Introduction
Multiple Myeloma (MM) is a plasma cell malignancy.
To date, MM remains an incurable disease (1). Despite the development of novel drugs
for MM, patients have a median survival of 5 years due to the
resistance to these agents (1).
Identifying novel targets for MM treatment is crucial. The
hepatocyte growth factor (HGF) has been demonstrated to promote MM
cell growth (2). Binding of HGF to
the tyrosine-protein kinase (c-Met) receptor activates a signaling
cascade, which regulates growth and survival of cancer cells
(3). c-Met and its ligand HGF are
overexpressed in the human MM cell lines JJN-3, U-266, OH-2, JW and
primary human MM cells (4).
Additionally, HGF/c-Met signaling promotes survival and drug
resistance of MM cells in vitro and in vivo (5). Blocking of the HGF/c-Met signaling
pathway inhibits MM cell proliferation (6,7).
NK4, a splice variant of HGF in which the heavy
chain consists of the N-terminal domain and the four kringle
domains, is a specific antagonist of HGF that competes with HGF for
c-Met receptor binding to inhibit interactions between HGF and
c-Met (8). For the current study,
the RPMI 8226 MM cell line in which HGF and c-Met are highly
expressed was chosen and adenovirus-mediated overexpression of NK4
was studied to examine antimyeloma effects of NK4 and to
investigate underlying mechanisms.
Materials and methods
Cell culture and transduction
RPMI 8226 cells were purchased from American Type
Culture Collection (Manassas, VA, USA) and cultured in RPMI 1640
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified atmosphere containing 5%
CO2. The construction of adenovirus (Ad) vectors Ad-NK4
and Ad-green fluorescent protein (GFP) was performed as described
previously (9). Transduction of RPMI
8226 cells with Ad-NK4 or Ad-GFP (Ad-Control) was conducted
according to a previously reported protocol (10). Cells were collected 48 h after
transduction for further analysis.
Reverse transcription polymerase chain
reaction (RT-PCR) analysis
Total RNA was isolated from untreated,
Ad-NK4-transduced and Ad-Control-transduced RPMI 8226 cells using
TRIzol reagent (Beyotime Institute of Biotechnology, Haimen,
China). mRNA was reverse transcribed to cDNA and the PCR was
performed using the Thermoscript RT-PCR System kit (Gibco; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocols.
Primers were as follows: NK4 forward 5′-CACGGAAGAGGAGATGAGA-3′ and
reverse 5′-AGGCCTGGCAAGCTTCATTA-3′; GAPDH forward
5′-AGCCTCAAGATCATCAGC-3′ and reverse 5′-GAGTCCTTCCACGATACC-3′. PCR
amplification consisted of initial denaturation at 94°C for 2 min,
then 35 cycles as follows: 15 sec at 94°C for denaturation, 30 sec
at 58°C for annealing and 45 sec at 72°C for elongation. Bands
separated on 1.5% agarose gels were stained by ethidium bromide and
quantified with an image analyzer using Gel-Pro analyzer 4.0
software (Media Cybernetics, Rockville, MD, USA).
Cell proliferation assay
Untreated, Ad-NK4-transduced and
Ad-Control-transduced RPMI 8226 cells were seeded in quadruplicate
at a density of 2×103 cells/well in 96-well plates and
cultured at 37°C for 24, 48 and 72 h. RPMI 1640 medium without
cells was provided as the blank control. Subsequently, 20 µl MTT
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) substrate (5 mg/µl
in PBS) was added to each well and the plates were incubated at
37°C for 4 h. Following a centrifugation at 400 × g at 4°C for 5
min, supernatants were carefully removed and 200 µl dimethyl
sulfoxide was added to each well. Insoluble crystals were dissolved
and a colorimetric analysis was performed at 570 nm. The inhibition
rate was calculated as follows: 100%-[optical density (OD) Ad-NK4
group/OD blank control]%.
Flow cytometry analysis
A total of 48 h after transduction, 2×105
untreated, Ad-NK4-transduced and Ad-Control-transduced RPMI 8226
cells were washed twice with ice-cold PBS and fixed with 70%
ethanol at 4°C overnight. Following washing with PBS, cells were
incubated in 0.5 ml PBS containing 50 µg/ml RNase A for 30 min at
37°C and then propidium iodide (PI) was added to achieve the final
concentration of 50 µg/ml and incubated for 30 min on ice in the
dark following the protocols of a propidium iodide staining kit
(Sigma-Aldrich; Merck KGaA). Samples were subjected to flow
cytometry analysis (Coulter Epics XL; Beckman Coulter, Inc., Brea,
CA, USA). The percentage of apoptotic cells (sub-G1) and cells in
the G0/G1, S and G2/M phases were
calculated.
Western blot analysis
Following transduction, untreated, Ad-NK4-transduced
and Ad-Control-transduced RPMI 8226 cells were collected by
centrifugation at 400 × g at 4°C for 5 min and lysed. Protein
concentration was determined using a bicinchoninic acid (BCA)
Protein Assay kit (Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol. Equal amounts of protein (20 µg/lane) were
separated on 10% SDS/PAGE gels and transferred onto polyvinylidene
fluoride membranes, which were blocked with TBST containing 5%
skimmed milk at 4°C overnight, and then incubated with primary
antibodies for cyclin D1 (cat. no. sc-56302), apoptosis regulator
Bcl-2 (Bcl-2; cat. no. sc-7382), apoptosis regulator BAX (Bax; cat.
no. sc-20067), cyclin-dependent kinase 4 (CDK4; cat. no. sc-70831),
phosphorylated (p)-serine/threonine-protein kinase mTOR (p-mTOR;
cat. no. sc-293133), NK4 (cat. no. sc-166724), cyclin-dependent
kinase inhibitor 1B (P27; cat. no. sc-53906; all 1:500; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), RAC-α
serine/threonine-protein kinase (Akt; cat. no. sc-5298), p-Akt
(cat. no. sc-271966; both 1:1,000; Santa Cruz Biotechnology, Inc.),
cleaved caspase-3 (cat. no. 9661), cleaved caspase-9 (cat. no.
