Introduction
Intrapancreatic splenic tissue, including splenosis
and intrapancreatic accessory spleen (IPAS), often appears in the
pancreatic tail (1,2). Splenosis is usually caused by the
autotransplantation of splenic tissue, which frequently occurs
following splenectomy or spleen trauma; accessory spleens are the
congenital foci of healthy splenic tissue that have separated from
the main body of the spleen (3,4).
However, intrapancreatic splenic tissue is challenging to identify
using medical imaging (5).
Intrapancreatic splenic tissue is often misdiagnosed as various
pancreatic tumors, including islet cell tumor, solid
pseudopapillarytumor, hypervascular metastasis and ductal
adenocarcinoma (5). Accurate
preoperative diagnosis is of great importance to avoid unnecessary
surgery or biopsy.
Several studies have provided certain analyses on
the imaging features of intrapancreatic splenic tissue and their
correlation with the pathological findings (6–16). The
CT scans in these studies demonstrated a well-circumscribed,
enhancing mass in the tail of the pancreas that revealed similar
enhancement patterns as the spleen. The specimens contained
well-demarcated dark-red nodules surrounded by pancreatic tissue.
The nodular lesions were elastic and soft, and composed of lymphoid
follicles and splenic pulp. Although IPASs have been reported, the
majority of the studies were case reports, and lacked comprehensive
conclusions about imaging features. To the best of our knowledge,
the performance of diffusion-weighted magnetic resonance imaging
(DWI) in the detection of IPAS has not yet been reported. In the
present study, the computed tomography (CT) and magnetic resonance
imaging (MRI) examination results of 9 cases of intrapancreatic
spleen were investigated, with a particular focus on the features
of DWI.
Materials and methods
Patients
The present retrospective review of patient data was
approved by the Ethics Committee of Ningbo University Hospital
(Ningbo, China). From July 2010 to July 2015, the clinical and
pathological records, as well as the imaging examinations of
patients at the Affiliated Hospital of the Medical School of Ningbo
University (Ningbo, China) and the Changhai Hospital of the Second
Military Medical University (Shanghai, China) were reviewed. In
total, 9 patients were diagnosed with intrapancreatic splenic
tissue, including 8 cases of IPAS and 1 case of
intrapancreaticsplenosis. The types of clinicoradiological
examinations conducted for each patient are presented in Table I.
 | Table I.Clinical and radiological data. |
Table I.
Clinical and radiological data.
| Case | Sex | Age (years) | Symptoms and/or
associated diseases | CA199a (U/ml) | Serum CT
sequences | MRI sequences | Confirmation
methods and results |
|---|
| 1 | M | 56 | None | 5.3 | Plain and
multiphase enhancement | T1WI, T2WI, DWI,
multiphase enhancement | Surgical pathology,
IPAS |
| 2 | M | 44 | Upper abdominal
pain and melena | 16.4 | Plain and
multiphase enhancement | T1WI, T2WI,
multiphase enhancement | Surgical pathology,
IPAS |
| 3 | M | 57 | Cirrhosis,
splenomegaly and hepatocellular carcinoma | 2.7 | Plain and
multiphase enhancement | T1WI, T2WI, DWI,
multiphase enhancement | Followed up 17
months, IPAS |
| 4 | M | 55 | Cirrhosis and
splenomegaly | 4.2 | Plain and
multiphase enhancement | T1WI, T2WI, DWI,
multiphase enhancement | Followed up 21
months, IPSP |
| 5 | M | 50 | Xiphoid pain and
gastroscopy confirmed duodenal ulcer | 12.9 | Plain and
multiphase enhancement | T1WI, T2WI, DWI,
multiphase enhancement | Surgical pathology,
IPAS |
| 6 | F | 65 | Cirrhosis and
splenomegaly | 13.7 | Plain and
multiphase enhancement | None | Followed up for 29
months, IPAS |
| 7 | F | 63 | None | 3.6 | Plain and
multiphase enhancement | T1WI, T2WI, DWI,
multiphase enhancement | Followed up 38
months, IPAS |
| 8 | F | 61 | None | 6.1 | Plain and
multiphase enhancement | T1WI, T2WI,
multiphase enhancement | Followed up 23
months, IPAS |
| 9 | F | 62 | None | NA | Plain and
multiphase enhancement | T1WI, T2WI, DWI,
multiphase enhancement | Followed up 20
months, IPAS |
The 8 patients diagnosed with IPAS were aged between
44 and 65 years (mean age, 56.4±6.9 years), included 4 males and 4
females, and had no trauma to the spleen or history of surgery. The
serum cancer antigen 19-9 data for the patients were all within the
normal range (0–35 µg/ml). The levels of blood glucose and
pancreatic enzymes were also normal. Among the 8 patients with
IPAS, 2 patients were suspected of having a pancreatic tail tumor
when examined by ultrasound imaging due to upper abdominal pain.
Another 2 patients had chronic liver dysfunction, one of whom was
suspected of having hepatocellular carcinoma as their serum
a-fetoprotein level was elevated (>1,000 µg/l) compared with the
normal range (0–20 µg/l). The remaining 4 patients did not have any
abdominal discomfort or complaints, and were diagnosed with
pancreatic tail tumors by abdominal sonography during health
examinations. Among the 8 patients with IPAS, 3 patients were
confirmed to have IPAS by the examination of a surgical specimen
obtained by distal pancreatectomy and splenectomy. These surgeries
were performed due to the possibility of neoplastic lesions,
although accessory spleen was also radiologically suspected. The
remaining 5 patients were suspected of having IPAS on the basis of
imaging characteristics and clinical follow-up for 17–38 months
(Table I).
The single case of intrapancreaticsplenosis was
observed in the pancreatic tail. The patient (male, 55 years old)
had a history of splenectomy due to cirrhosis with hypersplenism 23
months previously. No abnormalities of the pancreatic tail were
detected by preoperative imaging and intraoperative exploration. No
notable changes of the pancreatic tail lesions were identified on
the follow-up MRI results at 15 months (Table I).
Pathological analysis
In the 3 cases where PAS was confirmed by
pathological analysis, histological sections of the IPAS were
prepared by fixation with 10% formaldehyde at 25°C for 24 h and
embedding in paraffin, and then were stained with hematoxylin and
eosin. Then, the 3-µm-thick sections were treated with 1% periodic
acid solution for 10 min at room temperature, washed with PBS for 5
min, stained with Schiff solution for 10 min and eosin for 2 min at
25°C, and washed with PBS for 10 min. When the section turned red,
they were stained with hematoxylin at 60°C for 1 min. The diagnosis
was made by one experienced pathologist under a light microscope at
a magnification of ×100.
