Introduction
Articular cartilage tissue lacks blood supply and
has a limited regeneration capacity. An osteochondral defect occurs
when the subchondral bone is damaged due to abnormal mechanical
loading (1–3). This type of defect is relatively common
and notably reported in ~20% of all arthroscopic procedures
(4), and if left untreated or
treated incorrectly, osteoarthritis (OA) will occur. Patients
develop pain, locking of the joint and/or other symptoms, which may
lead to disability. Various methods have been reported for the
treatment of osteochondral defects, including the marrow
stimulation technique, osteochondral autograft/allograft
transplantation and autologous chondrocyte implantation (1–3).
Although the majority of methods have been proven to improve the
symptoms for a certain period of time, long-term efficacy and
physiological property recovery needs to be further examined
(4–6). Our research group has previously
fabricated and implanted three-dimensional porous scaffolds into
osteochondral defects in rabbits (7–10).
Although repair was observed, it was difficult for a single
scaffold to meet the needs for reconstructing the cartilage and
subchondral bone at the same time. Improved regenerative effects
are still under evaluation.
Two types of extracorporeal shock wave therapy
(ESWT) have been used in the musculoskeletal system, including
focused ESWT (fESWT) and radial ESWT (rESWT) (11). A larger number of studies have been
conducted on fESWT in the clinical setting and animal models, as
opposed to rESWT (12). As wave
patterns and tissue penetrating depth are different for fESWT and
rESWT, corresponding effects are expected to vary (12) and further investigations are
required. It has previously been reported that rESWT on
tendinopathy can be more effective compared with other types of
treatment, such as traditional physiotherapy and surgery (13), while its role in osteochondral
defects remains incompletely understood. Therefore, it can be
hypothesized that improved repair may be achieved when combining
scaffold transplantation with rESWT.
In the present study, a rabbit osteochondral defect
model was used to assess the morphology of articular cartilage and
subchondral bone according to the International Cartilage Repair
Society (ICRS) histological scoring system. Nitric oxide (NO)
levels in the synovial cavity were measured, and histological
sections were examined by hematoxylin-eosin (H&E) and Safranin
O/fast green staining. By comparing these parameters among the
untreated control, scaffold and scaffold plus rESWT groups, it was
concluded that rESWT combined with the scaffold improved the
osteochondral regeneration, which may be a result of the reduction
of NO levels in the synovial fluid.
Materials and methods
Animal model
The experimental protocol was reviewed and approved
by the Institutional Review Board of Beijing Jishuitan Hospital
(Beijing, China). A total of 15 male New Zealand White rabbits aged
5 months (mean weight, 2.7 kg; weight range, 2.5–2.9 kg) were
obtained from Xinglong Experimental Animal Farm (Beijing, China).
Animals were kept at 16–28°C with 40–70% humidity under a 12-h
light/dark cycle with free access to food and water.
Acclimatization for 7 days was allowed prior to experiments. Under
anesthesia performed by injection of 0.1% pentobarbital sodium (40
mg/kg), a model of osteochondral defects was established. During
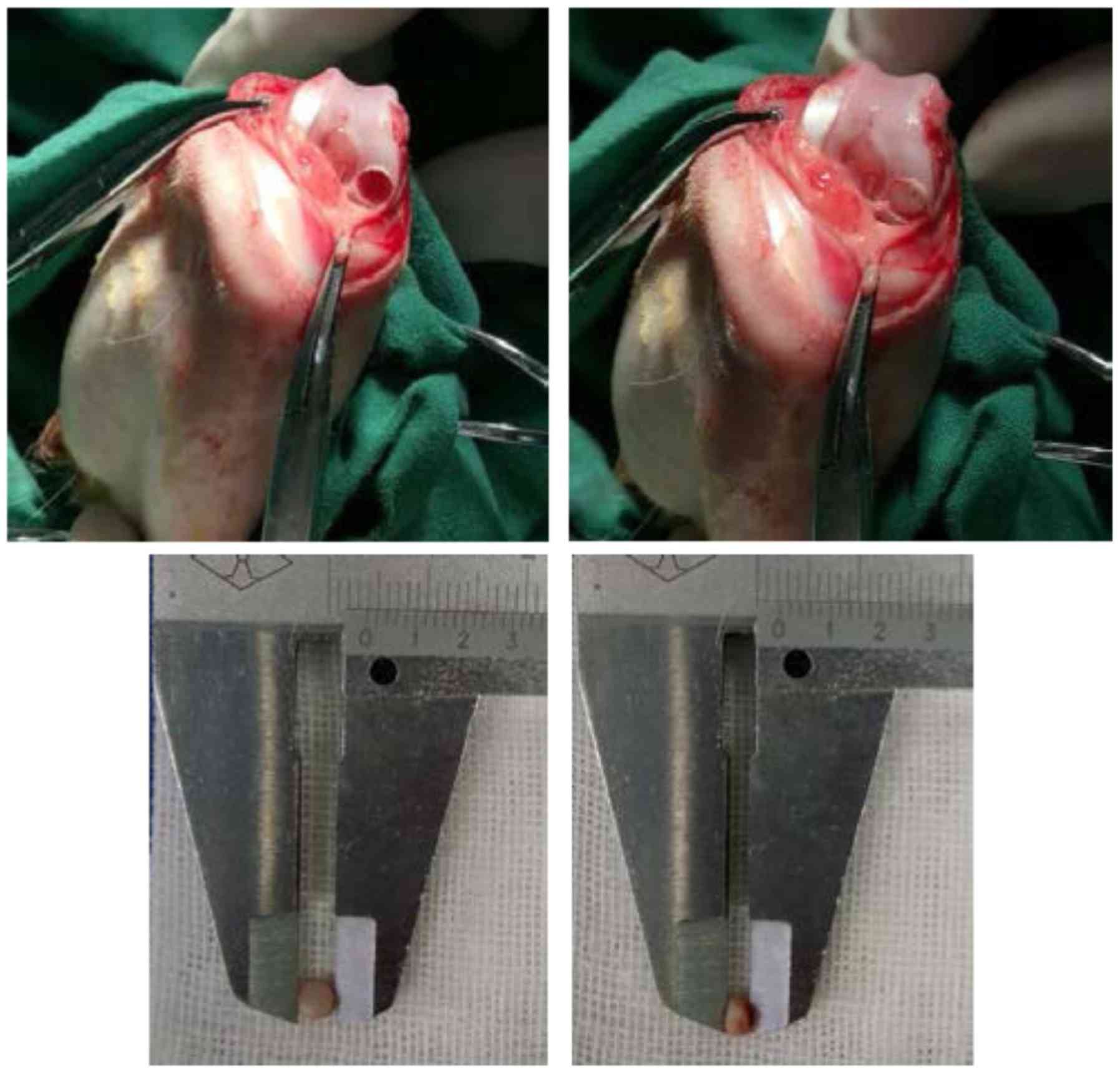
the surgery, a hole was drilled with a 3.5-mm diameter (the
diameter of the drill) and 2.21±0.24-mm depth in the weight-bearing
osteochondral surface of the femoral condyle (Fig. 1). Surgery was performed in the
bilateral knee joints of the rabbits; left knee joints were treated
12 weeks and right knee joints were treated for the last 6 weeks
only. Rabbits (n=5) were assigned the following groups: Control
group with osteochondral defects not receiving treatment; scaffold
group with osteochondral defects treated with a porous scaffold
implant, which was prepared by the authors from dermal tissues of a
calf back following decellularization, reconstruction,
