Introduction
Temporary blocking of renal blood flow is required
in some complex renal surgeries, such as renal parenchymal
lithotomy, renal transplantation and renal tumor resection.
Postoperative reperfusion may lead to increased injury of ische mic
renal tissue, which is called renal ischemia-reperfusion injury
(RIRI) (1). RIRI can lead to renal
failure and acute renal insufficiency, causing high morbidity and
mortality. A study has shown that the mechanism of RIRI is very
complicated, and many internal factors, such as vascular
obstruction, inflammatory mediators, enhanced acidic environment
interstitial edema, as well as calcium and oxygen-free radical
damage, were involved (2). A study
showed that adaptive immune system and innate immunity play an
important role in RIRI. Immune system intervention may have a
protective effect on RIRI (3).
Rapamycin, as a novel immunosuppressive agent, can bind mammalian
target of rapamycin (mTOR) via receptor FK50-binding protein-12 to
inhibit its signal transduction, thereby reducing the arrest of
immune cells in late G1 and suppressing immune response (4). The protective mechanism of rapamycin on
ischemia-reperfusion injury is still unclear. In view of this, our
study investigated the protective effects of rapamycin on RIRI, and
explored the mechanism, so as to find a way to reduce renal
ischemia-reperfusion injury.
Materials and methods
Experimental animals and grouping
A total of 100 SPF C57BL/6 male mice weighing 20–25
g (8 weeks old) were purchased from Shanghai Laboratory Animal
Center, Chinese Academy of Sciences (Shanghai, China). The mice
were raised in cages at 20–25°C, with free access to food and
water. Mice were divided randomly into the normal control, sham
operation, model and experimental groups with 25 mice in each
group. The study was approved by the Ethics Committee of North
China University of Science and Technology (Tangshan, China).
Main reagents
Rapamycin was from Wyeth: Pfizer (Collegeville, PA,
USA); TUNEL Apoptosis Detection kit was purchased from Wuhan Boster
Biological Technology, Ltd. (Wuhan, China); RT-qPCR kit was
purchased from Takara Biotechnology Co., Ltd. (Dalian, China); rat
anti-mouse NKl.1 monoclonal antibody was purchased from R&D
Systems, Inc. (1:300; cat. no. 694370, Minneapolis, MN, USA).
Main equipment
Ultra-clean workbench (AIRTECH; Suzhou Purification
Equipment Co., Ltd., Suzhou, China); precision electronic balance
(Mettler-Toledo GmbH, Greifensee, Switzerland); UV
spectrophotometer (Shanghai Third Analytical Instrument Factory,
Shanghai, China); PCR instrument (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA); Cytomics™ FC 500 series flow cytometer and
AU680 automatic biochemical analyzer (both from Beckman Coulter,
Inc., Brea, CA, USA).
Animal model construction
RIRI model was constructed according to the methods
described in a previous study (5).
Mice were fasted for 12 h before operation but were allowed to
drink water. After anesthesia (injected with 10% chloral hydrate),
the mice were fixed in supine position. Skin was disinfected with
75% ethanol, and an incision was made along the middle line of the
abdomen. Left renal pedicle was separated and clipped with
non-invasive microvascular clip. Kidneys gradually changed from
bright red to dark purple. Clips were removed 30 min later, and the
rapid recovery of kidney color indicated that the model was
successfully established. The skin was closed and incision was
coated with bupivacaine for analgesia after operation. Bilateral
renal pedicle was not clipped in the sham operation group and other
steps were the same as the model group. Mice in the control group
were not treated.
Animal treatment
Rapamycin (3 mg/kg/day) gavage was performed to mice
in the experimental group 2 days and 1 day before surgical
operation and after successful establishment of the model. Mice in
each group were sacrificed at 24 h after operation, and blood,
spleen and left kidney were collected. The left kidney was cut into
two halves, one half was fixed in formaldehyde, and the other half
was stored in liquid nitrogen for RT-qPCR detection.
Semi-quantitative analysis of renal
pathological injury
After fixation, embedding, slicing and PAS staining,
semi-quantitative analysis of renal pathological injury was
performed. Ten visual fields of renal plexus junction were randomly
selected under high magnification microscope (Olympus, Tokyo,
Japan) (×400) to observe the degree of injury. Scoring standards
were: 0 for normal, lesion <25% for 1 point, 25–50% for 2
points, 51–75% for 3 points, and >75% for 4 points.