Imaging techniques
All 9 patients underwent CT scanning using a
Sensation Cardiac 64 CT scanner (Siemens Healthineers, Erlangen,
Germany; n=8) or a Brilliance 16 CT scanner (Philips Healthcare,
Amsterdam, The Netherlands; n=1). The sequences consisted of
pre-contrast, arterial, portal venous and delayed phases. Following
the injection of 100 ml nonionic contrast material (Ultravist 350;
Schering AG, Berlin, Germany) at a rate of 2.5–3 ml/sec,
triple-phase CT scans were acquired at 25, 60–70 and 120 sec from
the initiation of the intravenous injection of the contrast
material. The scanning parameters for the two CT scanners were
identical, as follows: Slice thickness, 5 mm without gap; tube
voltage, 120 kV; tube current, 200 mA; matrix 512×512.
A phased-array abdominal coil was applied to 8
patients with a 1.5-T Avanto MRI scanner (Siemens Healthineers) 3
days after the CT examination. For these patients, T1-weighted
imaging (T1WI), T2-weighted imaging (T2WI), triple-phase enhanced
scanning on the axial plane and true fast imaging with steady state
precession sequences in coronal images were conducted. DWI scanning
was also performed for 6 of the 8 patients.
The turbo spin-echo sequence was used for T2WI with
the following parameters: Repetition time (TR)/echo time (TE),
3,000–4,500 msec/70–90 msec; flip angle, 150°; matrix, 256×192; and
section thickness, 5 mm without intersection gap. The volumetric
interpolated breath-hold sequence was used for T1WI during the pre-
and post-contrast phases with the following parameters: TR/TE, 3.49
msec/1.02 msec; reconstruction thickness, 5 mm; and matrix,
256×128-192. The echo planar imaging sequence was used for DWI with
the following parameters: TR/TE, 2,100 msec/127 msec; section
thickness, 5 mm; matrix, 192×192; number of excitations, 4–6; and
b-values, 0, 100 and 600 sec/mm2. Triple-phase enhanced
MRI scans were acquired at 25, 60–70 and 120 sec from the
initiation of the intravenous injection of contrast material. A
power injector was used to administer gadolinium chelate
(Magnevist; Bayer AG, Leverkusen, Germany) at a dose of 0.1 mmol/kg
using an injection rate of 2.5–3 ml/sec.
Image analysis
All images were retrospectively evaluated in
consensus by two experienced abdominal radiologists who were
unaware the histopathological diagnosis. The following
morphological features of the tumors were recorded: Location, tumor
size (the maximum cross-sectional diameter of a tumor was defined
as the longest measured diameter in the axial scan images; and the
minimum as the shortest measured diameter), lesion contour, border
definition, density and signal intensity (SI) on CT and MRI images
compared with normal pancreas tissue, apparent diffusion
coefficient (ADC) values on DWI, and the pattern of contrast
enhancement. For the ADC values, the region of interest (ROI) was
circled. The ROI encompassed the maximum diameter of the pancreas
or lesion, and the area and shape of ROI were as consistent as
possible, avoiding the vascular and external structure of the
pancreas.
Statistical analysis
The ADC values of the IPAS, orthotopic spleen and
pancreatic tissue from the same patient were compared using one-way
analysis of variance followed by least significant difference
tests. SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA) was used
to conduct the analysis and P<0.05 was considered to indicate a
statistically significant difference.
Results
MRI and CT image features of IPAS
Lesion location, size and contour
Among the 8 patients with IPAS, only one lesion was
identified at the tail of the pancreas in each case. Lesions were
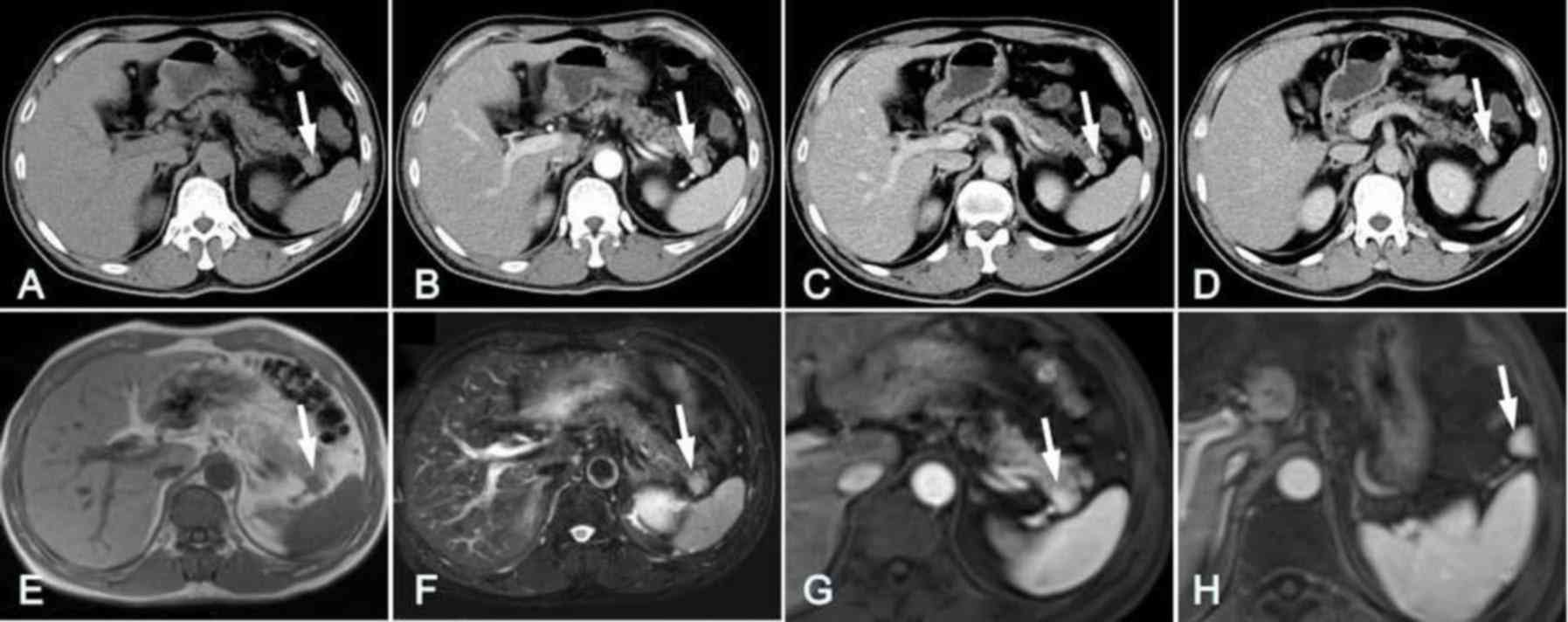
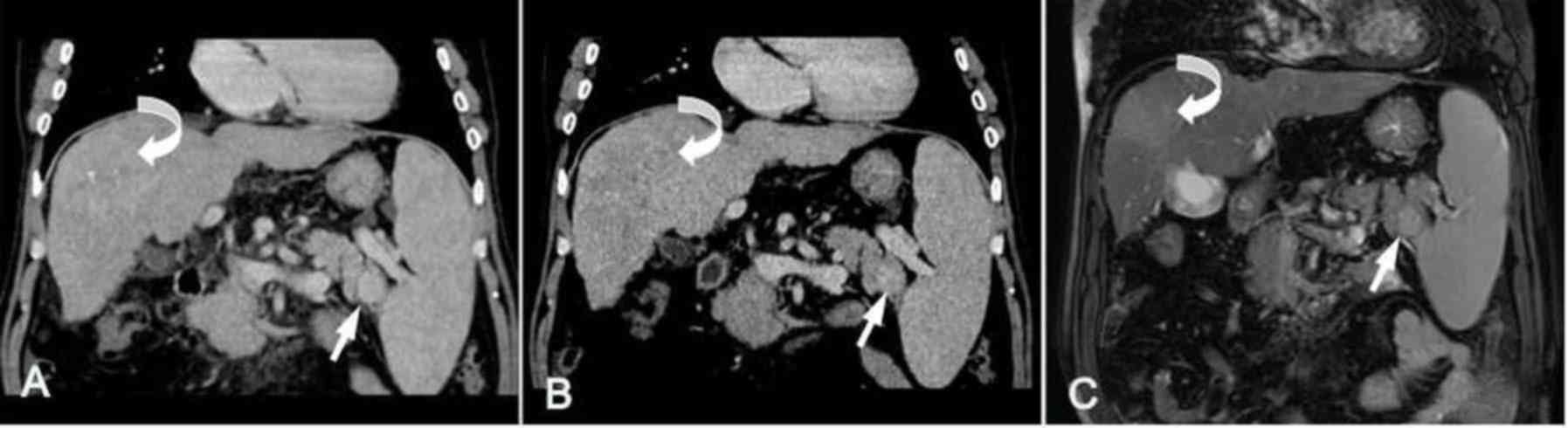
located in the rear of the pancreatic tail with a straight edge or
backward bulging in 5 cases (Fig.
