Introduction
Diabetes mellitus (DM) is a metabolic disease that
is characterized by insulin secretion defects (1,2). DM is
also an heterogeneous disease, which can be classified into type 1
and type 2 DM (3). Diabetic
cardiomyopathy is often caused by metabolic disorders and
microvascular lesions in patients with DM, and diabetic
cardiomyopathy is responsible for inflammation and apoptosis of
myocardial tissue (2–4). Diabetic cardiomyopathy is a major risk
factor for the development of insulin resistance in patients with
DM (5). The occurrence of
hyperglycemia and metabolic syndrome may lead to the initiation and
development of diabetic cardiomyopathy in patients with DM
(6). These metabolic imbalances
induced by diabetic cardiomyopathy may result in disturbed
metabolism (7). Therefore, it is
essential to understand the potential mechanism of DM to treat
DM-induced metabolic syndrome in patients.
Tanshinone IIA (Tan-IIA) is a traditional Chinese
medicine, which is extracted from Danshen (Salvia
miltiorrhiza) and has been clinically used for the treatment of
human cardiovascular and inflammatory diseases (8,9). A
previous study demonstrated that Tan-IIA can inhibit human cancer
cell growth, and affects cell cycle regulation, cell proliferation,
apoptosis and DNA synthesis (10).
In addition, Tan-IIA significantly reduces
lipopolysaccharide-induced acute lung injury by inhibiting
inflammation and apoptosis in mice (11). Furthermore, renal fibrosis and
inflammation can be attenuated by Tan-IIA via altering the
expression of transforming growth factor-β/Smad and nuclear factor
(NF)-κB signaling pathways in nephrectomized rats (12). Notably, Tan-IIA may serve a
protective function against renal damage in type 2 DM through
improving renal function, which could be a new evidence-based
therapy for diabetic nephropathy (13).
In the present study, based on the link between
inflammation and DM, it was assumed that Tan-IIA may have
beneficial effects on type 2 DM rats. The potential mechanisms
mediated by Tan-IIA were investigated in a type 2 DM rat model. To
the best of our knowledge, this is the first study to demonstrate
that Tan-IIA inhibits inflammation and alleviates type 2 DM
symptoms through NF-κB-induced 5′ adenosine monophosphate-activated
protein kinase (AMPK) signaling in experimental rats.
Materials and methods
Ethics statement
The protocols were approved by the Ethics Committee
of Sichuan Province People's Hospital and Sichuan Academy of
Medical Sciences (Chengdu, China). Anesthesia was administered
using intravenous sodium pentobarbital (35 mg/kg, Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany).
Animal model
A total of 20 male Sprague-Dawley (SD) rats (age,
6–8 weeks; body weight, 320–340 g) were purchased from Charles
River Laboratories (Beijing, China). All rats were housed in a
temperature-controlled room (25±1°C) with a 12-h light/dark cycle.
All rats were given accessed to food and water ad libitum.
Type 2 DM was induced in SD rats (n=20) using streptozotocin and
high-fat diet, as described previously (14). The experimental rats with type 2 DM
were then divided into two groups (n=10 in each) and received
Tan-IIA (10 mg/kg; Sigma-Aldrich; Merck KGaA) or PBS (10 mg/kg, 120
µl) by intragastric administration once every 2 days. Healthy male
SD rats (n=10; age, 6–8 weeks; body weight, 320–340 g; Beijing
University, Beijing, China) that did not receive treatment were
used as controls and kept under the same conditions as the
experimental rats. The treatments were continued for 8 weeks, and
kidney weight (KW) and body weight (BW) were measured at the end of
week 8. Total cholesterol (TC), non-esterified fatty acids (NEFAs),
total triglyceride (TG) and total low density lipoprotein
cholesterol (LDL-C) were measured at the end of week 8 as described
previously (15).
Insulin tolerance tests
Experimental rats were fasted for 6 h after 8 weeks
of treatment with Tan-IIA or PBS. Type 2 DM rats were injected with
insulin (Sigma-Aldrich; Merck KGaA) intraperitoneally at 0.75 U/kg
body weight. Blood glucose levels were measured 40 min after the
insulin injection using a blood glucometer (Changsha Sannuo
Biological Sensing Technology Co., Ltd., Changsha, China).