9505), p-ribosomal protein S6 kinase β-1 (p70S6K; cat. no. 9208)
and p-eukaryotic initiation factor 4E binding protein 1 (cat. no.
9456; all 1:500; Cell Signaling Technology, Inc., Danvers, MA, USA)
for 2 h at room temperature. Following washing with TBST, membranes
were incubated with horseradish peroxidase-conjugated secondary
antibodies (cat. no. sc-2357; 1:1,500; Santa Cruz Biotechnology,
Inc.) for 2 h at room temperature. Blots were visualized by using
the enhanced chemiluminescence reagent kit (Beyotime Institute of
Biotechnology) and analyzed by using Quantity One 4.0 software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
DNA fragmentation assay
Following transduction, untreated, Ad-NK4-transduced
and Ad-Control-transduced RPMI 8226 cells were harvested by
centrifugation at 1,600 × g for 5 min at 4°C and washed twice using
cold PBS. DNA was extracted using the DNA ladder extraction kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's protocol and separated on 1% agarose gels. DNA
ladders were visualized using a Bio-Rad GelDoc XR System (Bio-Rad
Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation
and analyzed by SPSS software for Windows (version 19.0; IBM Corp.,
Armonk, NY, USA). All experiments were repeated at least three
times. The significance of differences between two groups was
analyzed by Student's t-test, and the significance of differences
among multiple groups was analyzed by analysis of variance followed
by post-hoc Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
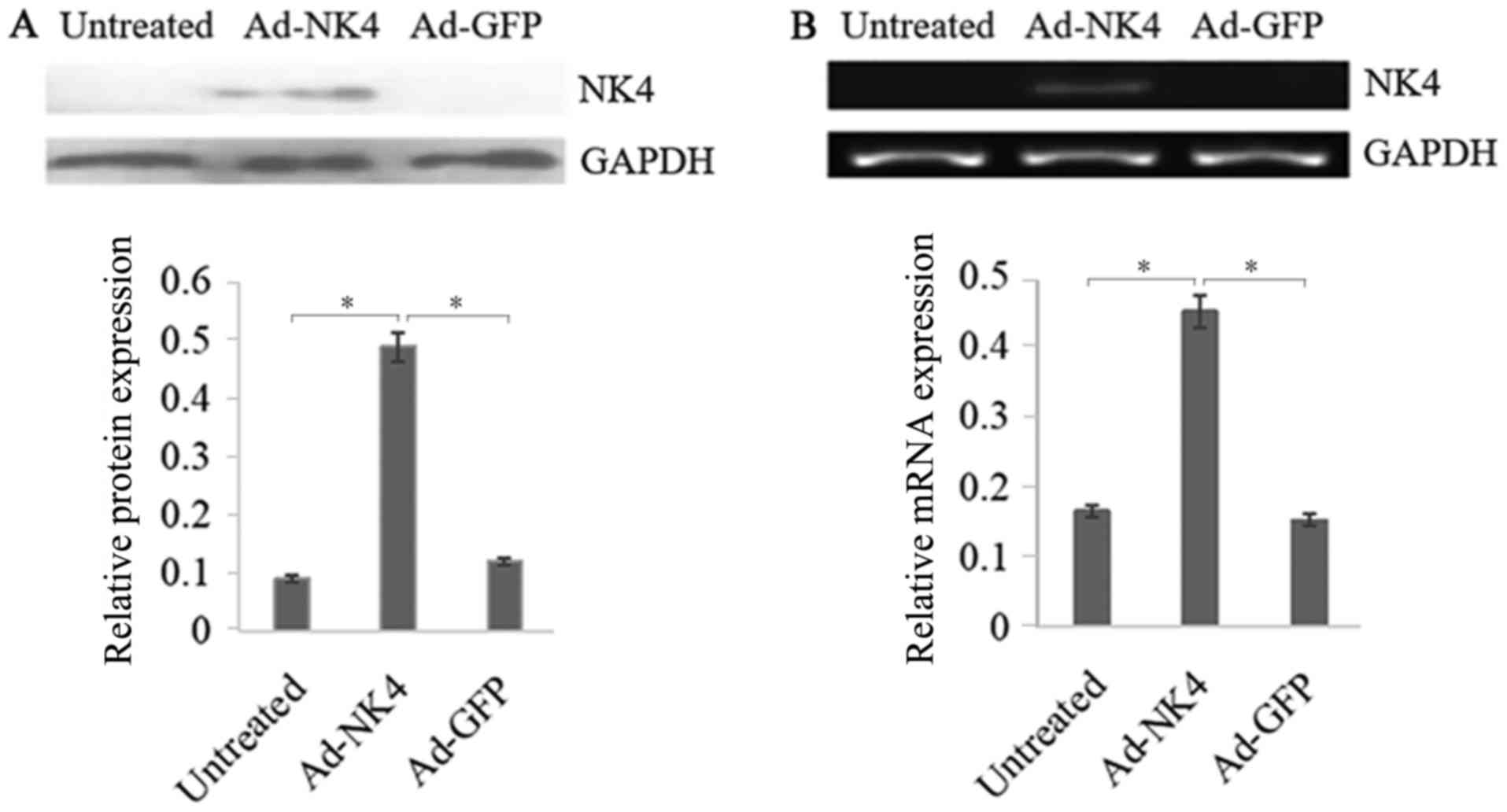
NK4 overexpression in RPMI 8226
cells
To confirm adenovirus-mediated overexpression of NK4
in RPMI 8226 cells, RT-qPCR and western blot analysis were
conducted. The results presented a significant increase in
overexpression of NK4 mediated by AD-NK4 in RPMI 8226 cells
compared with the untreated and Ad-GFP RPMI 8226 cells (Fig. 1).