Renal function
Serum creatinine (SCr) and blood urea nitrogen (BUN)
were detected using Beckman Coulter AU680 automatic biochemical
analyzer.
Cell apoptosis test
Kidneys were fixed overnight in 4% paraformaldehyde
solution, and embedded in paraffin. TUNEL method was used to detect
cell apoptosis according to the instructions. Red fluorescence
under microscope (Olympus)indicated TUNEL-positive cells. Six
non-repetitive visual fields (×200) were randomly selected to
calculate the positive apoptotic index (AI) according to the
formula: AI = (apoptotic cells/total cells) ×100%.
Flow cytometry
Blood, spleen and kidney tissues of mice were used
to make single cell suspension. After incubation with rat
anti-mouse CD3 and NKl.1 monoclonal antibodies (1:300; cat. nos.
17A2 and 694370, R&D Systems, Inc., at 4°C for 1 h, flow
cytometry was used to detect the ratio of NKT cells. CD3 and NKl.1
double-positive cells were defined as NKT cells.
RT-qPCR detection of expression levels
of CXC chemokine ligand 10 (CXCL10), hypoxia-inducible factor-1α
(HIF-1α) and vascular endothelial growth factor (VEGF) mRNA
Total RNA was extracted from kidneys using TRIzol.
After purification, RNA samples were used in reverse transcription
to synthesize cDNA. Primers used in PCR reactions were: β-actin
forward, 5′-AGGCATCCTGACCCTGAAGTA-3′ and reverse,
5′-GAGGCATACAGGGACAACACAG-3′; CXCL10 forward,
5′-ACTGCATCCATATCGATGAC-3′ and reverse, 5′-TTCATCCTGCAATGATCTC-3′;
HIF-1α forward, 5′-AAGTCTAGGGATGCAGCAC-3′ and reverse,
5′-CAAGATCACCAGCATCTAG-3′; VEGF forward,
5′-ATTGAGACCCTGGTGGACATC-3′ and reverse, 5′-TCCTTTCCTCGAACTGATT-3′.
Expression level of each gene was normalized to endogenous control