ELISA
Serum was isolated from 3 ml peripheral blood using
centrifugation at 4,000 × g for 15 min at 4°C. The concentrations
of serum parameters were analyzed following treatment to identify
mice in which chronic renal failure was induced by type 2 DM. ELISA
kits were used to determine interleukin (IL)-6 (M6000B; R&D
Systems, Inc., Minneapolis, MN, USA), IL-10 (DY417; R&D
Systems, Inc.), C-reactive protein (CRP; MCRP00; R&D Systems,
Inc.), tumor necrosis factor (TNF)-α (MTA00B; R&D Systems,
Inc.), blood urea nitrogen (BUN; MBUN002; R&D Systems, Inc.)
and creatinine serum levels (KGR005; R&D Systems, Inc.) in
rats. The procedures were performed according to the manufacturer's
protocols. The final results were recorded at 450 nm on an ELISA
plate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Western blot analysis
Rats were sacrificed and renal tissues were isolated
from rats as described previously (16) for further analysis at the end of week
8. Renal cells were homogenized in 1X radioimmunoprecipitation
assay buffer (Sigma-Aldrich; Merck KGaA) and western blotting was
performed to analyze the protein expression. Briefly, protein
concentrations were examined using a BCA protein assay (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and protein
samples (40 µg) were separated by 15% SDS-PAGE. Proteins were then
blotted on a nitrocellulose membrane and blocked with 5% skimmed
milk for 1 h at 37°C. Membranes were incubated with primary
antibodies against AMPK (1:2,000; cat. no. ab32047; Abcam,
Cambridge, MA, USA), NF-κB p65 (1:2,000; cat. no. ab16502; Abcam),
IL-6 (1:2,000; cat. no. ab9324; Abcam), IL-10 (1:2,000; cat. no.
ab33471; Abcam), TNF-α (1:1,000; cat. no. ab6671; Abcam), CRP
(1:2,000; cat. no. ab70010; Abcam), insulin receptor substrate
(IRS)-1 (1:2,000; cat. no. ab52167; Abcam) and β-actin (1:2,000,
cat. no. ab8226; Abcam) for 12 h at 4°C. Membranes were then
incubated with horseradish peroxidase-conjugated goat anti-rabbit
IgG mAb (1:5,000; PV-6001; OriGene Technologies, Inc., Beijing,
China) for 2 h at 37°C. The blots were visualized using a
chemiluminescence detection system (Invitrogen; Thermo Fisher
Scientific, Inc.). All the experiments were performed in
triplicate. Densitometric quantification of the immunoblot data was
performed by Quantity-One software (version 2.0; Bio-Rad
Laboratories, Inc.).
NF-κB overexpression
Renal cells (1×105) were isolated from
experimental type 2 DM rats before treatments as described
previously (17) and were cultured
in in RPMI 1640 medium supplemented with 10% heat-inactivated fetal
bovine serum (FBS; both Thermo Fisher Scientific, Inc.) in 6-well
plates for 12 h at 37°C until 85% confluence. The media was then
removed from the culture plate, followed by three PBS washes. Renal
cells were transfected with 100 pmol lentivirus-NF-κB (pNF-κB) or
lentivirus-vector (pControl; both Invitrogen; Thermo Fisher
Scientific, Inc.) using Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
NF-κB-overexpressed renal cells were used for further analysis
following transfection for 48 h at 37°C.