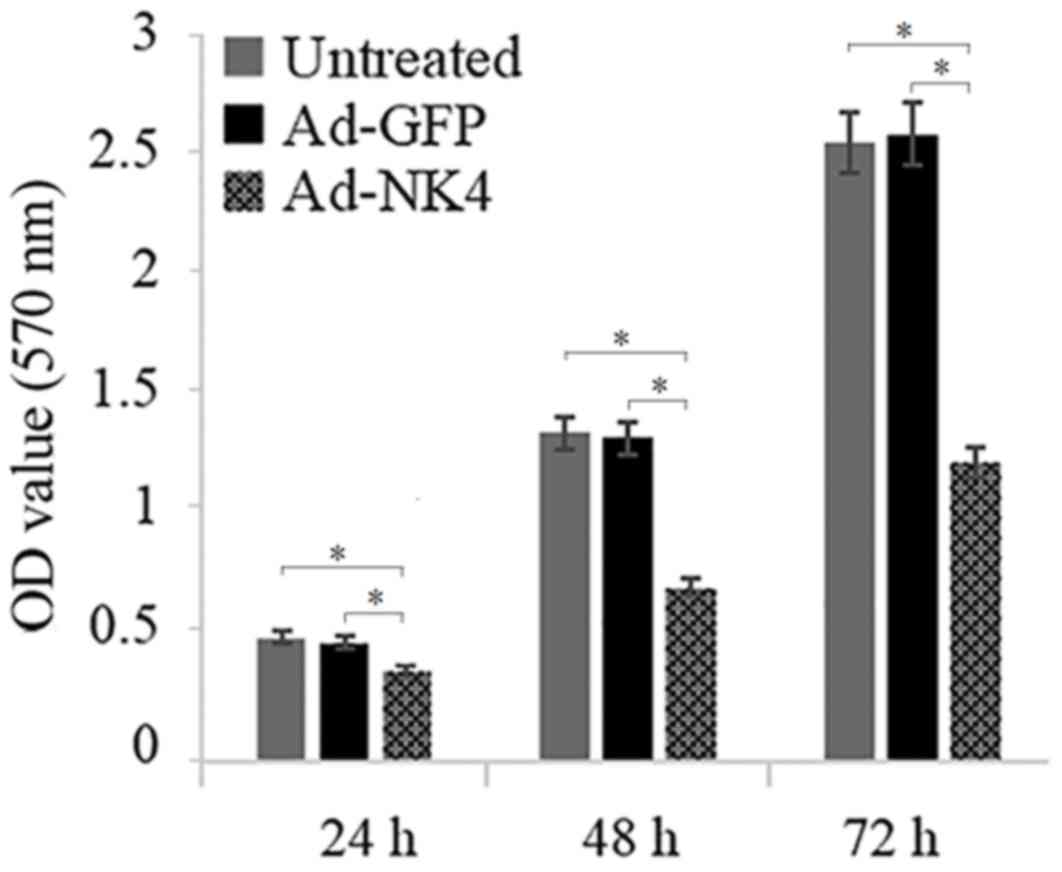
NK4 inhibits proliferation of RPMI
8226 cells
It was investigated whether NK4 decreased the
proliferation of RPMI 8226 cells. As presented in Fig. 2, compared with the RPMI 8226/Ad-GFP
and the untreated RPMI 8226 group, the proliferation ability of
RPMI 8226/Ad-NK4 was inhibited by 33.51±1.76%, 52.79±1.88% and
54.24±2.76% at 24, 48 and 72 h, respectively.