cross-linking and surface-modification as described previously
(7–10); and scaffold + rESWT group with
osteochondral defects treated with a scaffold implant and
rESWT.
Shock wave treatment
Radial shock waves were applied using a STORZ device
(STORZ Medical, Tägerwilen, Switzerland). At 2 weeks after surgery,
rabbits in the scaffold plus rESWT group were anesthetized and then
placed in a lateral position. A coupling gel was smeared to the
skin surrounding the surgical area. The knee joint of each rabbit
was subjected to 500 shock wave impulses at 1.5 bar every time
(Fig. 2). Immediately after rESWT
application, the knee joint was examined for swelling, ecchymosis
or hematoma. All animals were sacrificed 12 weeks following the
initial surgery.
NO quantification
Prior to euthanasia, the rabbits were anesthetized
and the synovial cavity of knee joints was washed with 0.5 ml
sterile saline. The synovial exudates were collected by aspiration
and stored at −80°C prior to the assay. During the measurement, all
samples were centrifuged at 1,000 × g for 20 min at 4°C.
Concentrations of NOX (nitrite/nitrate) in the synovial
fluid were determined using a method based on the enzymatic
conversion of nitrate to nitrite by nitrate reductase, followed by
colorimetric detection of nitrite as an azo dye product of the
Griess reaction (Molecular Probes; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Briefly, 130 µl deionized water, 150 µl of the
sample and 20 µl Griess reagent were respectively added into each
well of a 96-well plate, and incubated for 30 min at room
temperature. The absorbance of each sample was then measured
spectrophotometrically at 540 nm. The concentration of
NOX was determined through the standard curve of nitrite
concentration (1–100 µM) against the absorbance at 540 nm.
Macroscopic observation
The femoral condyles of rabbits in each group were
observed without microscopic aid and images were captured with a
Nikon D850 camera (Nikon Corporation, Tokyo, Japan). Macroscopic
evaluation was conducted according to the ICRS scoring system
(14). Briefly, the degree of defect
repair, integration to border zone and macroscopic appearance were
scored on a scale of 0–4; normal tissue achieves a score of 12.
Histological analyses
The femoral condyles were dissected into small
sections, and the regenerated/defect part was fixed in 4%
paraformaldehyde for 3–4 days. Subsequently, the tissues were
decalcified in 10% EDTA solution (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for 28 days and sectioned into 4-µm slices.
Sections were stained with conventional hematoxylin and eosin
(H&E). Sections were incubated with hematoxylin for 8–10 min
and then 0.5% eosin for 20 sec, both at room temperature. Safranin
O/fast green staining kit (Beijing Solarbio Science &
Technology Co., Ltd.) was used to determine the extent of cartilage
repair and observe the cartilaginous specific matrix secretion
according to the manufacturer's instructions. All the procedures of
Safranin O/fast green staining were performed at room temperature.
Following dewaxing with xylene (3×10 min), sections were stained
with Weigert solution for 3–5 min and washed with distilled water
(2×1 min). After differentiation for 15 sec with acid
differentiation solution, sections were incubated with fast green
for 5 min and then into safranin O for 2 min. Each step was
performed with 1 ml solutions followed by washing with distilled
water (2×1 min). After staining with fast green and safranin O,
sections were washed with a weak acid solution for 1–2 min to
remove the residual stain, and then also washed with distilled
water 2 times for 1 min. All the solutions were provided in the
kit. Sections were then incubated into 95% alcohol and absolute
ethanol for dehydration, each step was performed twice for a few
seconds. Xylene was used for transparency prior to sealing the
sections. Sections were analyzed using a light microscope
(magnification, ×40). The normal cartilage matrix is positively
stained by Safranin O (red), while the injury cartilage that is
negatively stained is almost white or weakly red.