Immunohistochemical analysis
Renal tissues were obtained at week 8 and fixed in
10% formaldehyde for 2 h at 37°C. Paraffin-embedded renal tissue
sections (4-µm-thick) were prepared and epitope retrieval was
performed using water-bath heating for further analysis (18). Following dehydration in graded
ethanol (100, 95 and 85%) and xylene, tissue sections were
deparaffinized in xylene, rehydrated in descending ethanol series,
followed by blocking of endogenous peroxidase activity in 3%
hydrogen peroxide for 10 min at 37°C. The paraffin sections were
treated with hydrogen peroxide (3%) for 15 min and subsequently
were blocked with 5% bovine serum albumin (Sigma-Aldrich; Merck
KGaA) for 30 min at 37°C. The tissue sections were incubated with
rabbit anti-mouse CD3 (1:1,000; cat. no. ab1669, Abcam) and CD19
(1:1,000; cat. no. ab25232, Abcam) at 4°C for 12 h. Sections were
then incubated with an Alexa 488-labeled goat anti-rabbit IgG
antibody (cat. no. A-11034; 1:400; Molecular Probes; Thermo Fisher
Scientific, Inc.) at 37°C for 2 h. Stained sections were examined
using an inverted laser scanning microscope (LSM 410; Zeiss AG,
Oberkochen, Germany). The semi-quantification of immunoreactivity
on each slide was evaluated using ImageJ software (version 1.02;
National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are expressed as the mean ± standard error of
mean. All data were analyzed using SPSS version 16.0 (SPSS, Inc.,
Chicago, IL, USA). Groups were compared using one-way analysis of
variance followed by a Student-Newman-Keuls test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Tan-IIA treatment improves glucose
metabolism and insulin resistance in type 2 DM rats
As indicated in Fig.
1A-C, Tan-IIA treatment significantly decreased blood glucose
and serum levels of BUN and creatinine in type 2 DM rats compared
with the control group (P<0.01). Tan-IIA treatment significantly
improved insulin resistance for type 2 DM rats compared with the
control group (Fig. 1D; P<0.01 at
40 min, P<0.05 at 80 and 120 min). Notably, compared with the
control, Tan-IIA treatment resulted in higher KW (Fig. 1E; P<0.01) and BW (Fig. 1F; P<0.05). These results suggested
that Tan-IIA treatment is beneficial for the improvement of glucose
metabolism and insulin resistance in type 2 DM rats.
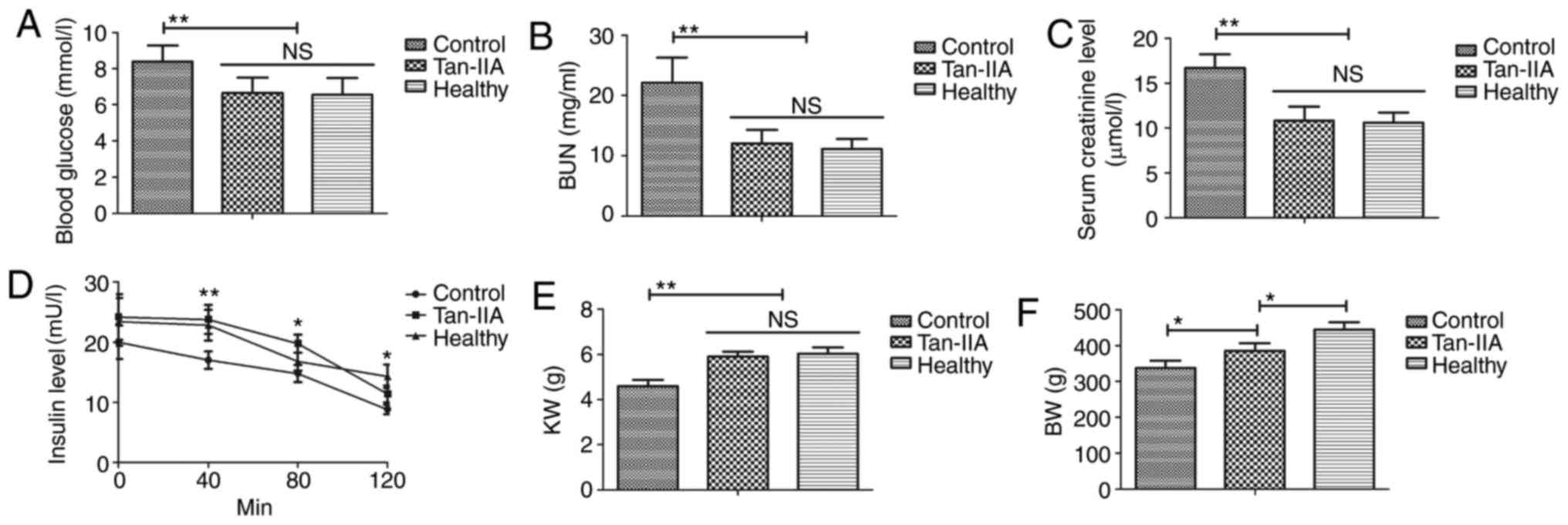 | Figure 1.Tan-IIA treatment improves glucose
metabolism and insulin resistance in type 2 DM rats. Effect of
Tan-IIA treatment on (A) blood glucose, (B) serum BUN, (C) serum
creatinine, (D) insulin resistance, (E) KW and (F) BW in type 2 DM
rats. Data are expressed as the mean ± standard error of the mean
of three independent experiments. *P<0.05, **P<0.01 vs.