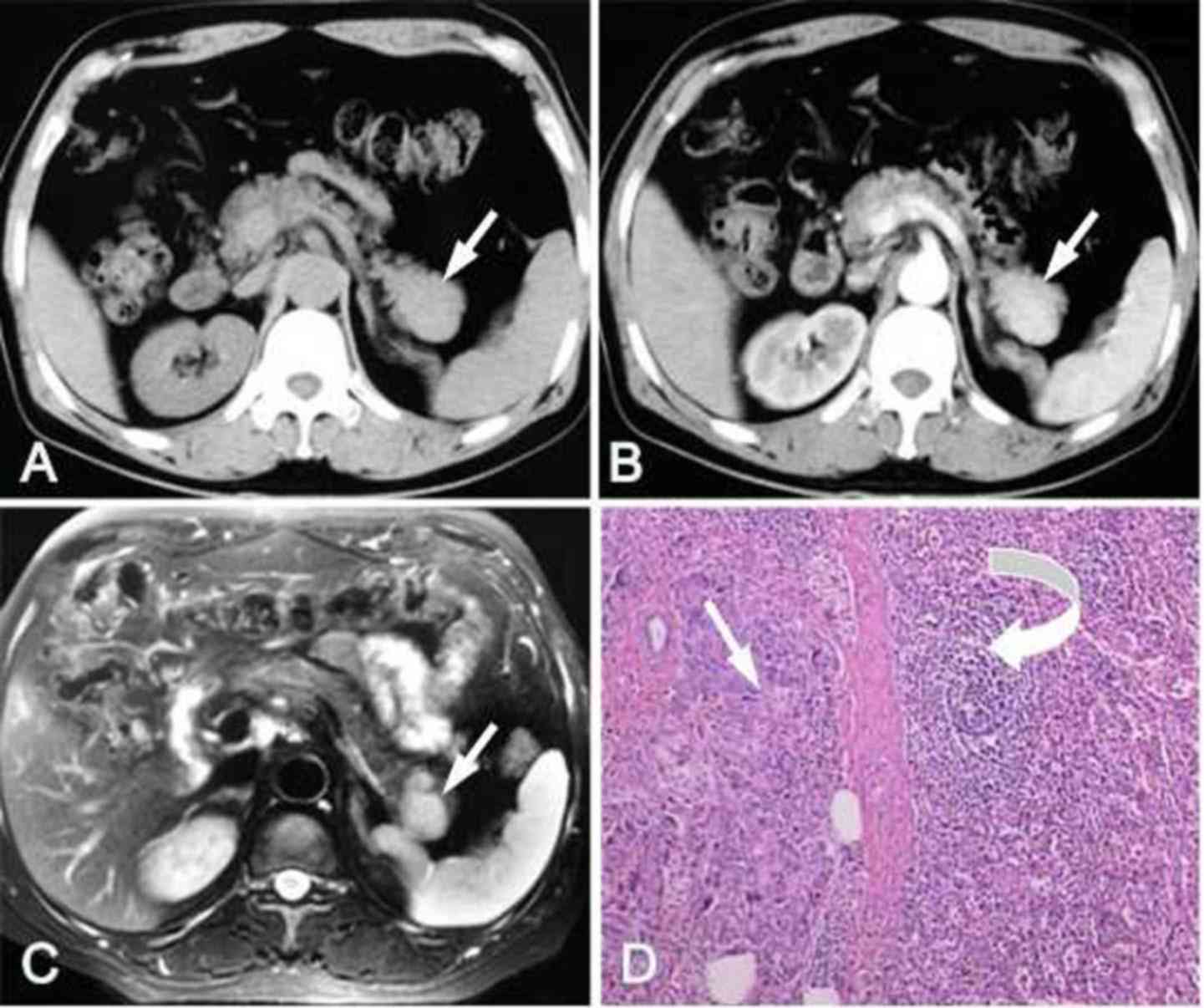
1), occupied the middle of the pancreatic tail with mild
forward and backward bulging in 2 cases (Fig. 2), and located in the forepart of the
pancreatic tail and projected forward prominently in 1 case
(Fig. 3). The shape of the lesions
was round (n=3; Fig. 1), oval (n=4;
Fig. 2) or triangular (n=1; Fig. 3). The largest diameter of the lesions
was 2.3±1.0 cm (range, 1.3–3.7 cm) and the shortest was 1.4±0.6 cm
(range, 0.9–2.4 cm).
Lesion density, signal and enhancement
features
Unenhanced CT images indicated that the lesions in
the 8 patients with IPAS were solid nodules with homogeneous
density, equal to that of the main spleen. In comparison with
pancreatic parenchyma, the lesions in 6 patients had slightly
higher attenuation (Figs. 1A and
2A) and the lesion in 1 patient had
slightly lower attenuation (Fig.