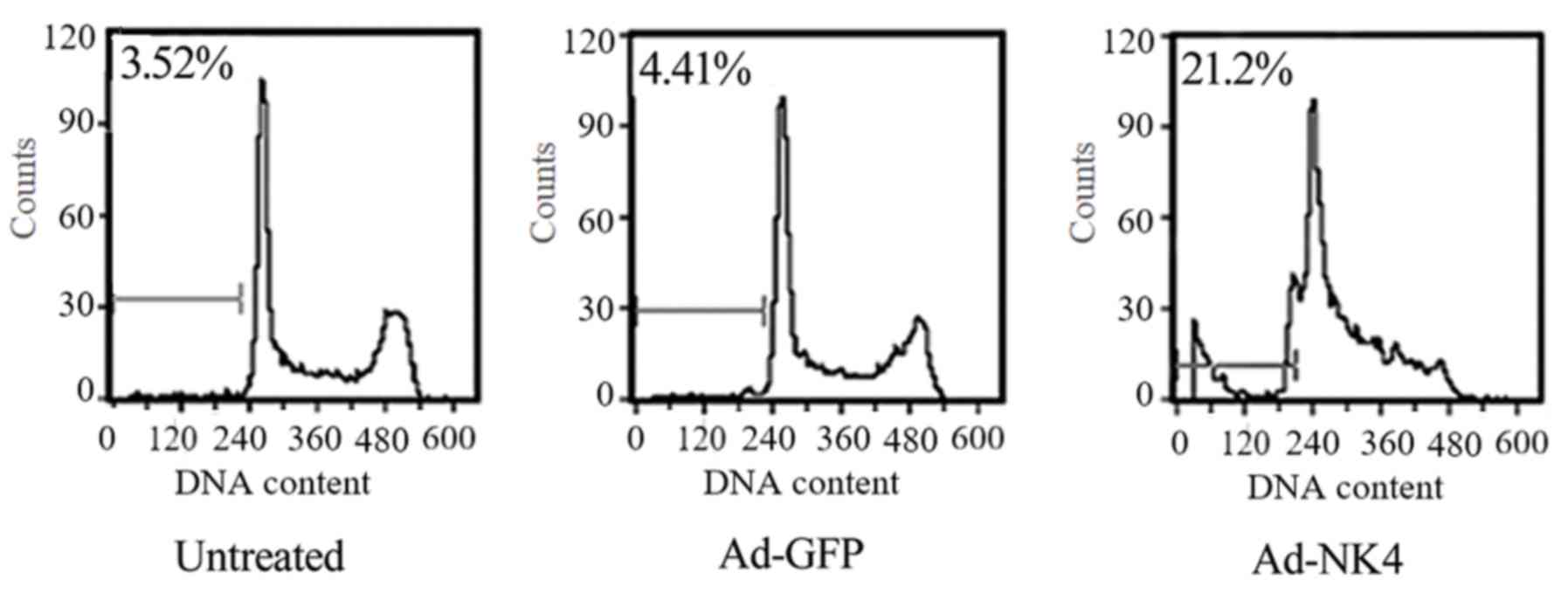
NK4 induces
G0/G1 arrest and apoptosis in RPMI 8226
cells
To elucidate how NK4 affects cell proliferation,
cell cycle and apoptosis were analyzed. The percentage of cells in
G0/G1 phase in RPMI 8226/Ad-NK4 (53.13±2.37%)
was increased significantly compared with the RPMI 8226/Ad-GFP
(24.41±1.76%) and the untreated RPMI 8226 group (21.18±1.98%; data
not shown). The percentage of cells in S phase in RPMI 8226/Ad-NK4
(23.12±1.65%) was decreased significantly compared with the RPMI
8226/Ad-GFP (47.31±1.87%) and the untreated RPMI 8226 group
(49.66±2.07%; data not shown). In order to determine the apoptosis
in RPMI 8226 cells, a flow cytometry-based analysis of PI-stained
cells and DNA fragmentation electrophoresis was performed. As
presented in Fig. 3, the apoptotic
rate in RPMI 8226/Ad-NK4 (21.23±1.76%) was increased significantly
compared with the RPMI 8226/Ad-GFP (4.51±0.29%) and the untreated
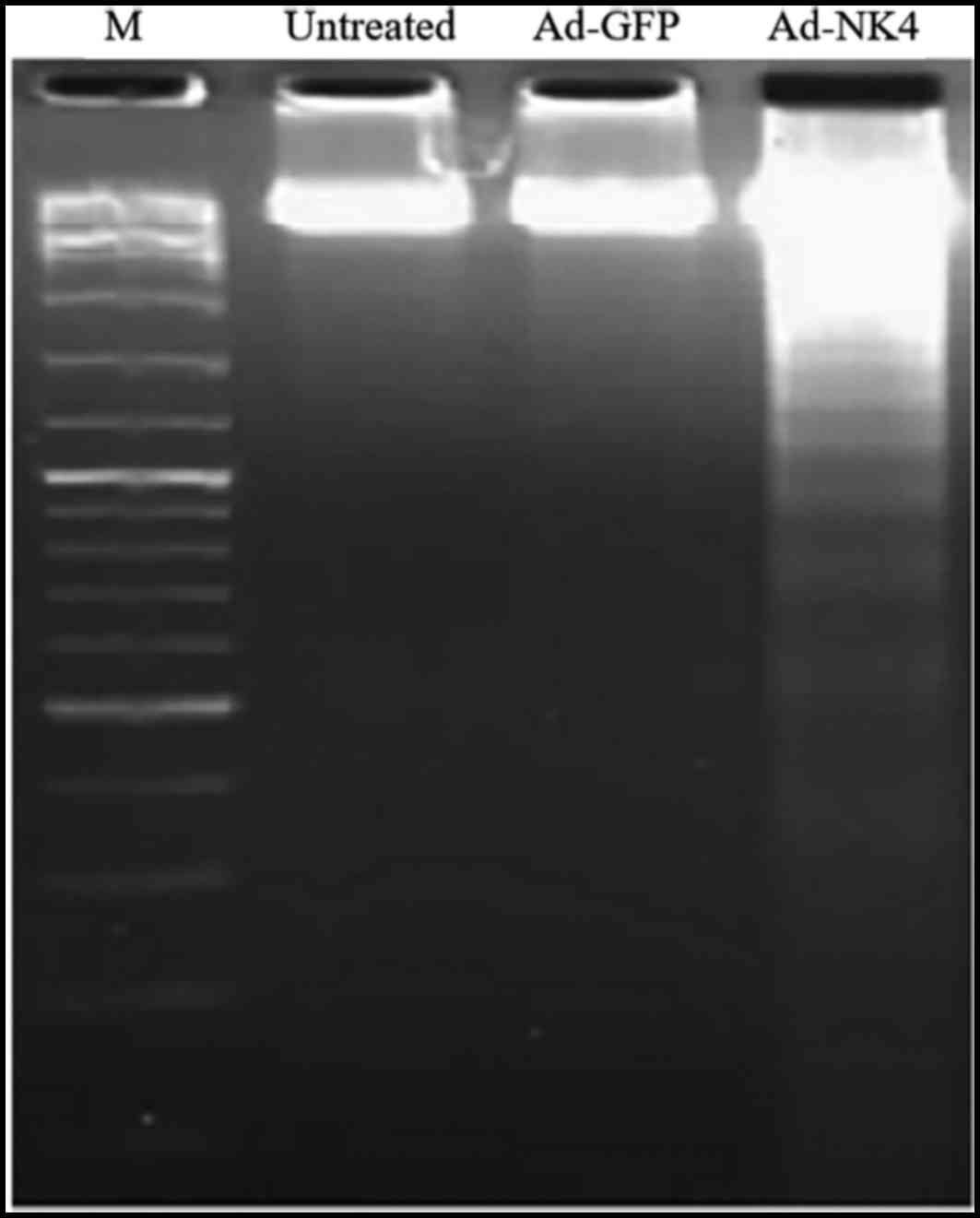
RPMI 8226 group (3.7±0.32%). The DNA fragmentation assay
demonstrated that DNA isolated from RPMI 8226 cells transduced by
Ad-NK4 ran like a ladder on agarose electrophoresis, indicating