Statistical analyses
Data are expressed as the mean ± standard
derivation. One-way analysis of variance followed by post hoc
testing with Fisher's least significant difference was used to
analyze experimental data with the IBM SPSS software (version 21.0;
IBM Corp., Armonk, NY, USA). In the current study, statistically
significant differences were indicated by P<0.05.
Results
rESWT leads to lower NOX
concentrations in the synovial fluid
The total concentration of NOX, including
nitrite and nitrate, in the synovial fluid was measured with the
Griess method. NOX concentrations were significantly
lower in the scaffold (19.2±3.0 µmol/l) and scaffold plus rESWT
groups (18.8±4.0 µmol/l) as compared with the control group
(25.8±3.1 µmol/l) at 6 weeks after surgery. However, no significant
difference was detected among the three groups at 12 weeks after
surgery (Table I).
 | Table I.NO concentration in the synovial fluid
(n=5). |
Table I.
NO concentration in the synovial fluid
(n=5).
|
| NO concentration
post-surgery (µmol/l) |
|---|
|
|
|
|---|
| Group | 6 weeks | 12 weeks |
|---|
| Control |
25.8±3.1 |
10.2±2.9 |
| Scaffold |
19.2±3.0a |
8.0±1.4 |
| Scaffold plus
rESWT |
18.8±4.0a |
8.4±2.4 |
rESWT improves macroscopic
osteochondral appearance
In order to identify whether rESWT helps promote
osteochondral regeneration, the gross appearance of the three
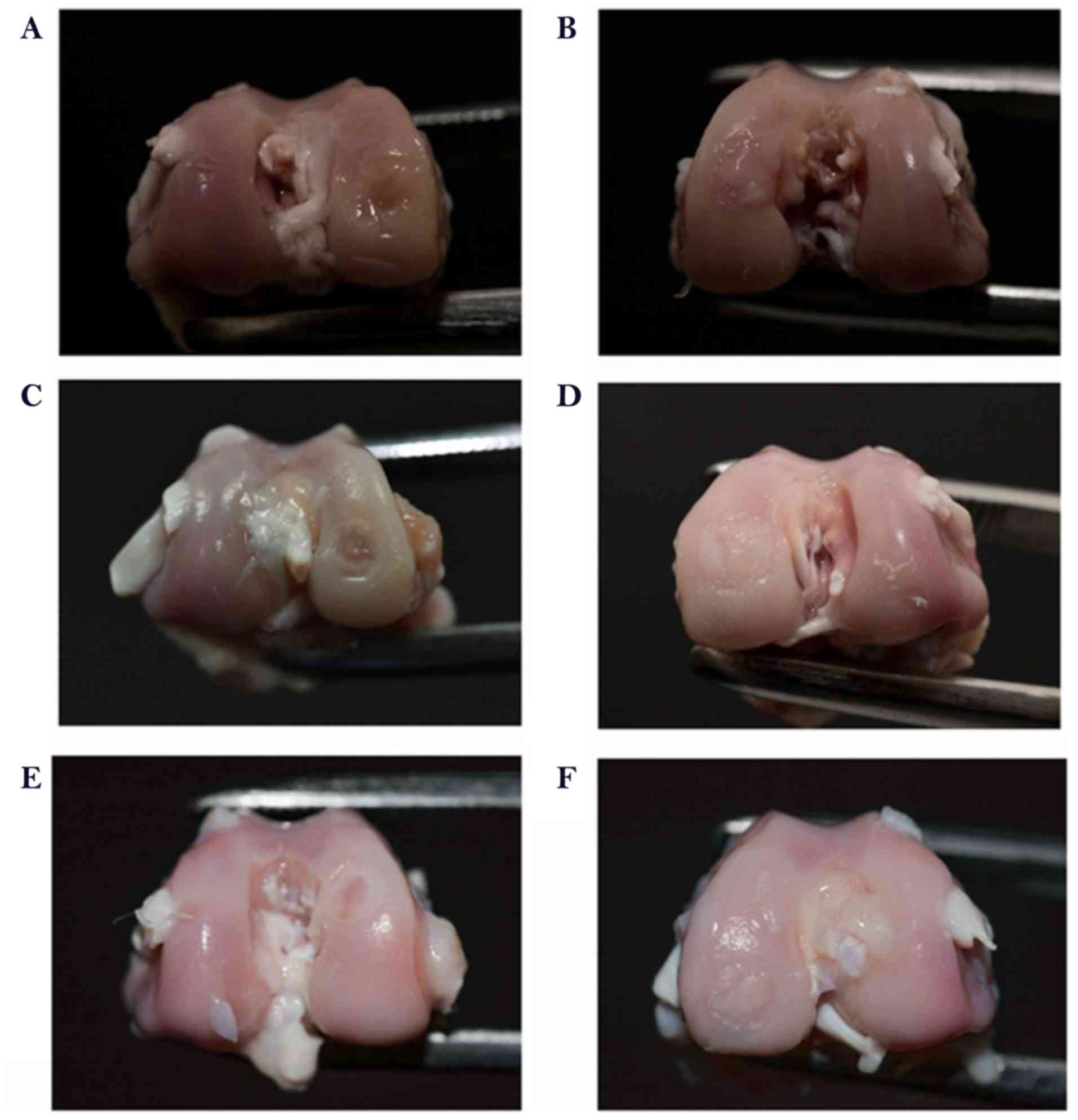
groups at 6 or 12 weeks after surgery was compared.