β-actin using 2−ΔΔCq method (6).
Statistical analysis
Statistical analyses were performed using SPSS 18.0.
(SPSS, Inc., Chicago, IL, USA). Count data were processed by
χ2 test. Measurement data were expressed as (x±SD).
ANOVA was used for comparison between multiple groups and the post
hoc test was SNK test. were processed using parallel t-test.
P<0.05 was considered to be statistically significant.
Results
Semi-quantitative analysis of renal
pathological injury
Clear renal tubule and glomeruli structure, and no
obvious pathological changes were found in the control group. In
the model group, renal tubular structure was damaged, epithelial
cells were exfoliated and necrotic, basement membrane was exposed,
and vacuolar degeneration was also observed. Only a small number of
epithelial cells were exfoliated in the experimental group, and
proximal renal tubule swelling was also observed. Kidney injury was
significantly lower in the experimental group than in the model
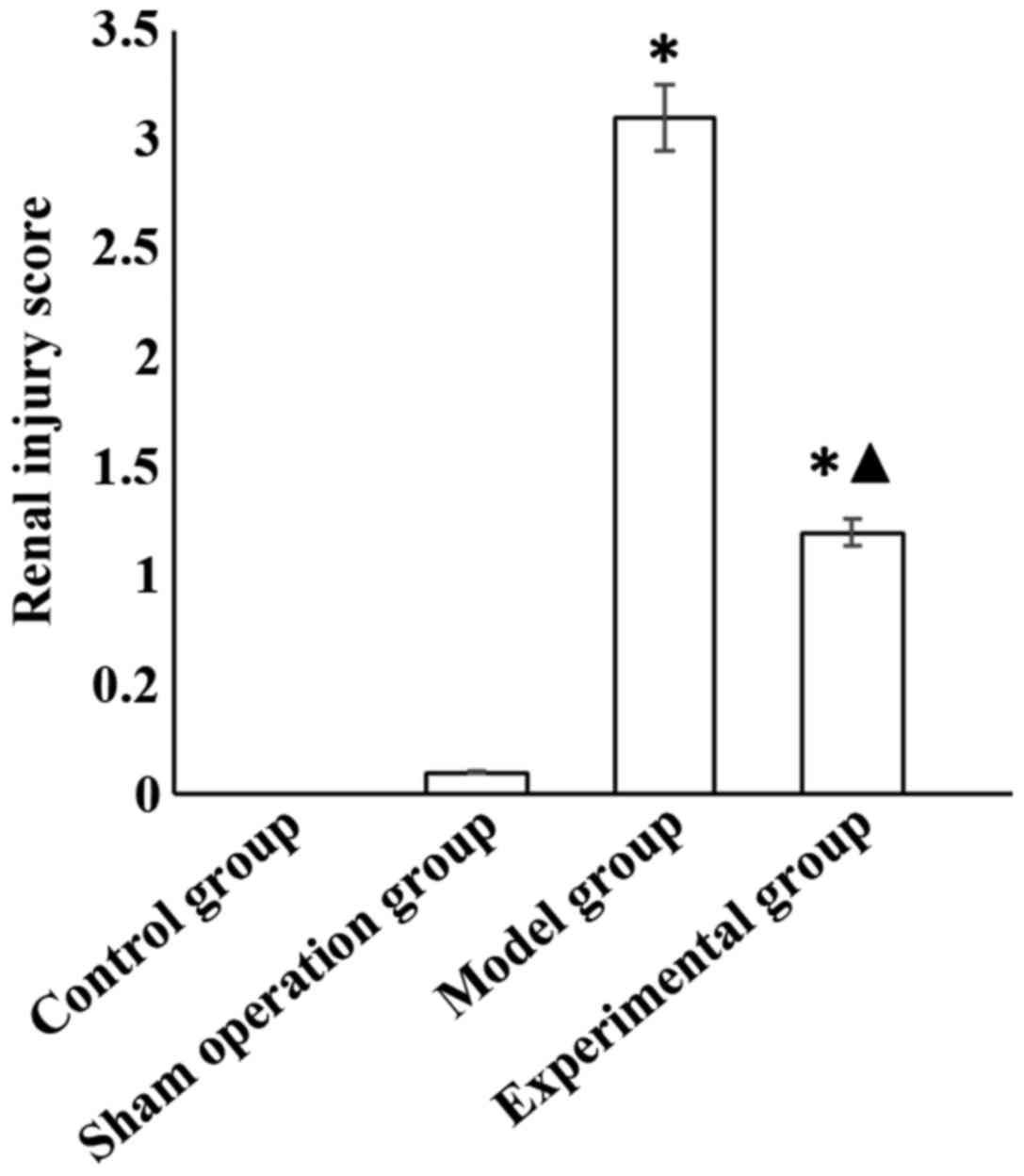
group. Renal injury score was higher in the model and experimental
groups than in the sham operation group (p<0.05), and the score
of the experimental group was significantly lower than that of the
model group (p<0.05) (Fig.
1).
Renal function
Serum levels of SCr and BUN in the model and
experimental groups were significantly higher than those in the
sham operation group (p<0.05), and were significantly lower in
the experimental group than those in the model group (p<0.05)
(Table I).
 | Table I.Comparison of serum levels of SCr and
BUN among groups (x±SD). |
Table I.
Comparison of serum levels of SCr and
BUN among groups (x±SD).