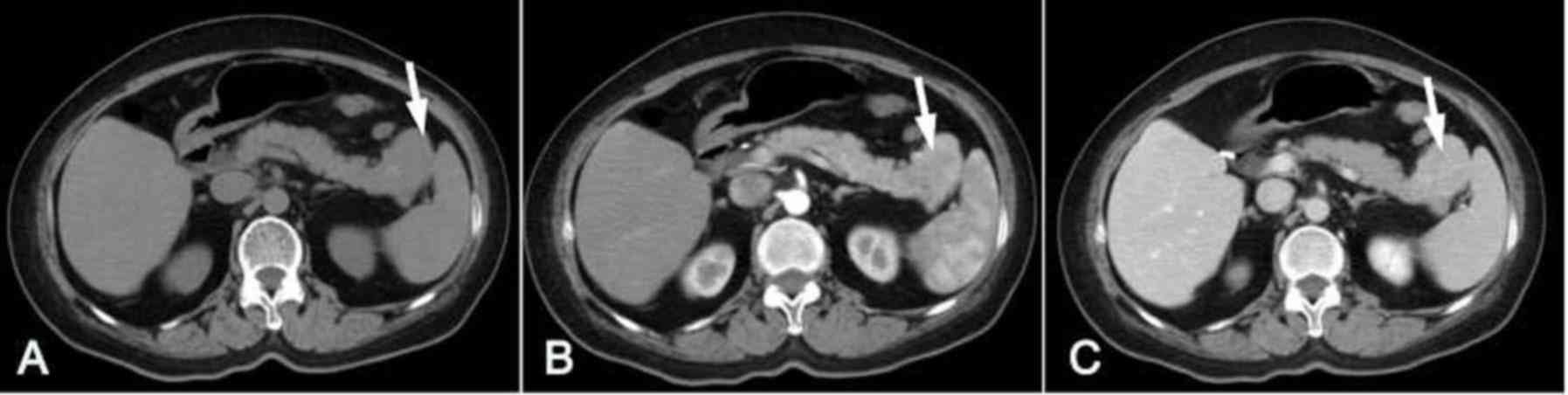
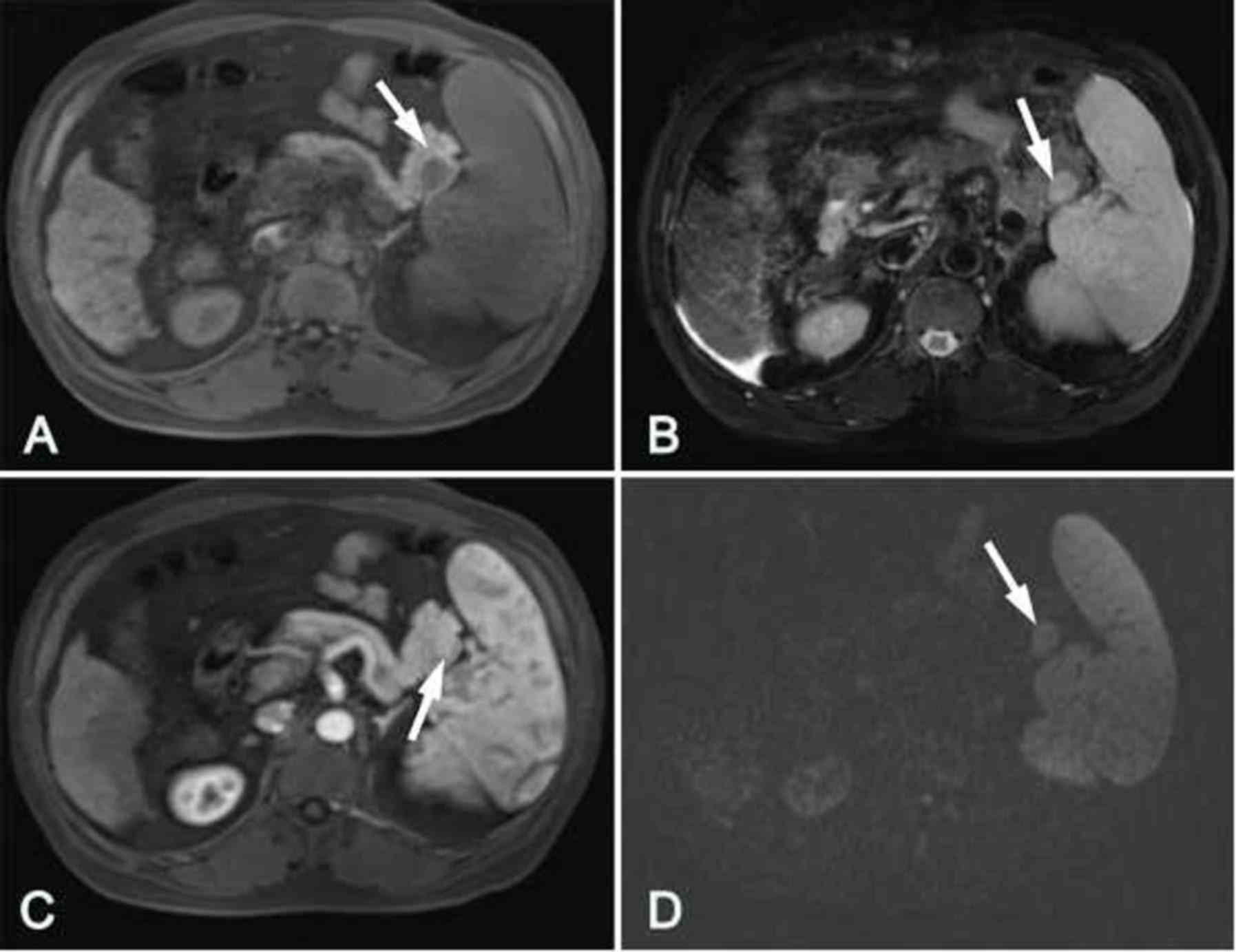
3A). However, in 1 patient (case 1; Fig. 4) the lesion had a similar attenuation
level to the main spleen and was difficult to detect (Fig. 4A).
In the arterial phase, heterogeneous enhancement of
the lesion was shown in 3 patients and ‘zebra-patterned’
enhancement was exhibited in 5 patients; in the portal venous
phase, heterogeneous enhancement was shown in all 8 patients; and
in the delayed phase, the degree of enhancement was reduced
(Figs. 1, 4 and 5). The
characteristic enhancement pattern of the lesions in these 8
patients was similar to that of the orthotopic spleen. In
comparison with the pancreatic parenchyma, the IPAS of 1 patient
exhibited a similar attenuation level (Fig. 4B and C) and the other 7 IPAS lesions
had a higher attenuation than the surrounding pancreas in the three
phases (Figs. 1–3 and 5).
In the MRI images, the IPAS for 7 patients exhibited
low intensity signals in T1WI with and without fat suppression, but
high intensity signals in T2WI compared with the surrounding
pancreatic parenchyma, and the SI of the IPAS was comparable to
that of the orthotopic spleen (Figs.
1 and 4–6).