Macroscopically, in the control group, the surface of the defect
area was evidently lower in comparison with the normal tissue at 6
weeks after surgery. However, the scaffold and the scaffold plus
rESWT groups exhibited marked improvement in osteochondral
appearance after 6 and 12 weeks (Fig.
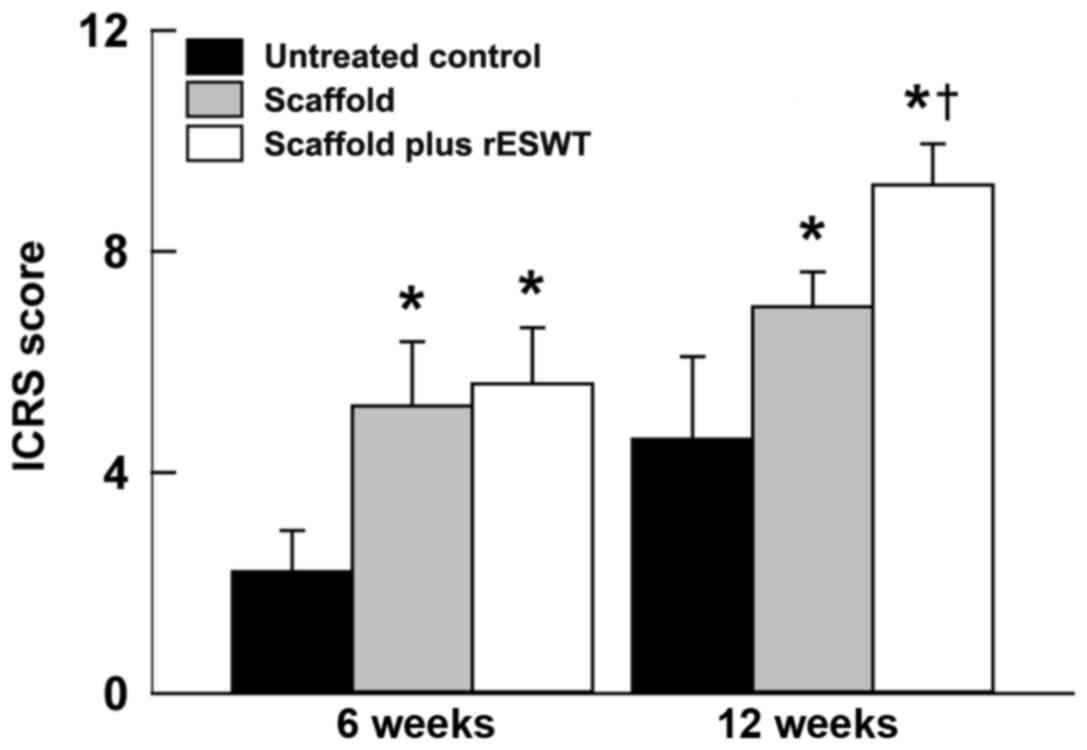
3A-F). The ICRS scores also revealed similar results
(P<0.05; Fig. 4). Additionally,
the scaffold plus rESWT group displayed much better repair
characteristics after 12 weeks, but not after 6 weeks of surgery
(Figs. 3E and F, and 4). All these results indicated a
significant therapeutic effect of rESWT used in combination with
the scaffold.
rESWT results in improved
osteochondral regeneration
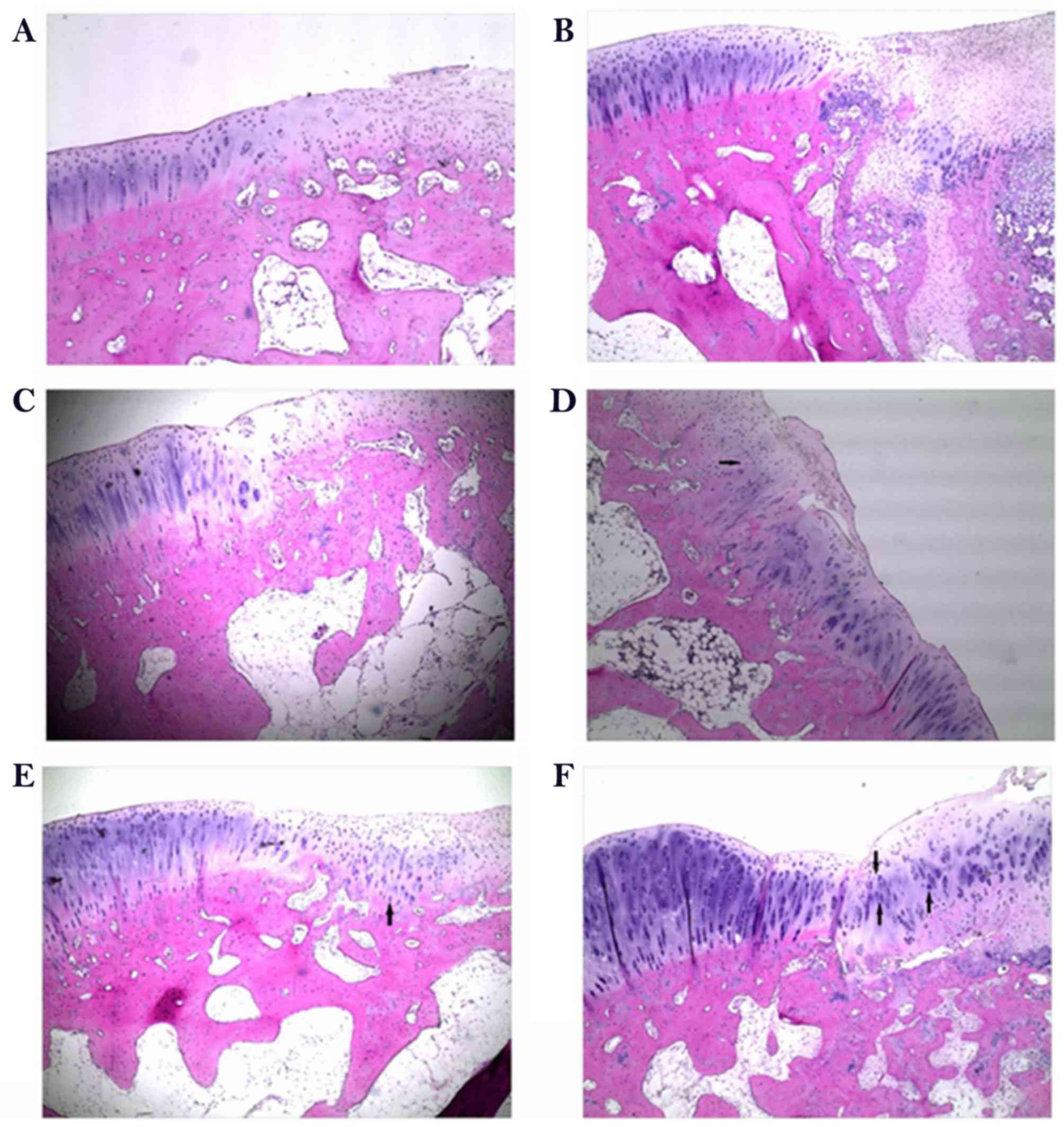
To determine the effect of rESWT on the
osteochondral defect of knee joints, H&E staining was
performed. As presented in Fig. 5,
the defect area was covered by fibrous tissue both at week 6 and
week 12 in the control group (Fig. 5A
and B) and limited repair was observed in the scaffold group at
6 weeks following surgery when implanted with porous scaffold
(Fig. 5C). A limited number of small
rounded chondrocyte-type cells were detected beneath the defect
area and at the interface with the normal tissue at 12 weeks
following surgery (Fig. 5D). An
increased number of rounded chondrocyte-type cells were detected
and an increased cartilaginous-type surface area was presented by
rESWT treatment group both at week 6 and week 12 (Fig. 5E and F). The results were further
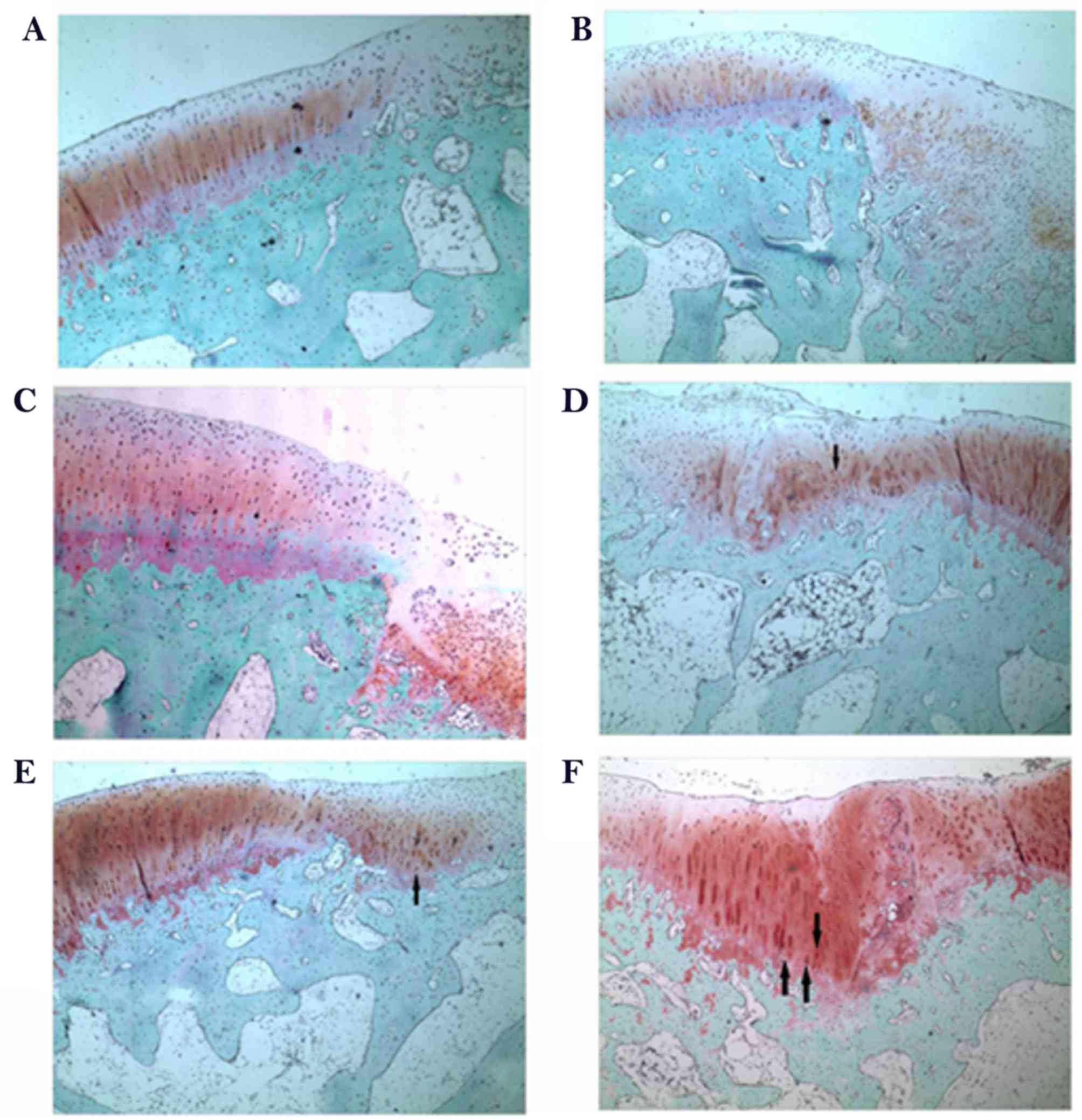
confirmed by Safranin O/fast green staining (Fig. 6). Safranin O/fast green staining was
used to access the cartilage matrix content. The results exhibited
that Safranin O staining was negative in the control group both at
week 6 and week 12 (Fig. 6A and B)
and weak-positive in the scaffold group, beneath the defect area
and at the interface with the normal tissue both at week 6 and week
12 (Fig. 6C and D) and a limited
number of small rounded chondrocyte-type cells were detected at 12
weeks following surgery (Fig. 6D).
In the scaffold plus rESWT group, an increased amount of
chondrocyte-type cells were observed. Safranin O staining was
positive at week 6 (Fig. 6E) and a
larger proportion of cartilaginous-matrix tissue was positively