apoptosis in these cells (Fig.
4).
NK4 regulates cell cycle and
apoptosis-associated proteins in RPMI 8226 cells
To further elucidate how NK4 affects cell cycle and
apoptosis, western blot analysis was performed. As presented in
Fig. 5A, protein levels of CDK4 and
cyclin D1 were significantly downregulated in RPMI 8226/Ad-NK4
compared with the RPMI 8226/Ad-GFP and the untreated RPMI 8226.
Protein levels of p27 were significantly upregulated in RPMI
8226/Ad-NK4 cells compared with the RPMI 8226/Ad-GFP and untreated
RPMI 8226 cells. As presented in Fig. 5B
and C, protein levels of Bax, cleaved caspase-9 and cleaved
caspase-3 significantly increased, whereas protein levels of Bcl-2
decreased in RPMI 8226/Ad-NK4 cells compared with the RPMI
8226/Ad-GFP and the untreated RPMI 8226 cells.
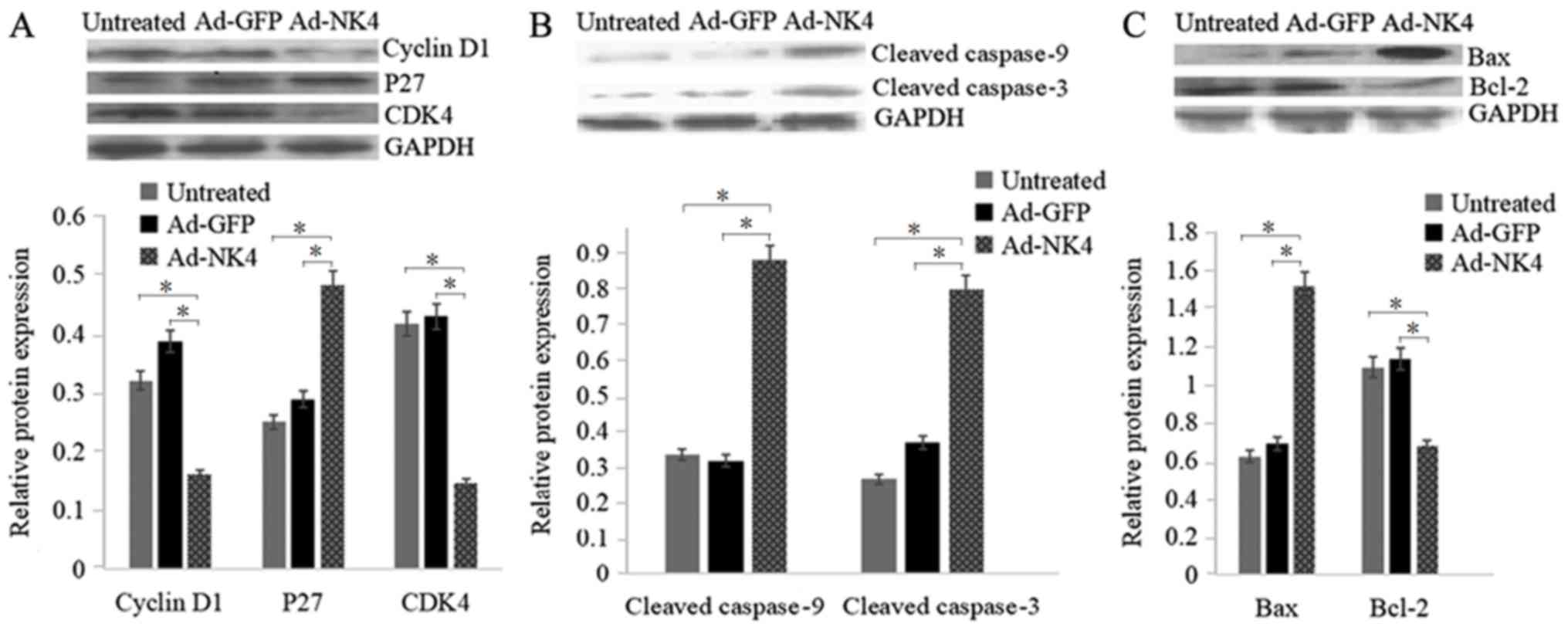 | Figure 5.NK4 modulates the expression of cell
proliferation and apoptosis-associated proteins. RPMI 8226 cells
were untreated, treated with Ad-GFP or Ad-NK4 for 48 h and protein
levels were detected by western blot analysis. (A) Cell
cycle-associated proteins, including cyclin D, P27 and CDK4. (B)
Apoptosis-associated proteins, including cleaved caspase 3 and 9.