control group. NS, not significant; DM, diabetes mellitus; Tan-IIA,
Tanshinone IIA; BUN, blood urea nitrogen; KW, kidney weight; BW,
body weight. |
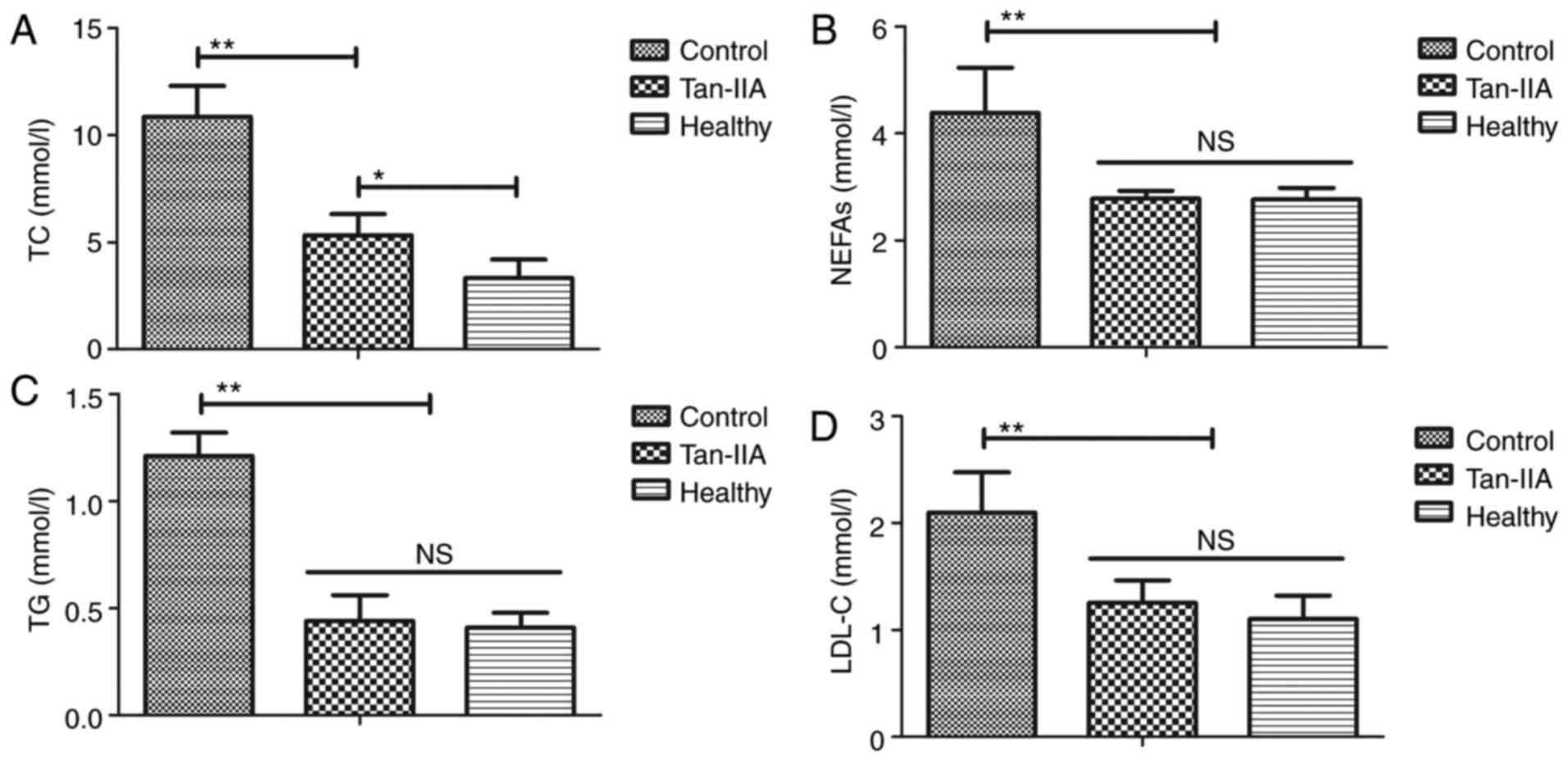
Tan-IIA treatment improves lipid
metabolism for type 2 DM rats
As indicated in Fig.