stained with Safranin O at week 12 (Fig.
6F).
Discussion
Severe cartilage defects are often combined with the
destruction of subchondral bone and are known as osteochondral
defects. If left untreated, an osteochondral defect will gradually
expand to the surrounding normal cartilage, causing OA (15). While a number of treatment options
currently present good short-term functional restoration, the
majority of the treatments result in fibrocartilage with inferior
mechanical properties and diminished long-term durability (1,16).
Therefore, to further improve the osteochondral regeneration, a
combination of different methods is actively investigated currently
(17).
A scaffold is one of the three main components of
tissue engineering, along with cells and growth factors (18,19). A
porous scaffold was fabricated by our group and received a Chinese
Invention Patent (patent no. ZL 2011 1 0099363.1) (7–10), was
prepared from the dermal tissues of a calf back following
decellularization, reconstruction, cross-linking and
surface-modification. Although this scaffold promotes osteochondral
regeneration, its effect was not fully satisfactory in terms of
functional recovery. Previously, ESWT has been reported to serve an
important role in treating a number of orthopedic injuries
(20,21). The Food and Drug Administration of
USA has approved ESWT for the treatment of proximal plantar
fasciitis, and clinical trials of lateral epicondylitis of the
elbow, calcific tendinitis of the shoulder and nonunion fractures
(20,21). To date, an increasing number of
studies have demonstrated the significant effect of ESWT on bone
and tendon regeneration in animals and patients (13). There are also several studies
reporting the therapeutic effect of ESWT on OA, osteochondritis
dissecans or cartilage injury (22–25);
however, the mechanisms underlying its effect remain largely
unknown.
In the present study, rESWT was used to treat
osteochondral defects. Promising results were obtained in the
scaffold plus rESWT group, compared with the scaffold group, as
demonstrated by the higher ICRS scores and improved gross
appearance, which were much more evident at 12 weeks after
surgery.
Nitric oxide (NO) is one of the main biological
mediators under acoustic stimulation by shock waves (26). It has been confirmed that the
systemic concentrations of NO increased at 1 month after ESWT was