(C) Proteins of the Bcl-2 family, including Blc-2 and Bax. GAPDH
served as loading control. Data were obtained from three
independent experiments. *P<0.05. Ad, adenovirus; GFP, green
fluorescent protein; P27, cyclin-dependent kinase inhibitor 1B;
CDK4, cyclin-dependent kinase 4; Bcl-2, apoptosis regulator Bcl-2;
Bax, apoptosis regulator BAX; NK4, a splice variant of hepatocyte
growth factor in which the heavy chain consists of the N-terminal
domain and the four kringle domains. |
NK4 regulates the Akt/mTOR signaling
pathway in RPMI 8226 cells
To clarify the role of NK4 on the Akt/mTOR signaling
pathway and the proliferation and apoptosis of RPMI 8226 cells,
western blot analysis was performed to validate the status of
Akt/mTOR signaling. As presented in Fig.
6, levels of p-AKT, p-mTOR, p-p70S6K and p-4BEP-1 significantly
decreased in RPMI 8226/Ad-NK4 cells compared with the RPMI
8226/Ad-GFP and the untreated RPMI 8226 cells. No significant
difference in the expression level of total Akt in RPMI 8226/Ad-NK4
cells compared with the RPMI 8226/Ad-GFP and the untreated RPMI
8226 cells was observed.
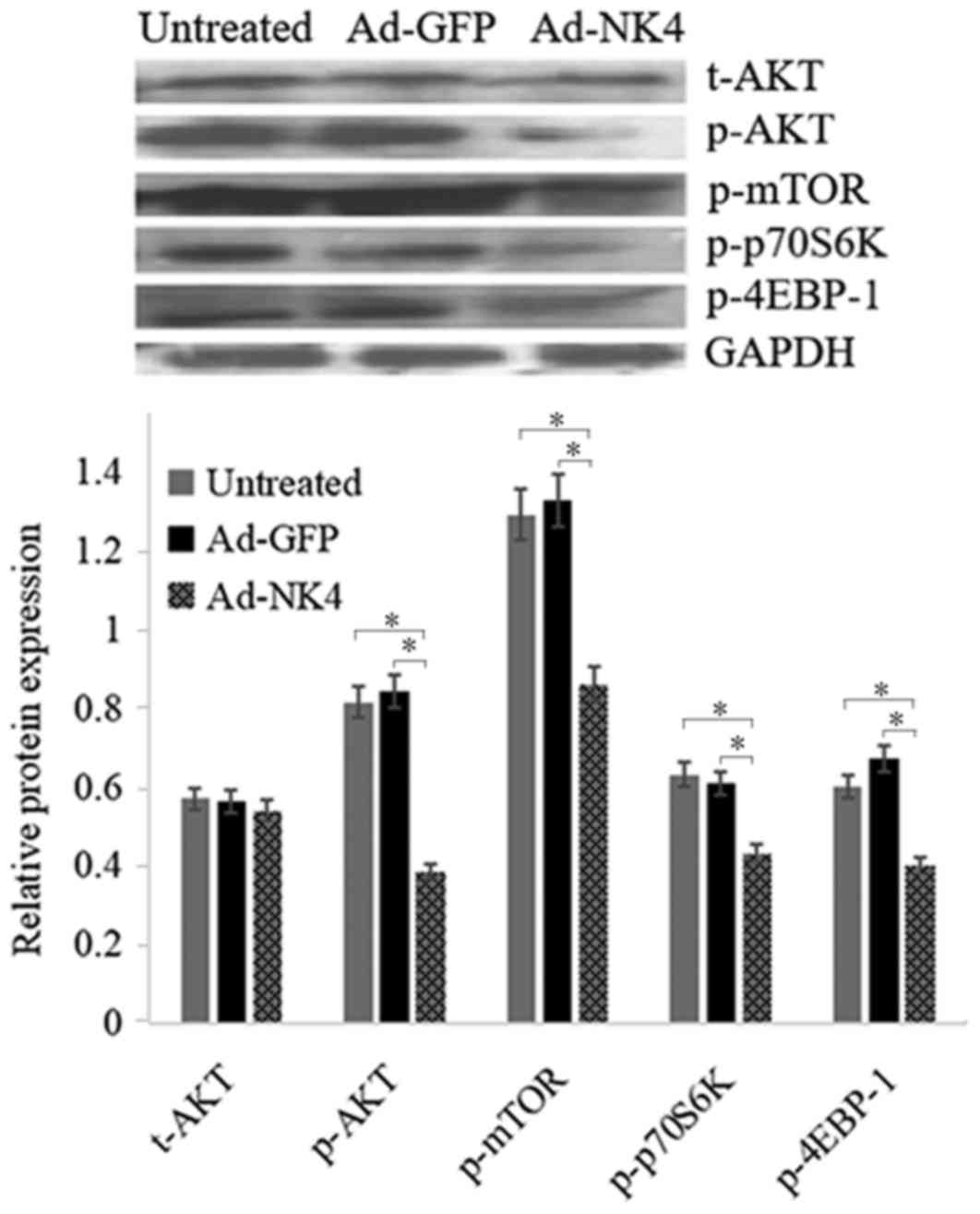 | Figure 6.NK4 inhibits the Akt/mTOR signaling
pathway. RPMI 8226 cells were untreated, treated with Ad-GFP or
Ad-NK4 for 48 h and protein levels of t-Akt, p-Akt, p-mTOR,
p-p70S6K and p-4EBP-1 were detected by western blot analysis. GAPDH
served as loading control. Data were obtained from three
independent experiments. *P<0.05. Ad, adenovirus; GFP, green
fluorescent protein; p, phosphorylated; Akt, RAC-α
serine/threonine-protein kinase; mTOR, serine/threonine-protein
kinase mTOR; p70S6K, ribosomal protein S6 kinase beta-1; 4E-BP1,
eukaryotic initiation factor 4E binding protein 1; t-Akt, total
Akt; NK4, a splice variant of hepatocyte growth factor in which the
heavy chain consists of the N-terminal domain and the four kringle
domains. |
Discussion
Previous studies indicated that NK4 inhibits
proliferation and induces apoptosis in several cancer cell lines
(11–13). However, antimyeloma activities of NK4
remain to be identified. The current study demonstrated that NK4
possessed antimyeloma effects in vitro. NK4 significantly
inhibited the proliferation of human MM RPMI 8226 cells. However,
Ad-mediated overexpression of NK4 was not sufficient to completely
inhibit the proliferation of MM RPMI 8226 cells, as treated cells
still grew at 72 h. It has previously been reported that Ad-NK4