In the arterial phase, heterogeneous enhancement of
the lesion was observed for 2 patients and ‘zebra-patterned’
enhancement was observed for 5 patients. In the portal venous
phase, heterogeneous enhancement was shown for 7 patients, whereas
in the delayed phase, the degree of enhancement was reduced. These
results are similar to those obtained by CT. In comparison with the
pancreas, the IPAS in 1 patient exhibited a similar degree of
enhancement to the pancreas in the 3 phases, whereas the SI of the
other 6 IPAS cases was higher than the pancreas in the 3 phases
(Figs. 1G and 6C).
On DWI examination, the IPAS lesions in 5 patients
presented high intensity signals when b-values of 0, 100 and 600
sec/mm2 were used. When the b-value was 600
sec/mm2, the lesions clearly had higher SI and lower ADC
values compared with the pancreas, and similar SI and ADC values to
the orthotopic spleen (Figs. 4E and
6D). In these patients, the ADC
value of the IPAS was 0.868±0.046 mm2/sec, and the ADC
value of the orthotopic spleen was 0.870±0.045 mm2/sec;
the difference between these two values was not significantly
different (t=0.620, P=0.587). The ADC value of the pancreatic
tissue was 1.404±0.081×10−3 mm2/sec, which
was significantly higher compared with that of the IPAS (P<0.01;
Table II).
 | Table II.DWI signal strength and ADC values
for the IPAS, pancreatic tissue and orthotopic spleen. |
Table II.
DWI signal strength and ADC values
for the IPAS, pancreatic tissue and orthotopic spleen.
| Variable | IPAS | Pancreatic
tissue | Orthotopic
spleen |
|---|
| DWI signal
strength | High | Low | High |
| ADC value
(×10−3 sec/mm2) | 0.868±0.046 |
1.404±0.081a | 0.870±0.045 |
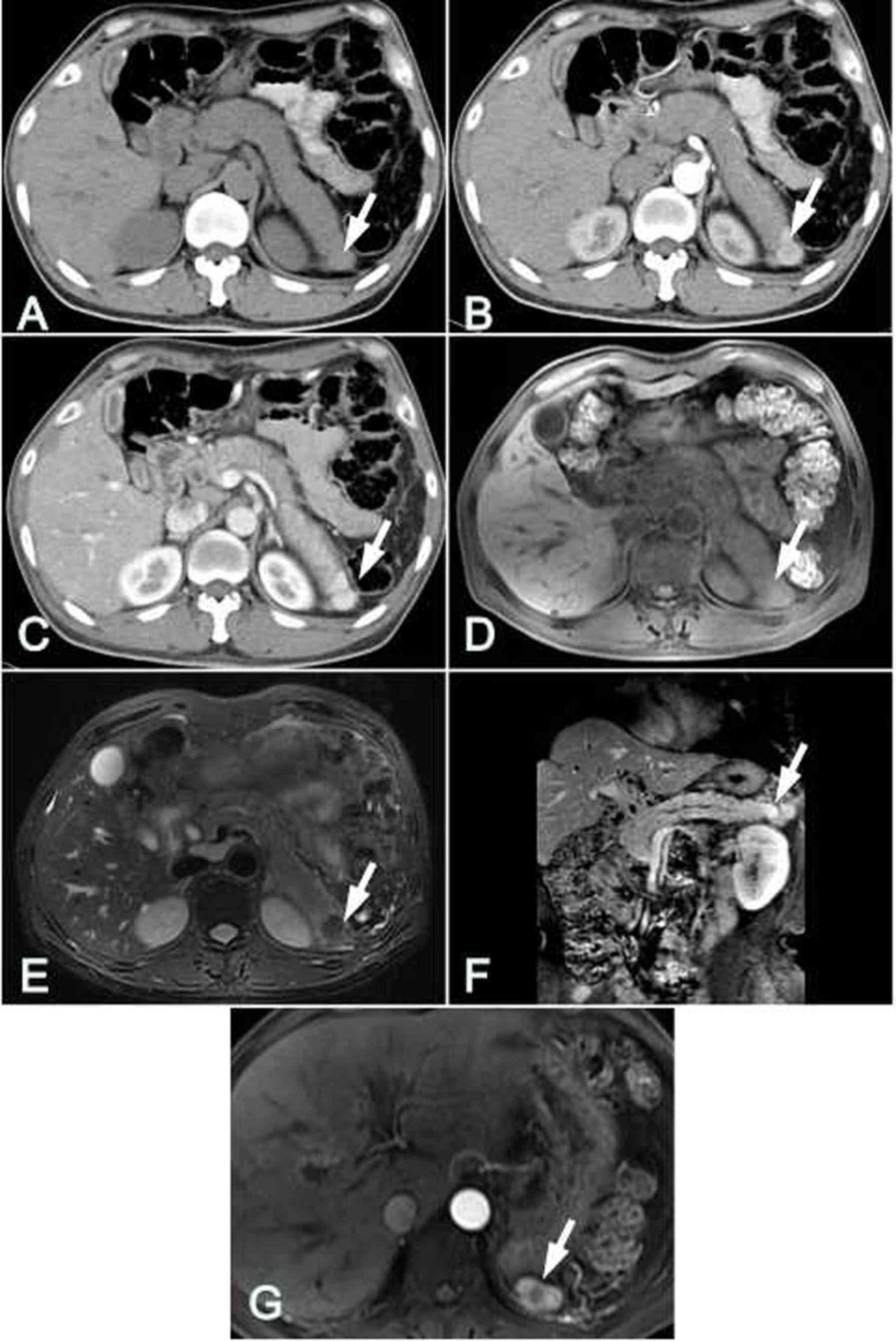
Accompanying presentations
In 2 of the 8 patients with IPAS, liver cirrhosis
and splenomegaly complications were present, and in 1 of these 2
patients, hepatocellular carcinoma was confirmed by hepatic
arteriography. The tumor exhibited a ‘quick wash-in and wash-out’
feature in dynamic enhanced CT imaging (Fig. 5). Accessory spleens were identified
in 2 patients, and these accessory spleens had similar imaging
features to those of the IPAS and orthotopic spleen (Fig. 1H).