2, it was observed that Tan-IIA treatment significantly reduced
serum levels of TC, NEFAs, TG and total LDL-C in type 2 DM rats
compared with the control group (P<0.01). These results
suggested that Tan-IIA treatment is beneficial for improving lipid
metabolism in type 2 DM rats.
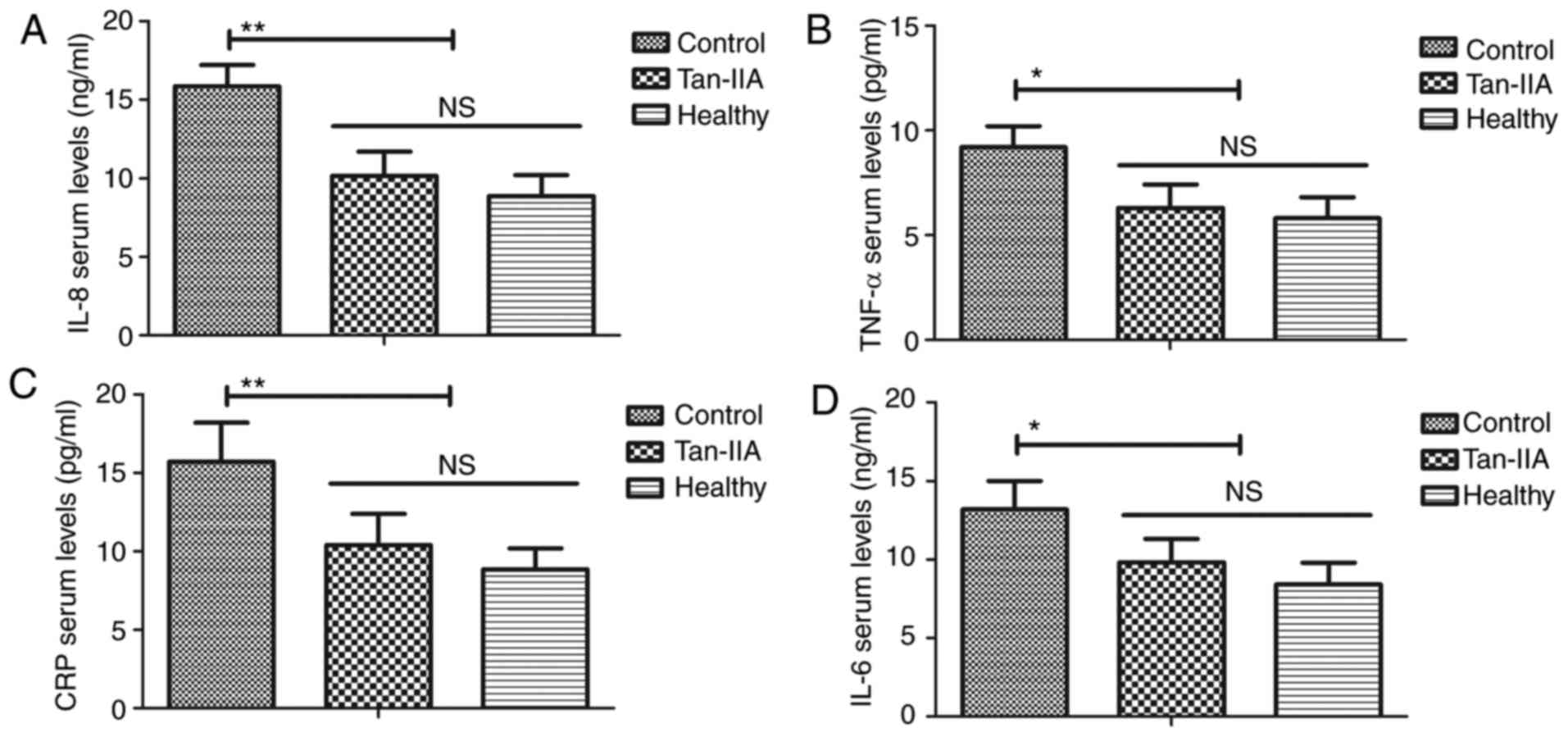
Tan-IIA decreases inflammation in type
2 DM rats
As indicated in Fig.