conducted for the treatment of on long bone nonunion (27). These results suggested the potential
role of NO in ESWT. NO is important in OA progression and mediates
the inflammatory responses. It is also involved in the apoptosis of
chondrocytes, stimulation of matrix metalloproteinases, and
degradation of collagen and proteoglycans (28–31). In
the current study, NO levels in the synovial cavity were
significantly reduced subsequent to treatment using a scaffold
alone or scaffold plus rESWT at 6 weeks after surgery compared with
the control group, while no significant difference was detected
between the two groups. However, the mean value was lower when
rESWT was used; since NO may serve a role in treatment with rESWT,
further examination should be conducted in the future. Furthermore,
the NO levels were not markedly different among the three groups at
12 weeks after surgery. This finding was not consistent with the
observations of the study by Zhao et al (31), which mentioned that NO production was
reduced in a rabbit OA model at 4 and 8 weeks after ESWT. The NO
level in this previous OA model was markedly higher in comparison
with that in the model of the present study. The discrepancy in
these results may be due to the following reasons: Firstly, the OA
model established in the previous study was induced by anterior
cruciate ligament transaction, while the current study model was
induced by drilling a hole in femoral condyle; secondly, the
previous authors selected 4 and 8 weeks as the time points of NO
detection, whereas 6 and 12 weeks were selected in the present
study; thirdly, although rESWT was used in the two studies,
different manufacturer, frequency and energy flux density may have
caused different results; and finally, the use of a different
method and kit may have also given rise to this discrepancy. NO is
an important factor in osteochondral injury. It is routinely
examined through quantification of the concentrations of
nitrite/nitrate (NOX) using a method based on the enzymatic
conversion of nitrate to nitrite facilitated by a nitrate reductase
and followed by colorimetric detection of nitrite using a Griess
reaction (32). As the detection
procedure and incubation durations are not consistent between
various laboratories, the concentrations of NOX will differ.