| Groups | SCr (µmol/l) | BUN (mmol/l) |
|---|
| Control | 18.56±5.26 | 13.02±3.01 |
| Sham operation | 20.12±4.35 | 14.58±2.98 |
| Model |
62.19±8.38a |
39.09±4.36a |
| Experimental |
39.85±7.45a,b |
25.35±3.86a,b |
Cell apoptosis
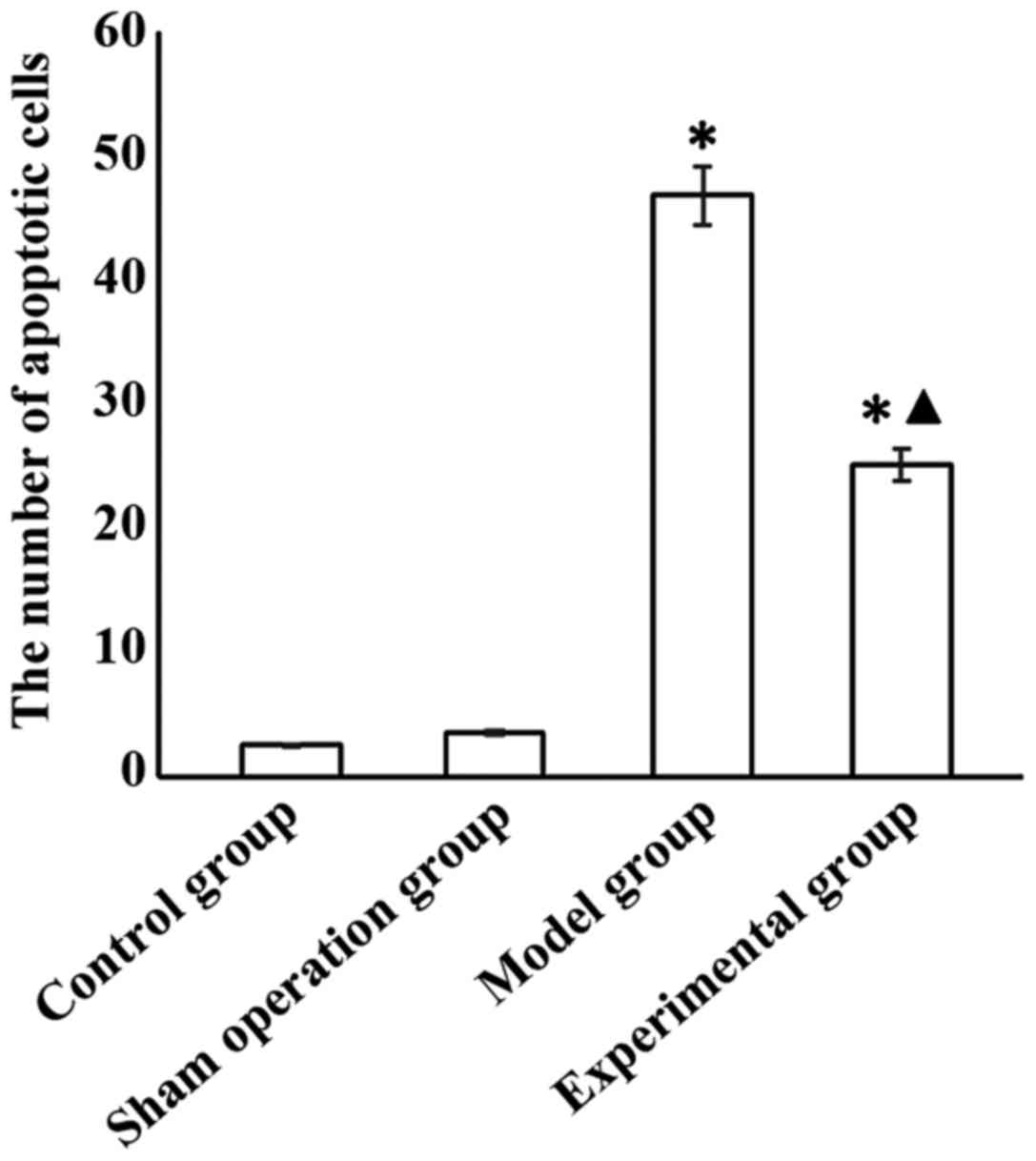
The number of apoptotic cells in the model and
experimental groups was significantly higher than that in sham
operation group (p<0.05), and was significantly lower in the
experimental than in the model group (p<0.05) (Fig. 2).