3, it was demonstrated that Tan-IIA treatment significantly
decreased levels of IL-8 (P<0.01), TNF-α (P<0.05), CRP
(P<0.01) and IL-6 (P<0.05) compared with the control group.
These results suggested that Tan-IIA treatment decreases
inflammation in type 2 DM rats.
Tan-IIA treatment reduces inflammation
via the NF-κB-induced AMPK signaling pathway
The possible mechanism of Tan-IIA in renal cells in
type 2 DM was investigated. The results identified that Tan-IIA
treatment significantly decreased expression levels of NF-κB p65
(P<0.05; Fig. 4A) and
significantly elevated AMPK expression levels in renal cells
(P<0.01; Fig. 4A). Western blot
analysis also indicated that Tan-IIA significantly elevated
expression levels of IRS-1 (P<0.01; Fig. 4B) and significantly decreased
immunocyte precipitation in renal cells (P<0.01; Fig. 4C). Western blotting indicated that
NF-κB overexpression (pNF-κB) increased NF-kB p65 expression
compared with the control and abolished Tan-IIA-increased AMPK
levels in renal cells (both P<0.01; Fig. 4D). NF-κB overexpression also
increased and blocked Tan-IIA-inhibited IL-8, TNF-α, CRP and IL-6
in renal cells isolated from rats (all P<0.01; Fig. 4E). These results suggested that
Tan-IIA treatment reduces inflammation via an NF-κB-induced AMPK
signaling pathway in renal cells of a type 2 DM rat model.
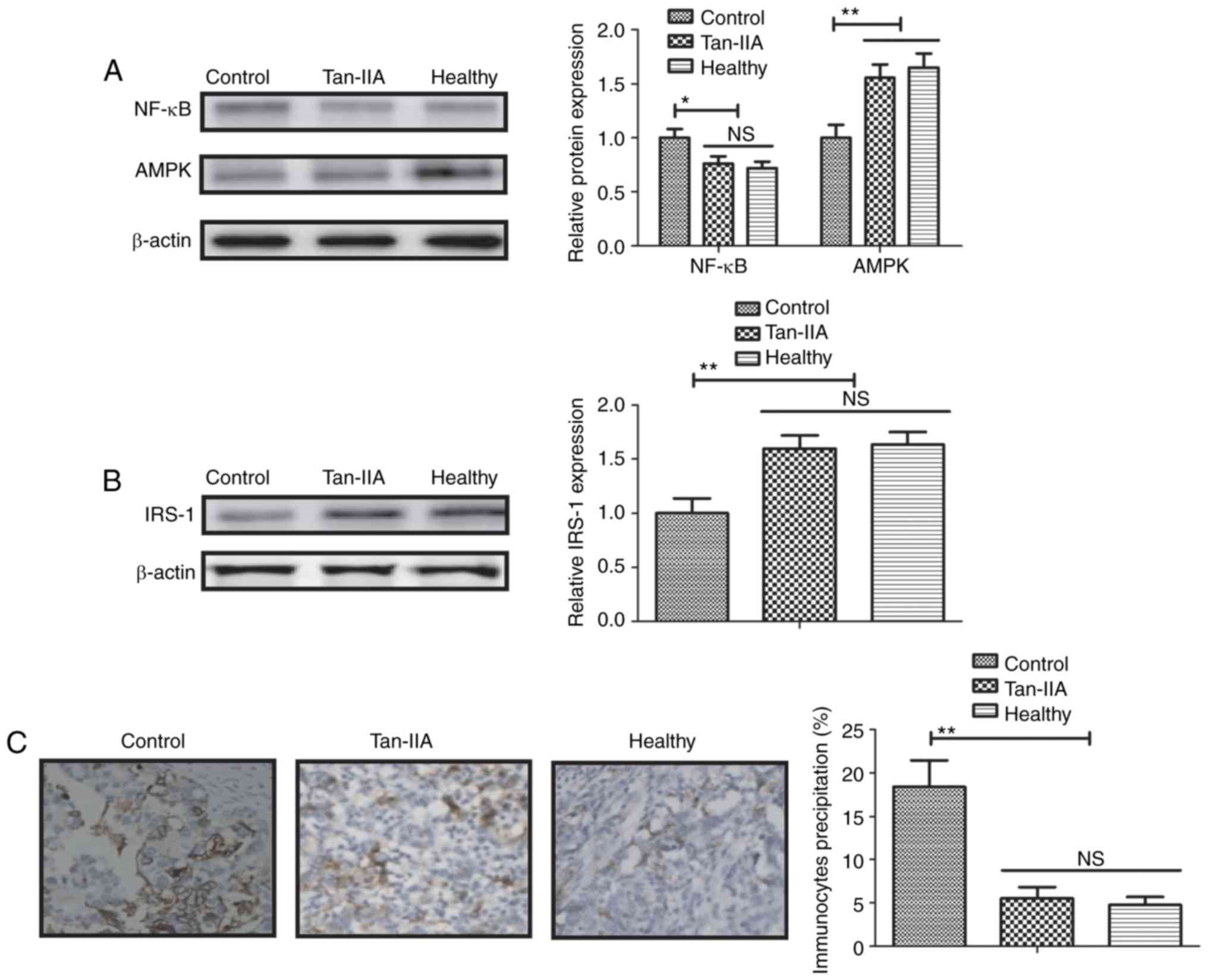 | Figure 4.Tan-IIA treatment improves type 2 DM
via the NF-κB-induced AMPK signaling pathway. (A) Effect of Tan-IIA
treatment on the expression level of NF-κB and levels of AMPK in