Furthermore, several biomarkers have been reported to be involved
in the pathogenesis of osteochondral injury or OA, such as
prostaglandin E2 (33,34), and the results of the present study
will be further verified using such biomarkers in the future. To
further clarify the underlying molecular mechanisms, other effects
of rESWT on chondrocytes in vitro will also be assessed.
The current study observed that rESWT promoted
osteochondral regeneration in rabbits, which may in part be due to
the reduction of NO level in the synovial fluid in the knee joint;
however, the exact molecular mechanisms remain unknown. In the
present study, only a single dose was assessed and one treatment of
rESWT was conducted; therefore, it is unclear whether other energy
levels or multiple applications of rESWT have the same effect. The
time point at which rESWT was conducted may also be an important
factor, and certain researchers have suggested performing rESWT on
knee OA prior to surgical treatment (25). Therefore, further in vitro and
in vivo studies need to be conducted in the future, both
basic and clinical, although the important role of rESWT on
osteochondral regeneration is evident, and this strategy may serve
as a valuable alternative supplement.
In conclusion, due to inadequate blood supply and
limited regeneration ability of cartilage, there is a lack of
effective treatments for osteochondral defects. Emerging studies
have revealed the excellent therapeutic efficacy of ESWT on bone
regeneration. Similarly, the results of the present study indicated
that rESWT combined with the use of a scaffold was able to improve
osteochondral regeneration. At 6 weeks after surgery, NO levels in
the scaffold and the scaffold plus rESWT groups were significantly
reduced in the synovial cavity, whereas no significant difference
was detected at 12 weeks after surgery. Furthermore, the ICRS
scores and histological examination also revealed a much improved
osteochondral regeneration following treatment with scaffold plus
rESWT. These data suggest that rESWT improved osteochondral
regeneration when combined with a scaffold and that it may be a
useful treatment for osteochondral defects.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Capital Public
Health Project (grant no. Z171100000417025) and the National
Natural Science Foundation of China (grant no. 91543124).
Availability of data and materials
All data generated or analyzed during the present
study are included in this article.
Authors' contributions
YL, LS and HQ designed the project and supervised
the study. HQ, SJ and LC performed the experiments. CY and YL
analyzed the data and performed statistical analysis. HQ, SJ and CY
generated the figures. HQ and YL wrote the manuscript. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The experimental protocol was reviewed and approved
by the Institutional Review Board of Beijing Jishuitan Hospital
(Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huey DJ, Hu JC and Athanasiou KA: Unlike
bone, cartilage regeneration remains elusive. Science. 338:917–921.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marcacci M, Filardo G and Kon E: Treatment
of cartilage lesions: What works and why? Injury. 44 Suppl
1:S11–S15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Filardo G, Andriolo L, Balboni F, Marcacci
M and Kon E: Cartilage failures. Systematic literature review,
critical survey analysis, and definition. Knee Surg Sports
Traumatol Arthrosc. 23:3660–3669. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okano T, Mera H, Itokazu M, Okabe T, Koike
T, Nakamura H and Wakitani S: Systemic administration of
granulocyte colony-stimulating factor for osteochondral defect
repair in a rat experimental model. Cartilage. 5:107–113. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khorshidi S and Karkhaneh A: A review on
gradient hydrogel/fiber scaffolds for osteochondral regeneration. J
Tissue Eng Regen Med. 12:e1974–e1990. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kon E, Roffi A, Filardo G, Tesei G and
Marcacci M: Scaffold-based cartilage treatments: With or without
cells? A systematic review of preclinical and clinical evidence.
Arthroscopy. 31:767–775. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qi H, Jie YS, Chen L, Li QH, Gao XS and
Sun L: Repair of articular defects in the knee with a cell-free
scaffold. Orthopaedic Journal of China (Chinese). 23:1303–1309.
2015.
|
|
8
|
Qi H, Jie YS, Chen L, Jiang J, Gao XS and
Sun L: Preparation of acellular dermal matrix as a kind of scaffold
for cartilage tissue engineering and its biocompatibility. Zhongguo
Xiu Fu Chong Jian Wai Ke Za Zhi. 28:768–772. 2014.(In Chinese).
PubMed/NCBI
|
|
9
|
Qi H, Sun L, Chen L, Jie YS and Gao XS:
Technics of hair removal-decellularization-reconstruction to build
cartilage repair carrier. Orthopaedic J China (Chinese).
22:151–157. 2014.
|
|
10
|
Qi H, Jie YS, Chen L, Tao JF, Jiang J and
Sun L: Comparison of scaffolds for chondrocyte implantation with
two kinds of cross-linking agents. Chin Med Biotechnol (Chinese).
8:408–413. 2013.
|
|
11
|
Imamura M, Alamino S, Hsing WT, Alfieri
FM, Schmitz C and Battistella LR: Radial extracorporeal shock wave
therapy for disabling pain due to severe primary knee
osteoarthritis. J Rehabil Med. 49:54–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hochstrasser T, Frank HG and Schmitz C:
Dose-dependent and cell type-specific cell death and proliferation
following in vitro exposure to radial extracorporeal shock waves.