Percentage of NKT cells
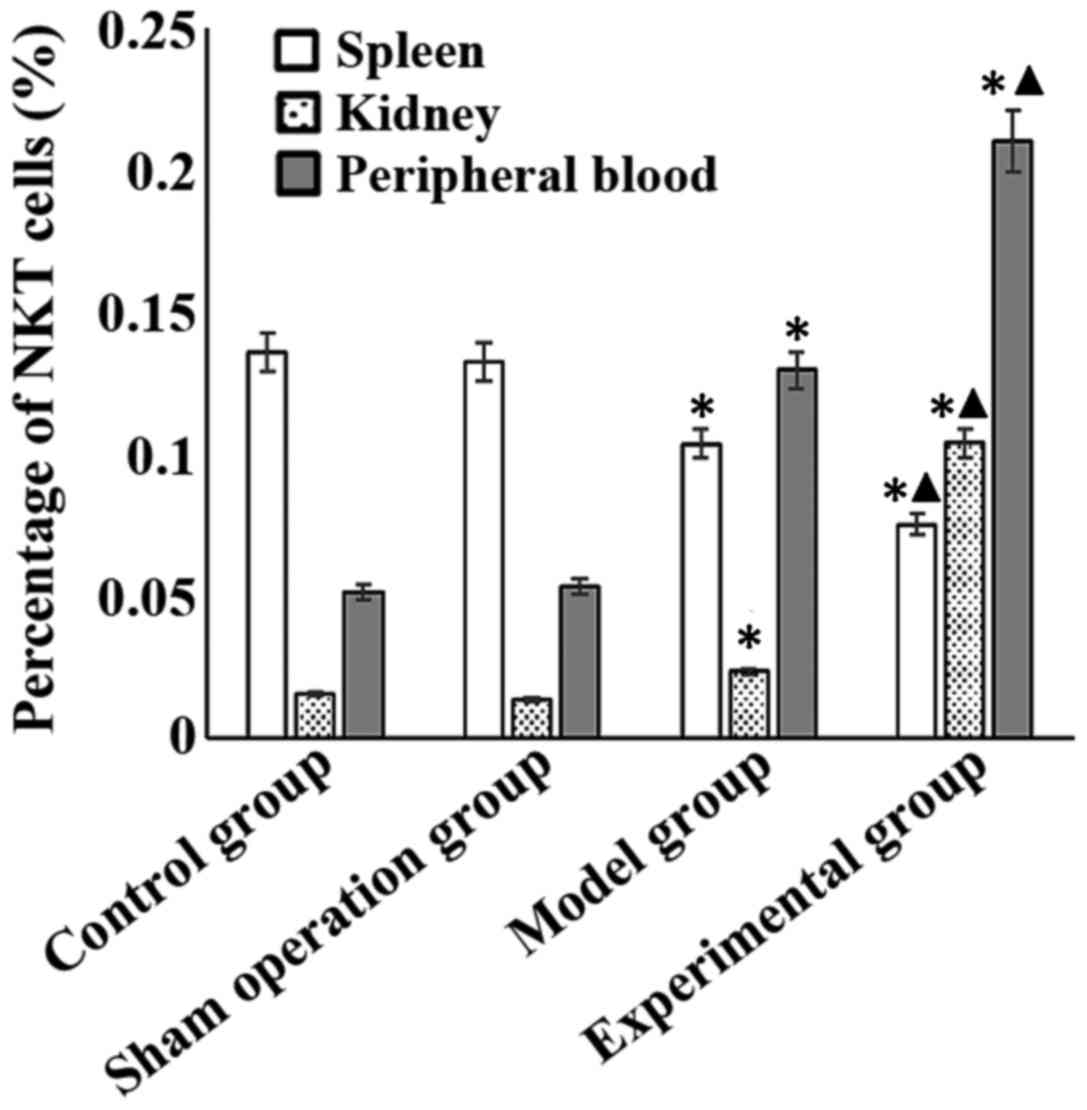
Percentage of NKT cells in spleen of the model and
experimental groups was significantly lower than that in the sham
operation group (p<0.05), and was significantly lower in the
experimental than that in the model group (p<0.05). Percentage
of NKT cells in kidney and peripheral blood of the model and
experimental groups was significantly higher than that in the sham
operation group (p<0.05), and was significantly higher in the
experimental than that in the model group (p<0.05) (Fig. 3).
Expression levels of CXCL10, HIF-1α
and VEGF mRNA in different groups
The expression levels of HIF-1α and VEGF mRNAs in
the model and experimental groups were significantly higher than
those in the sham-operated group (p<0.05), and were
significantly lower in the experimental group than those in the
model group (p<0.05). The expression level of CXCL10 mRNA in the
model and experimental groups was significantly lower than that in
the sham operation group (p<0.05), and was significantly higher
in the experimental group than that in the model group (p<0.05)
(Table II).
 | Table II.Expression levels of CXCL10, HIF-1α
and VEGF mRNA in the different groups (x±SD). |
Table II.
