vigneri R, Goldfine ID and Pezzino V:
Insulin-like growth factor-I receptors are overexpressed and
predict a low risk in human breast cancer. Cancer Res.
53:3736–3740. 1993.PubMed/NCBI
|
|
17
|
Bahhnassy A, Mohanad M, Shaarawy S, Ismail
MF, El-Bastawisy A, Ashmawy AM and Zekri AR: Transforming growth
factor-β, insulin-like growth factor I/insulin-like growth factor I
receptor and vascular endothelial growth factor-A: Prognostic and
predictive markers in triple-negative and non-triple-negative
breast cancer. Mol Med Rep. 12:851–864. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kelly GM, Buckley DA, Kiely PA, Adams DR
and O'Connor R: Serine phosphorylation of the insulin-like growth
factor I (IGF-1) receptor C-terminal tail restrains kinase activity
and cell growth. J Biol Chem. 287:28180–28194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gradishar WJ, Anderson BO, Balassanian R,
Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A,
Giordano SH, et al: Breast cancer version 2.2015. J Natl Compr Canc
Netw. 13:448–475. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hartog H, Horlings HM, van der Vegt B,
Kreike B, Ajouaou A, van de Vijver MJ, Marike Boezen H, de Bock GH,
van der Graaf WT and Wesseling J: Divergent effects of insulin-like
growth factor-1 receptor expression on prognosis of estrogen
receptor positive versus triple negative invasive ductal breast
carcinoma. Breast Cancer Res Treat. 129:725–736. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yerushalmi R, Gelmon KA, Leung S, Gao D,
Cheang M, Pollak M, Turashvili G, Gilks BC and Kennecke H:
Insulin-like growth factor receptor (IGF-1R) in breast cancer
subtypes. Breast Cancer Res Treat. 132:131–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
23
|
Friedrichs K, Gluba S, Eidtmann H and
Jonat W: Overexpression of p53 and prognosis in breast cancer.
Cancer. 72:3641–3647. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao S, Chen SS, Gu Y, Jiang EZ and Yu ZH:
Expression and clinical significance of sushi domain-containing
protein 3 (SUSD3) and insulin-like growth factor-I receptor
(IGF-IR) in breast cancer. Asian Pac J Cancer Prev. 16:8633–8636.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heskamp S, Boerman OC, Molkenboer-Kuenen
JD, Wauters CA, Strobbe LJ, Mandigers CM, Bult P, Oyen WJ, van der
Graaf WT and van Laarhoven HW: Upregulation of IGF-1R expression
during neoadjuvant therapy predicts poor outcome in breast cancer
patients. PLoS One. 10:e01177452015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mauro L, Naimo GD, Ricchio E, Panno ML and
Andò S: Cross-Talk between adiponectin and IGF-IR in breast cancer.
Front Oncol. 5:1572015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Surmacz E: Growth factor receptors as
therapeutic targets: Strategies to inhibit the insulin-like growth
factor I receptor. Oncogene. 22:6589–6597. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Su B, Wong C, Hong Y and Chen S: Growth
factor signaling enhances aromatase activity of breast cancer cells
via post-transcriptional mechanisms. J Steroid Biochem Mol Biol.
123:101–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Casa AJ, Potter AS, Malik S, Lazard Z,
Kuiatse I, Kim HT, Tsimelzon A, Creighton CJ, Hilsenbeck SG, Brown
PH, et al: Estrogen and insulin-like growth factor-I (IGF-I)
independently down-regulate critical repressors of breast cancer
growth. Breast Cancer Res Treat. 132:61–73. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Corcoran C, Rani S, Breslin S, Gogarty M,
Ghobrial IM, Crown J and O'Driscoll L: miR-630 targets IGF1R to
regulate response to HER-targeting drugs and overall cancer cell
progression in HER2 over-expressing breast cancer. Mol Cancer.
13:712014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ye XM, Zhu HY, Bai WD, Wang T, Wang L,
Chen Y, Yang AG and Jia LT: Epigenetic silencing of miR-375 induces
trastuzumab resistance in HER2-positive breast cancer by targeting
IGF1R. BMC Cancer. 14:1342014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vogel CL, Cobleigh MA, Tripathy D, Gutheil
JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF,
Burchmore M, et al: Efficacy and safety of trastuzumab as a single
agent in first-line treatment of HER2-overexpressing metastatic
breast cancer. J Clin Oncol. 20:719–726. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nahta R, Yuan LX, Zhang B, Kobayashi R and
Esteva FJ: Insulin-like growth factor-I receptor/human epidermal
growth factor receptor 2 heterodimerization contributes to
trastuzumab resistance of breast cancer cells. Cancer Res.
65:11118–11128. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen C, Zhang Y, Zhang Y, Li J, Tsao SW
and Zhang MY: Superior antitumor activity of a novel bispecific
antibody cotargeting human epidermal growth factor receptor 2 and
type I insulin-like growth factor receptor. Mol Cancer Ther.
13:90–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Papa A, Caruso D, Tomao S, Rossi L,
Zaccarelli E and Tomao F: Triple-negative breast cancer:
Investigating potential molecular therapeutic target. Expert Opin
Ther Targets. 19:55–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mancini P, Angeloni A, Risi E, Orsi E and
Mezi S: Standard of care and promising new agents for triple
negative metastatic breast cancer. Cancers (Basel). 6:2187–2223.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Masuda H, Baggerly KA, Wang Y, Zhang Y,
Gonzalez-Angulo AM, Meric-Bernstam F, Valero V, Lehmann BD,
Pietenpol JA, Hortobagyi GN, et al: Differential response to
neoadjuvant chemotherapy among 7 triple-negative breast cancer
molecular subtypes. Clin Cancer Res. 19:5533–5540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shimizu C, Hasegawa T, Tani Y, Takahashi
F, Takeuchi M, Watanabe T, Ando M, Katsumata N and Fujiwara Y:
Expression of insulin-like growth factor 1 receptor in primary
breast cancer: Immunohistochemical analysis. Hum Pathol.
35:1537–1542. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Law JH, Habibi G, Hu K, Masoudi H, Wang
MY, Stratford AL, Park E, Gee JM, Finlay P, Jones HE, et al:
Phosphorylated insulin-like growth factor-i/insulin receptor is
present in all breast cancer subtypes and is related to poor
survival. Cancer Res. 68:10238–10246. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Haffty BG, Yang Q, Reiss M, Kearney T,
Higgins SA, Weidhaas J, Harris L, Hait W and Toppmeyer D:
Locoregional relapse and distant metastasis in conservatively
managed triple negative early-stage breast cancer. J Clin Oncol.
24:5652–5657. 2006. View Article : Google Scholar : PubMed/NCBI
|