Imaging findings of pancreatic
splenosis
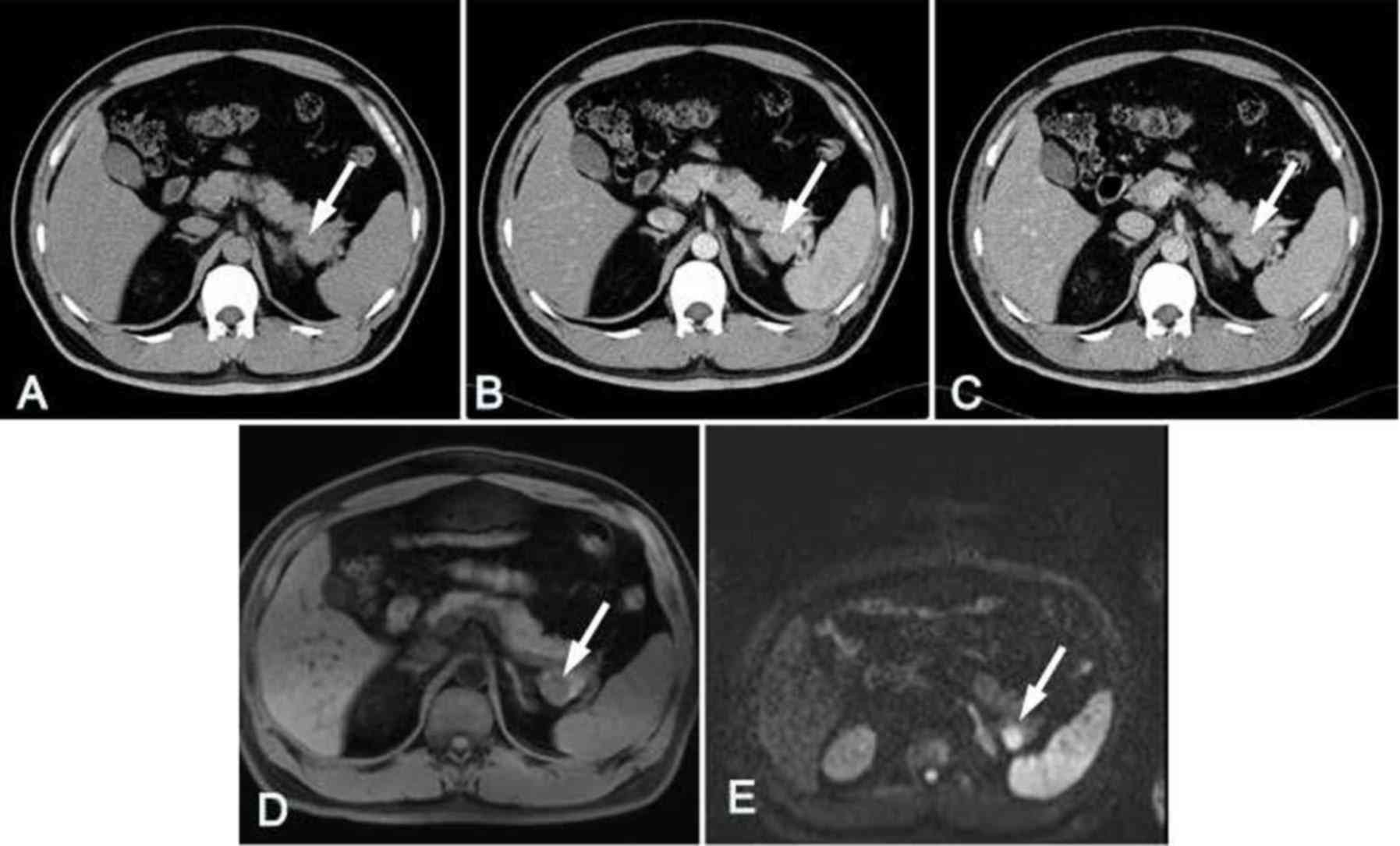
Splenosis in the pancreatic tail was observed in 1
patient who had undergone splenectomy. On the unenhanced CT image
(CT value, 65 HU), the lesion exhibited high density and had a
largest cross-sectional diameter of 2.2×1.2 cm. In comparison with
the normal pancreas, the lesion appeared to have a slightly higher
SI in T1WI, and a lower SI in T2WI and DWI when a b-value of 600
sec/mm2 was used. Dynamic contrast-enhanced CT and MRI
images demonstrated that the enhancement pattern of splenosis was
similar to that of the normal spleen. In this case, several splenic
nodules scattered in the upper left abdominal cavity were also
detected with similar density, signals and enhancement patterns to
those of the tail lesion (Fig.
7).
Pathological findings
The initial pathological diagnosis for 3 of the 9
patients was pancreatic tail tumor. Thus, resections of the distal
pancreas and spleen were performed. In each case, the pancreatic
tail specimen contained a round, smooth, dark-red nodule with a
clear boundary. Cross-sectioning of the IPAS specimen revealed a
reddish nodule surrounded by a fibrotic capsule, which separated it
from the adjacent yellowish pancreatic tissue. Histological
analysis revealed that the IPAS was composed of red and white pulp,
which was similar to that of the normal spleen. The red pulp
comprised numerous vascular sinuses. The lymphoid follicles and
cells of the reticuloendothelial system lay between these sinuses,
which constituted the white pulp (Fig.
2D).
Discussion
There have been few reports of splenosis in solid
organs, and splenosis occurs most frequently in the liver according
to these reports (17,18). These cases have often been
misdiagnosed as primary liver cancer and thus been treated using
surgical resection (17,18). Fiamingo et al (19) presented the first case of
laparoscopic resection of pancreatic splenosis, in which the
patient had a history of splenectomy. In that study, the clinical
history and imaging findings of 2 patients with splenosis of the
pancreatic tail were similar to the single patient with splenosis
in the present study. It may be speculated that splenic tissue was
brought into the pancreatic tail by splenectomy. Generally, the
imaging features of intrapancreaticsplenosis are consistent with
those of normal spleen. However, the case in the present study
exhibited high density on pre-contrast CT images, slightly higher
SI in T1WI and lower SI in T2WI and DWI, which was inconsistent
with normal spleen. It is hypothesized that these unusual features
might be associated with hemosiderosis caused by cirrhosis.
IPAS is not a rare condition; in an autopsy study of
2,700 patients, 61 of 364 (17%) cases of accessory spleen in the
pancreatic tail were detected (3).
The tail of the pancreas was the second most common site of
accessory splenunculi (16–20%) (3,4,20,21),
which was diagnosed by pathological analysis following surgery
(22).
Prior to the regular use of CT and MRI scanning,
IPAS was difficult to detect as symptoms are seldom and the
consequences are not clinically significant (7). However, since abdominal multi-slice
computed tomographic (MSCT) scans are now widely used in healthcare
examinations and systemic surveys, IPAS cases have become easier to
detect.
In the absence of secondary lesions, ectopic spleen
in the pancreas usually does not require any aggressive treatment,
unlike endocrine tumor and pancreatic adenocarcinoma (5,9,15). Therefore, accurate diagnosis using
preoperative imaging tools is important. However, comprehensive
radiologic data on IPAS or pancreatic splenosis is lacking, and
progressive investigation is urgently required. Thus, the present
study describes the radiological features of 9 patients with
intrapancreatic spleen and reviews other cases described in
previous reports.
In the current study, the IPAS was observed to be a
round or oval, smooth nodule with clear boundary surrounded by
pancreatic tissue and a diameter of 1.3–3.7 cm. These
characteristics were consistent with those reported in previously
studies (2,11,23).
The density and SI of the intrapancreatic spleen
were consistent with those of the orthotopic spleen in the majority
of cases (8/9 cases). In comparison with the pancreatic parenchyma,
the attenuation of the intrapancreatic spleen in the CT images was
generally higher than that of the pancreas. The SI of the
intrapancreatic spleen was low in T1WI and high in T2WI and DWI.