enhances the chemosensitivity of MM RPMI 8226 cells to bortezomib,
the first proteasome inhibitor to be used as anti-cancer drug
(10). These results suggest that a
combined therapy with Ad-NK4 and chemotherapeutic agents may be an
effective treatment of MM.
Cell proliferation inhibition is known to be
associated with the induction of apoptosis and cell cycle arrest
(14,15). Flow cytometry analysis in the present
study demonstrated that NK4 induced apoptosis and cell cycle arrest
at G0/G1 in RPMI 8226 cells. A DNA fragmentation assay confirmed
the induction of apoptosis by NK4. Members of the Bcl-2 family,
including anti- and proapoptotic regulators, serve key roles in
cell survival and apoptosis (16).
It was reported that NK4 increased 5-fluorouracil sensitivity in
cholangiocarcinoma cells by modulating the expression of the Bcl-2
family (17). In the present study,
the antiapoptotic protein Bcl-2 was downregulated, whereas
proapoptotic protein Bax was upregulated by NK4, suggesting that
NK4 modulated the expression of Bcl-2 family members in RPMI 8226
cells. Caspase family proteins are executors of apoptosis (18). The present study demonstrated that
the expression of cleaved caspase-3 and caspase-9 significantly
increased in NK4-treated RPMI 8226 cells compared with untreated or
Ad-GFP-treated cells. In addition, the expression levels of cyclin
D1 and CDK4, two cell cycle-promoting proteins, were downregulated
whereas the levels of p27, a growth suppressor protein, were
upregulated by NK4, suggesting that NK4 induced cell cycle arrest.
In conclusion, these results demonstrated that NK4 induced
apoptosis and arrested cell cycle in RPMI 8226 cells.
Using recombinant Ad containing NK4 cDNA, Du et
al (19) reported that NK4
inhibits growth and induces apoptosis in MM cells. These effects
may be associated with the inhibition of the activation of c-Met,
extracellular signal-regulated kinase1/2, signal transducer and
activator of transcription factor 3 and Akt (19). Akt/mTOR signaling serves a crucial
role in the regulation of cell proliferation, cell cycle and
apoptosis (20). A previous study
revealed that knockdown of c-Met enhances the sensitivity to
bortezomib in human MM U266 cells by inhibiting Akt/mTOR activation
Therefore, in the present study, the mechanism by which NK4
exhibits anti-MM activity was examined and the results demonstrated
that NK4 may inhibit Akt⁄mTOR signaling in MM cells.