Expression levels of CXCL10, HIF-1α
and VEGF mRNA in the different groups (x±SD).
| Groups | CXCL10 | HIF-1α | VEGF |
|---|
| Control | 0.3589±0.040 | 0.0107±0.0005 | 0.0143±0.0012 |
| Sham operation | 0.3615±0.039 | 0.0106±0.006 | 0.0146±0.0011 |
| Model |
0.2315±0.041a |
0.0156±0.007a |
0.0198±0.0013a |
| Experimental |
0.2935±0.038a,b |
0.0123±0.008a,b |
0.0163±0.0014a,b |
Discussion
As a hyperperfusion organ, kidney is sensitive to
ischemia reperfusion. Pathogenesis of RIRI is very complex, and the
factors which affect RIRI can interact with each other. So the
mechanism of RIRI is still unclear. How to reduce RIRI is now a hot
research field. As a novel immunosuppressive agent, the effects of
rapamycin on kidney transplantation have been confirmed (7). However, the mechanism of the protective
effects of rapamycin on ischemia-reperfusion injury remains
controversial.
Results of this study showed that rapamycin could
significantly reduce the levels of SCr and BUN after renal
ischemia-reperfusion in mice to improve renal function, so as to
reduce the rate of tubular apoptosis. Histopathological examination
also confirmed that rapamycin has a protective effect on RIRI. Khan
et al (8) reported that
rapamycin had a protective effect on liver ischemia reperfusion
injury in mice, which is consistent with the results of our study.
Lui et al (9) reported that
in the early stage of RIRI, rapamycin could reduce the degree of
renal damage through its anti-apoptotic effect, and in the late
stage of RIRI, rapamycin could aggravate ischemia-reperfusion
injury by inhibiting tissue repair. Ischemia-reperfusion injury
refers to the increased degree of ischemic tissue injury after
reperfusion. Rapamycin is a macrolide antibiotic isolated from the
culture medium of actinomycetes. Besides the application in the
treatment of fungal infections and tumors, rapamycin has also been
used in organ transplantation (10).
Rapamycin blocks various signaling pathways through different
cytokine receptors and blocks the progression of lymphocytes from
the G1 phase to the S phase, thereby producing an immunosuppressive
effect (11,12).
NKT cell is a kind of immune cell. In recent years,
studies on NKT cell have attracted increasing attention. A previous
study showed that NKT cells play an important role in
ischemia-reperfusion injury, in which NKT cells can be recruited
from spleen through peripheral blood to target organ damage
(13). Results of our study showed
that the percentage of NKT cells in spleen of the model and
experimental groups was significantly lower than that in the sham
operation group (p<0.05), and the percentage was also
significantly lower in the experimental group than that in the
model group (p<0.05). The percentage of NKT cells in kidney and
peripheral blood of the model and experimental groups was
significantly higher than that in the sham operation group
(p<0.05), and the percentage was also significantly higher in
the experimental group than that in the model group (p<0.05),
indicating that rapamycin can promote moving of NKT cells from
spleen through the peripheral blood to kidney, leading to the
higher percentage of NKT cells in kidney after ischemia-reperfusion
injury, so as to protect the kidney. This may be one of the
mechanisms by which rapamycin exerts a protective effect on
RIRI.
HIF-1α is a cytokine that is widely distributed in
various tissues and is highly sensitive to the concentration of
oxygen. Under normoxic conditions, HIF-1α gene is continuously
transcribed and translated, but is also degraded by ubiquitous
degradation, so the level of HIF-1α in the body is very low
(14). Under hypoxic conditions, the
degradation of HIF-1α was inhibited and the level of HIF-1α was
increased. HIF-1α can form a dimer with HIF-1β to upregulate VEGF
expression, promote angiogenesis, and improve oxygen supply, so as
to make tissues adapt to hypoxia (15). Our data showed that the expression
levels of HIF-1α and VEGF mRNAs in the model and experimental
groups were significantly higher than those in the sham operation
group (p<0.05), and the expression levels in the experimental
group was significantly lower than those in the model group
(p<0.05), indicating that rapamycin may have an anti-apoptotic
effect by inhibiting the expression of HIF-1α. To date, >50
chemokines have been identified, among which chemokine CXC is a
group of cytokines closely related to angiogenesis. CXC can be
divided into ELR and non-ELR chemokines. The former has the
function of promoting angiogenesis, while the latter has the effect
on inhibiting angiogenesis (10).
Our data show that the CXCL10 mRNA expression level in the model
and experimental groups was significantly lower than that in the
sham operation group (p<0.05), and the expression level in the
experimental group was significantly higher than that in the model
group (p<0.05), indicating that rapamycin can significantly
upregulate the expression of CXCL9, which may be involved in the
recruitment of NKT cells to kidney after ischemia-reperfusion
injury (16).