Notably, high b-value (b=600 sec/mm2) DWI images
provided greater soft tissue contrast than did T2WI with regard to
SI. Thus, it offered superior diagnostic data for the depiction and
characterization of the intrapancreatic spleen, even with inferior
spatial resolution. The density and SI of the intrapancreatic
spleen may be affected by pathological changes of the spleen, such
as hemosiderosis, hypersplenismleukemia and lymphoma. For example,
in 1 case in the current study, the CT density and MRI SI of the
intrapancreatic spleen were inconsistent with those of normal
spleen tissue, which was considered to be a result of substantial
deposition of hemosiderin due to cirrhosis.
In the majority of cases, the enhancement patterns
of the intrapancreatic spleen were consistent with those of the
orthotopic or normal spleen and the enhancement degree was higher
than that of the pancreas in three dynamic phases, which was
consistent with the findings of Kim et al (2). The heterogeneous or zebra-patterned
enhancement features, which are caused by different rates of flow
through the cords of the red and white pulp in the arterial phase,
may assist in diagnosis (24).
Inhomogeneous enhancement patterns may be detected more frequently,
even in small IPAS, by the application of MSCT, fast MRI sequences
and high-power contrast agent delivery techniques, which enable the
differentiation of IPAS from other hypervascular pancreatic tumors
(2). However, Park et al
(25) reported a case of ectopic
spleen with different CT and MRI enhancement patterns from those of
normal spleen, which was misdiagnosed as pancreatic tumor and then
surgically resected. The attenuation and SI of IPAS may be lower
than those of the pancreas in the arterial and pancreatic phases
when splenic enhancement is retarded, such as in patients with
liver cirrhosis (26). Therefore,
for suspected cases with atypical dynamic enhancement patterns, DWI
scanning is potentially useful to distinguish the lesion.
Nuclear medicine, such as 99mTc-sulfur
colloid and 99mTc heat-damaged red blood cell
scintigraphy, can be utilized as a confirmatory modality when IPAS
or splenosis is suspected. It has been reported that pancreatic
ectopic spleen can specifically take up
99mTc-heat-damaged red blood cells or sulfur colloid at
a normal spleen tissue dose, which is helpful for the detection and
definitive diagnosis of ectopic splenic tissue (14–16).
A small proportion of cases of intrapancreatic
spleen may occur secondary to other diseases, including epidermoid
cyst and inflammatory pseudotumor, have been detected in
intrapancreatic spleens on rare occasions (27–29). The
diagnosis of intrapancreatic spleen is challenging when secondary
lesions are present.
In the present study, 3 cases were complicated with
liver cirrhosis, and 1 of these 3 patients had secondary
hepatocellular carcinoma. The detection of intrapancreatic spleen
appeared to be more likely in patients with cirrhosis due to
repeated imaging follow-up. Intrapancreatic spleen has often been
misdiagnosed as pancreatic tumor causing patients to undergo
unnecessary surgery (5,12,15,19,25).
Thus, it is recommended that the diagnosis of intrapancreatic
spleen is included in the differential diagnosis of pancreatic
hypervascular masses, such as pancreatic neuroendocrine tumors,
solid pseudopapillarytumors and hypervascular metastases.
Neuroendocrine tumors, such as islet cell tumors,
are often observed as small, round and hypervascular features in
the arterial phase of CT and MRI images (9). However, nonfunctional neuroendocrine
tumors are typically large and secondary to cystic necrosis, while
IPAS usually presents as a small and homogeneous nodule. Unlike
IPAS, islet cell tumors do not have a characteristic enhancement
pattern (30). In DWI conducted
using a high b-value, some endocrine neoplasms may present high SI
and low ADC values (31), whereas
IPAS exhibit homogeneous high signals in DWI.
Solid pseudopapillarytumor of the pancreas
predominantly occurs in females aged 20–40 years according to a
previous study by the present research team (32). In CT and MRI images, the lesions
typically present as well-defined round shaped masses with a
diameter >5 cm. In non-enhanced CT or MRI, the lesions exhibit
heterogeneous density or SI. The solid parts of the tumor often
present mild peripheral heterogeneous enhancement, and the degree
of enhancement is usually lower than that of the pancreatic
parenchyma.
Primary malignancies, such as lung cancer, breast
cancer, and gastrointestinal and renal tumors, frequently
metastasize to the pancreas. Ng et al (33) reported that renal cell carcinoma was
the most common malignancy metastasizing to the pancreas, where the
metastasis was solitary or multiple, well-defined and progressively
enlarged. Pancreatic metastases of renal carcinoma were observed to
exhibit rapid enhancement in the arterial phase, but were not easy
to identify in the portal phase and were even less visible in the
120-sec delayed phase. Although it was difficult to distinguish
hypervascular metastasis from intrapancreatic spleen, a clinical
history of known malignancy helped in the diagnosis (33).
In conclusion, a diagnosis of IPAS should be
considered when a solidary lesion in the pancreatic tail has
similar characteristics to the orthotopic spleen in pre-contrast
and post-contrast enhanced CT and MRI images. Clearly elevated SI
consistent with that of the spleen in DWI conducted using a high
b-value is suggestive of a diagnosis of IPAS, and helps in the
differential diagnosis. For intrapancreaticsplenosis, in addition
to the above imaging features, a history of spleen trauma or
splenectomy is crucial to the diagnosis.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Natural Science
foundation of Zhejiang Province (grant no. Y13H070008), the
Medicine and Health Science and Technology Project of Zhejiang
Province (grant nos. 2013KYA182 and 2012KYB176) and the Natural
Science Foundation of Ningbo (grant nos. 2017A610146 and
2010A610052).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QD and ZR collected the cases, analysed the imaging
features and drafted the paper. JW designed the study, analyzed the
data and revised the paper. XM and JZ collected the cases and
analysed imaging features. GS and HG collected the cases and
analysed the data. CZ revised the paper. HJ performed the
pathological examinations.
Ethics approval and consent to
participate
The present retrospective review of patient data was
approved by the Ethics Committee of Ningbo University Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Movitz D: Accessory spleens and
experimental splenosis. Principles of growth. Chic Med Sch Q.
26:183–187. 1967.PubMed/NCBI
|
|
2
|
Kim SH, Lee JM, Han JK, Lee JY, Kim KW,
Cho KC and Choi BI: Intrapancreatic accessory spleen: Findings on
MR Imaging, CT, US and scintigraphy, and the pathologic analysis.
Korean J Radiol. 9:162–174. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Halpert B and Alden ZA: Accessory spleens
in or at the tail of the pancreas. A survey of 2700 additoonal
necropsies. Arch Pathol. 77:652–654. 1964.PubMed/NCBI
|
|
4
|
Halpert B and Gyorkey F: Lesions observed
in accessory spleens of 311 patients. Am J Clin Pathol. 32:165–168.