Sci Rep. 6:306372016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schmitz C, Császár NB, Milz S, Schieker M,
Maffulli N, Rompe JD and Furia JP: Efficacy and safety of
extracorporeal shock wave therapy for orthopedic conditions: A
systematic review on studies listed in the PEDro database. Br Med
Bull. 116:115–138. 2015.PubMed/NCBI
|
|
14
|
Mainil-Varlet P, Aigner T, Brittberg M,
Bullough P, Hollander A, Hunziker E, Kandel R, Nehrer S, Pritzker
K, Roberts S and Stauffer E: International Cartilage Repair
Society. Histological assessment of cartilage repair: A report by
the histology endpoint committee of the international cartilage
repair society (ICRS). J Bone Joint Surg Am. 85-A (Suppl)2:S45–S57.
2003. View Article : Google Scholar
|
|
15
|
Li X, Ding J, Wang J, Zhuang X and Chen X:
Biomimetic biphasic scaffolds for osteochondral defect repair.
Regen Biomater. 2:221–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dahlin RL, Kinard LA, Lam J, Needham CJ,
Lu S, Kasper FK and Mikos AG: Articular chondrocytes and
mesenchymal stem cells seeded on biodegradable scaffolds for the
repair of cartilage in a rat osteochondral defect model.
Biomaterials. 35:7460–7469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jeon JE, Vaquette C, Theodoropoulos C,
Klein TJ and Hutmacher DW: Multiphasic construct studied in an
ectopic osteochondral defect model. J R Soc Interface.
11:201401842014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Armiento AR, Stoddart MJ, Alini M and
Eglin D: Biomaterials for articular cartilage tissue engineering:
Learning from biology. Acta Biomater. 65:1–20. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maia FR, Carvalho MR, Oliveira JM and Reis
RL: Tissue engineering strategies for osteochondral repair. Adv Exp
Med Biol. 1059:353–371. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang CJ: An overview of shock wave therapy
in musculoskeletal disorders. Chang Gung Med J. 26:220–232.
2003.PubMed/NCBI
|
|
21
|
Wang CJ: Extracorporeal shockwave therapy
in musculoskeletal disorders. J Orthop Surg Res. 7:112012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lyon R, Liu XC, Kubin M and Schwab J: Does
extracorporeal shock wave therapy enhance healing of
osteochondritis dissecans of the rabbit knee? a pilot study. Clin
Orthop Relat Res. 471:1159–1165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Frisbie DD, Kawcak CE and McIlwraith CW:
Evaluation of the effect of extracorporeal shock wave treatment on
experimentally induced osteoarthritis in middle carpal joints of
horses. Am J Vet Res. 70:449–454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim JH, Kim JY, Choi CM, Lee JK, Kee HS,
Jung KI and Yoon SR: The dose-related effects of extracorporeal
shock wave therapy for knee osteoarthritis. Ann Rehabil Med.
39:616–623. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ochiai N, Ohtori S, Sasho T, Nakagawa K,
Takahashi K, Takahashi N, Murata R, Takahashi K, Moriya H, Wada Y
and Saisu T: Extracorporeal shock wave therapy improves motor
dysfunction and pain originating from knee osteoarthritis in rats.
Osteoarthritis Cartilage. 15:1093–1096. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Evens DM and Ralston SH: Nitric oxide and
bone. J Bone Miner Res. 11:300–305. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang CJ, Yang KD, Ko JY, Huang CC, Huang
HY and Wang FS: The effects of shockwave on bone healing and
systemic concentrations of nitric oxide (NO), TGF-beta1, VEGF and
BMP-2 in long bone non-unions. Nitric Oxide. 20:298–303. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abramson SB: Nitric oxide in inflammation
and pain associated with osteoarthritis. Arthritis Res Ther. 10
Suppl 2:S22008. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hancock CM and Riegger-Krugh C: Modulation
of pain in osteoarthritis: The role of nitric oxide. Clin J Pain.
24:353–365. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Studer R, Jaffurs D, Stefanovic-Racic M,
Robbins PD and Evans CH: Nitric oxide in osteoarthritis.
Osteoarthritis Cartilage. 7:377–379. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao Z, Ji H, Jing R, Liu C, Wang M, Zhai
L, Bai X and Xing G: Extracorporeal shock-wave therapy reduces
progression of knee osteoarthritis in rabbits by reducing nitric
oxide level and chondrocyte apoptosis. Arch Orthop Trauma Surg.
132:1547–1553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Du Q, Park KS, Guo Z, He P, Nagashima M,
Shao L, Sahai R, Geller DA and Hussain SP: Regulation of human
nitric oxide synthase 2 expression by Wnt beta-catenin signaling.
Cancer Res. 66:7024–7031. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nguyen LT, Sharma AR, Chakraborty C,
Saibaba B, Ahn ME and Lee SS: Review of prospects of biological
fluid biomarkers in osteoarthritis. Int J Mol Sci. 18:E6012017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li N, Rivéra-Bermúdez MA, Zhang M, Tejada
J, Glasson SS, Collins-Racie LA, Lavallie ER, Wang Y, Chang KC,
Nagpal S, et al: LXR modulation blocks prostaglandin E2 production
and matrix degradation in cartilage and alleviates pain in a rat
osteoarthritis model. Proc Natl Acad Sci USA. 107:pp. 3734–3739.
2010; View Article : Google Scholar : PubMed/NCBI
|