In conclusion, it was demonstrated that NK4
inhibited cell proliferation by inducing apoptosis and cell cycle
arrest in RPMI 8226 cells. The apoptotic effect of NK4 may be
associated with decreased Bcl-2 level, increased Bax and cleaved
caspase 3 and 9 levels and inhibiting Akt/mTOR signaling in RPMI
8226 cells. These findings suggest that NK4 may be a potential
agent for the treatment of MM. Further investigations are necessary
to explore the detailed mechanism of the underlying apoptotic
effect of NK4 on human MM cells both in vitro and in
vivo.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81500167) and Fujian
Provincial Clinical Key Subject Construction Project.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WQ and SX designed the study. WQ, HL and QY
performed the experiments. All authors read and approved the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bergsagel PL, Mateos MV, Gutierrez NC,
Rajkumar SV and San Miguel JF: Improving overall survival and
overcoming adverse prognosis in the treatment of cytogenetically
high-risk multiple myeloma. Blood. 121:884–892. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zaman S, Shentu S, Yang J, He J, Orlowski
RZ, Stellrecht CM and Gandhi V: Targeting the pro-survival protein
MET with tivantinib (ARQ 197) inhibits growth of multiple myeloma
cells. Neoplasia. 17:289–300. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Borset M, Lien E, Espevik T, Helseth E,
Waage A and Sundan A: Concomitant expression of hepatocyte growth
factor/scatter factor and the receptor c-MET in human myeloma cell
lines. J Biol Chem. 271:24655–24661. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mahtouk K, Tjin EP, Spaargaren M and Pals
ST: The HGF/MET pathway as target for the treatment of multiple
myeloma and B-cell lymphomas. Biochim Biophys Acta. 1806:208–219.
2010.PubMed/NCBI
|
|
5
|
Que W, Chen J, Chuang M and Jiang D:
Knockdown of c-Met enhances sensitivity to bortezomib in human
multiple myeloma U266 cells via inhibiting Akt/mTOR activity.
APMIS. 120:195–203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Que W and Chen J: Knockdown of c-Met
inhibits cell proliferation and invasion and increases
chemosensitivity to doxorubicin in human multiple myeloma U266
cells in vitro. Mol Med Rep. 4:343–349. 2011.PubMed/NCBI
|
|
7
|
Ferrucci A, Moschetta M, Frassanito MA,
Berardi S, Catacchio I, Ria R, Racanelli V, Caivano A, Solimando
AG, Vergara D, et al: A HGF/cMET autocrine loop is operative in
multiple myeloma bone marrow endothelial cells and may represent a
novel therapeutic target. Clin Cancer Res. 20:5796–5807. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mizuno S and Nakamura T: HGF-MET cascade,
a key target for inhibiting cancer metastasis: The impact of NK4
discovery on cancer biology and therapeutics. Int J Mol Sci.
14:888–919. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Que W and Chen J: Gateway technical
supported construction of a recombinant adenovirus vector pAd-NK4.
Chinese Pharmacol Bulletin. 27:467–472. 2011.
|
|
10
|
Que W and Chen J: Ad-NK4 enhances the
chemosensitivity of human multiple myeloma RPMI 8226 cells to
bortezomib. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 24:1079–1085.
2016.(In Chinese). PubMed/NCBI
|
|
11
|
Deng XB, Xiao L, Wu Y, Jin F, Mossman B,
Testa JR and Xiao GH: Inhibition of mesothelioma cancer stem-like
cells with adenovirus-mediated NK4 gene therapy. Int J Cancer.
137:481–490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramanujum R, Lin YL, Liu JK and He S:
Regulatory expression of MMP-8/MMP-9 and inhibition of
proliferation, migration and invasion in human lung cancer A549
cells in the presence of HGF variants. Kaohsiung J Med Sci.
29:530–539. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun YP, Zhang BL, Duan JW, Wu HH, Wang BQ,
Yu ZP, Yang WJ, Shan YF, Zhou MT and Zhang QY: Effect of NK4
transduction in bone marrow-derived mesenchymal stem cells on
biological characteristics of pancreatic cancer cells. Int J Mol
Sci. 15:3729–3745. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han L, Zhang Y, Liu S, Zhao Q, Liang X, Ma
Z, Gupta PK, Zhao M and Wang A: Autophagy flux inhibition, G2/M
cell cycle arrest and apoptosis induction by ubenimex in glioma
cell lines. Oncotarget. 8:107730–107743. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lima KG, Krause GC, da Silva EFG, Xavier
LL, Martins LAM, Alice LM, da Luz LB, Gassen RB, Filippi-Chiela EC,
Haute GV, et al: Octyl gallate reduces ATP levels and Ki67
expression leading HepG2 cells to cell cycle arrest and
mitochondria-mediated apoptosis. Toxicol In Vitro. 48:11–25. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kale J, Osterlund EJ and Andrews DW: BCL-2
family proteins: Changing partners in the dance towards death. Cell
Death Differ. 25:65–80. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ge X, Wang Y, Li Q, Yu H, Ji G and Miao L:
NK4 regulates 5-fluorouracil sensitivity in cholangiocarcinoma
cells by modulating the intrinsic apoptosis pathway. Oncol Rep.
30:448–454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang T, Ji D, Wang P, Liang D, Jin L, Shi
H, Liu X, Meng Q, Yu R and Gao S: The atypical protein kinase RIOK3
contributes to glioma cell proliferation/survival,
migration/invasion and the AKT/mTOR signaling pathway. Cancer Lett.
415:151–163. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Du W, Hattori Y, Yamada T, Matsumoto K,
Nakamura T, Sagawa M, Otsuki T, Niikura T, Nukiwa T and Ikeda Y:
NK4, an antagonist of hepatocyte growth factor (HGF), inhibits
growth of multiple myeloma cells: Molecular targeting of angiogenic
growth factor. Blood. 109:3042–3049. 2007.PubMed/NCBI
|
|
20
|
Lv XY, Ma L, Chen JF, Yu R, Li Y, Yan ZJ,
Cheng Y and Ma Q: Knockdown of DUXAP10 inhibits proliferation and
promotes apoptosis in bladder cancer cells via PI3K/Akt/mTOR
signaling pathway. Int J Oncol. 52:288–294. 2018.PubMed/NCBI
|