In summary, rapamycin can significantly upregulate
the expression of CXCL9 and promote recruitment of NKT cells from
spleen through peripheral blood to kidney. Rapamycin can also
inhibit the expression of HIF-1α, so as to have a protective effect
on RIRI.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ and SL conceived and designed the study. JZ
collected the data. FC was responsible for the analysis and
interpretation of the data. WZ drafted this manuscript. SL revised
the manuscript critically for important intellectual content. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
North China University of Science and Technology (Tangshan,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zandstra J, van Beuge MM, Zuidema J,
Petersen AH, Staal M, Duque LF, Rodriguez S, Lathuile AA, Veldhuis
GJ, Steendam R, et al: Microsphere-based rapamycin delivery,
systemic versus local administration in a rat model of renal
ischemia/reperfusion injury. Pharm Res. 32:3238–3247. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malek M and Nematbakhsh M: Renal
ischemia/reperfusion injury; from pathophysiology to treatment. J
Renal Inj Prev. 4:20–27. 2015.PubMed/NCBI
|
|
3
|
Kezić A, Stajic N and Thaiss F: Innate
immune response in kidney ischemia/reperfusion injury: Potential
target for therapy. J Immunol Res. 2017:63054392017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu J, Lu T, Yue S, Shen X, Gao F,
Busuttil RW, Kupiec-Weglinski JW, Xia Q and Zhai Y: Rapamycin
protection of livers from ischemia and reperfusion injury is
dependent on both autophagy induction and mammalian target of
rapamycin complex 2-Akt activation. Transplantation. 99:48–55.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feldman ME, Apsel B, Uotila A, Loewith R,
Knight ZA, Ruggero D and Shokat KM: Active-site inhibitors of mTOR
target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol.
7:e382009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using realtime quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guan X, Qian Y, Shen Y, Zhang L, Du Y, Dai
H, Qian J and Yan Y: Autophagy protects renal tubular cells against
ischemia/reperfusion injury in a time-dependent manner. Cell
Physiol Biochem. 36:285–298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khan S, Salloum F, Das A, Xi L, Vetrovec
GW and Kukreja RC: Rapamycin confers preconditioning-like
protection against ischemia-reperfusion injury in isolated mouse
heart and cardiomyocytes. J Mol Cell Cardiol. 41:256–264. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lui SL, Chan KW, Tsang R, Yung S, Lai KN
and Chan TM: Effect of rapamycin on renal ischemia-reperfusion
injury in mice. Transpl Int. 19:834–839. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Connell BJ, Gordon JR and Saleh TM:
ELR-CXC chemokine antagonism is neuroprotective in a rat model of
ischemic stroke. Neurosci Lett. 606:117–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baroja-Mazo A, Revilla-Nuin B, Ramírez P
and Pons JA: Immunosuppressive potency of mechanistic target of
rapamycin inhibitors in solid-organ transplantation. World J
Transplant. 6:183–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, He S, Du X, Jiang Y, Tian B and
Xu S: Rapamycin suppresses hypoxia/reoxygenation-induced islet
injury by up-regulation of miR-21 via PI3K/Akt signalling pathway.
Cell Prolif. 50:1–8. 2017. View Article : Google Scholar
|
|
13
|
Zimmerman MA, Martin A, Yee J, Schiller J
and Hong JC: Natural killer T cells in liver ischemia-reperfusion
injury. J Clin Med. 6:412017. View Article : Google Scholar
|
|
14
|
Hagen T: Oxygen versus reactive oxygen in
the regulation of HIF-1α: The balance tips. Biochem Res Int.
2012:4369812012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Coothankandaswamy V, Liu Y, Mao SC, Morgan
JB, Mahdi F, Jekabsons MB, Nagle DG and Zhou YD: The alternative
medicine pawpaw and its acetogenin constituents suppress tumor
angiogenesis via the HIF-1/VEGF pathway. J Nat Prod. 73:956–961.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Javedan G, Shidfar F, Davoodi SH, Ajami M,
Gorjipour F, Sureda A, Nabavi SM, Daglia M and Pazoki-Toroudi H:
Conjugated linoleic acid rat pretreatment reduces renal damage in
ischemia/reperfusion injury: Unraveling antiapoptotic mechanisms
and regulation of phosphorylated mammalian target of rapamycin. Mol
Nutr Food Res. 60:2665–2677. 2016. View Article : Google Scholar : PubMed/NCBI
|