1959. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matthaei H, Schmelzle M, Braunstein S,
Bölke E and Peiper M: Pancreatic incidentalomas: A growing clinical
challenge exemplified by an intrapancreatic accessory spleen. Wien
Klin Wochenschr. 123:186–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Churei H, Inoue H and Nakajo M:
Intrapancreatic accessory spleen: Case report. Abdom Imaging.
23:191–193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sica GT and Reed MF: Case 27:
Intrapancreatic accessory spleen. Radiology. 217:134–137. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tozbikian G, Bloomston M, Stevens R,
Ellison EC and Frankel WL: Accessory spleen presenting as a mass in
the tail of the pancreas. Ann Diagn Pathol. 11:277–281. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Uchiyama S, Chijiiwa K, Hiyoshi M,
Ohuchida J, Imamura N, Nagano M, Hidaka H, Yorita K, Akiyama Y and
Nishiura M: Intrapancreatic accessory spleen mimicking endocrine
tumor of the pancreas: Case report and review of the literature. J
Gastrointest Surg. 12:1471–1473. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spencer LA, Spizarny DL and Williams TR:
Imaging features of intrapancreatic accessory spleen. Br J Radiol.
83:668–673. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Low G, Panu A, Millo N and Leen E:
Multimodality imaging of neoplastic and nonneoplastic solid lesions
of the pancreas. Radiographics. 31:993–1015. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawamoto S, Johnson PT, Hall H, Cameron
JL, Hruban RH and Fishman EK: Intrapancreatic accessory spleen: CT
appearance and differential diagnosis. Abdom Imaging. 37:812–827.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dodds WJ, Taylor AJ, Erickson SJ, Stewart
ET and Lawson TL: Radiologic imaging of splenic anomalies. American
Journal of Roentgenology. 155:805–810. 2013. View Article : Google Scholar
|
|
14
|
Ota T, Tei M, Yoshioka A, Mizuno M,
Watanabe S, Seki M, Nakata H, Yamamoto I and Morita R:
Intrapancreatic accessory spleen diagnosed by technetium-99m
heat-damaged red blood cell SPECT. J Nucl Med. 38:494–495.
1997.PubMed/NCBI
|
|
15
|
Brasca LE, Zanello A, De Gaspari A, De
Cobelli F, Zerbi A, Fazio F and Del Maschio A: Intrapancreatic
accessory spleen mimicking a neuroendocrine tumor: Magnetic
resonance findings and possible diagnostic role of different
nuclear medicine tests. Eur Radiol. 14:1322–1323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Belkhir SM, Archambaud F, Prigent A and
Chaumet-Riffaud P: Intrapancreatic accessory spleen diagnosed on
radionuclide imaging. Clin Nucl Med. 34:642–644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim KA, Park CM, Kim CH, Choi SY, Park SW,
Kang EY, Seol HY and Cha IH: An interesting hepatic mass: Splenosis
mimicking a hepatocellular carcinoma (2003:9b). Eur Radiol.
13:2713–2715. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hilal Abu M, Harb A, Zeidan B, Steadman B,
Primrose JN and Pearce NW: Hepatic splenosis mimicking HCC in a
patient with hepatitis C liver cirrhosis and mildly raised alpha
feto protein; the important role of explorative laparoscopy. World
J Surg Oncol. 7:12009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fiamingo P, Veroux M, Da Rold A, Guerriero
S, Pariset S, Buffone A and Tedeschi U: A rare diagnosis for a
pancreatic mass: Splenosis. J Gastrointest Surg. 8:915–916. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Varga I, Galfiova P, Adamkov M, Danisovic
L, Polak S, Kubikova E and Galbavy S: Congenital anomalies of the
spleen from an embryological point of view. Med Sci Monit.
15:RA269–RA276. 2009.PubMed/NCBI
|
|
21
|
Mortelé KJ, Mortelé B and Silverman SG: CT
features of the accessory spleen. AJR Am J Roentgenol.
183:1653–1657. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hwang HS, Lee SS, Kim SC, Seo DW and Kim
J: Intrapancreatic accessory spleen: Clinicopathologic analysis of
12 cases. Pancreas. 40:956–965. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SH, Lee JM, Han JK, Lee JY, Kang WJ,
Jang JY, Shin KS, Cho KC and Choi BI: MDCT and superparamagnetic
iron oxide (SPIO)-enhanced MR findings of intrapancreatic accessory
spleen in seven patients. Eur Radiol. 16:1887–1897. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paterson A, Frush DP, Donnelly LF, Foss
JN, O'Hara SM and Bisset GS 3rd: A pattern-oriented approach to
splenic imaging in infants and children. Radiographics.
19:1465–1485. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park JS, Kim WJ, Jeong YG, Park YS, Koo
HC, Lee TI, Choi GC and Kim S: A case of intrapancreatic accessory
spleen mistaken as a pancreatic mass due to different enhancing
pattern from normal spleen. Korean J Gastroenterol. 58:357–360.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Blomley MJ, Kormano M, Coulden R,
Lim-Dunham J, Dawson P and Lipton MJ: Splenic blood flow:
Evaluation with computed tomography. Acad Radiol. 4:13–20. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Davidson ED, Campbell WG and Hersh T:
Epidermoid splenic cyst occurring in an intrapancreatic accessory
spleen. Dig Dis Sci. 25:964–917. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu S, Zhu L, Song Q and Chen K: Epidermoid
cyst in intrapancreatic accessory spleen: Computed tomography
findings and clinical manifestation. Abdom Imaging. 37:828–833.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okura N, Mori K, Morishita Y, Oda T, Tanoi
T and Minami M: Inflammatory pseudotumor of the intrapancreatic
accessory spleen: Computed tomography and magnetic resonance
imaging findings. Jpn J Radiol. 30:171–175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sukaiti R, Robinson K and Menias C:
Retrospective review of cross sectional imaging findings of
pancreatic non-functional islet cell tumor (NFICT) and its hepatic
metastases. Oman Med J. 26:39–42. 2011.PubMed/NCBI
|
|
31
|
Kurata Y, Kido A, Moribata Y, Kameyama K,
Himoto Y, Minamiguchi S, Konishi I and Togashi K: Diagnostic
performance of MR imaging findings and quantitative values in the
differentiation of seromucinous borderline tumour from
endometriosis-related malignant ovarian tumour. Eur Radiol.
27:1695–1703. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma XL, Wang JH, Jiang H, Lu JP and Liu Q:
Solid-pseudopapillary tumor of pancreas: Different types of imaging
features and their correlation with pathological findings. Zhonghua
Yi Xue Za Zhi. 92:170–174. 2012.(In Chinese). PubMed/NCBI
|
|
33
|
Ng CS, Loyer EM, Iyer RB, David CL, DuBrow
RA and Charnsangavej C: Metastases to the pancreas from renal cell
carcinoma: Findings on three-phase contrast-enhanced helical CT.
AJR Am J Roentgenol. 172:1555–1559. 1999. View Article : Google Scholar : PubMed/NCBI
|