renal cells. (B) Effect of Tan-IIA on the expression levels of
IRS-1 in renal cells. (C) Effect of Tan-IIA treatment decreases
immunocyte precipitation in renal cells. Magnification, ×40. (D)
Effect of NF-κB overexpression on NF-κB p65 and AMPK levels in
renal cells. (E) Effect of NF-κB overexpression on IL-8, TNF-α, CRP
and IL-6 expression in renal cells. Data are expressed as the mean
± standard error of the mean of three independent experiments.
*P<0.05, **P<0.01 vs. control group. NS, not significant; DM,
diabetes mellitus; Tan-IIA, Tanshinone IIA; AMPK, 5′ adenosine
monophosphate-activated protein kinase; NF-κB, nuclear factor-κB;
IL, interleukin; TNF, tumor necrosis factor; CRP, C-reactive
protein; pNF-κB, transfected with NF-κB overexpression vector;
pControl, transfected with control vector. |
Discussion
DM can lead to upregulation of inflammatory
cytokines, which results in metabolic syndrome (19). A previous study described a novel
mechanism for Tan-IIA in regulating vasorelaxation and may help to
better understand the cardiovascular protective action of Tan-IIA
(20). In the present study, the
beneficial effects of Tan-IIA treatment for DM rats were
investigated in vitro and in vivo. A dose of 10 mg/kg
was used to evaluate the efficacy of Tan-IIA for DM, as described
previously (21). The present
findings indicated that Tan-IIA administration could decrease
inflammatory cytokines, and alleviate glucose intolerance and
insulin resistance in experimental rats. The current study also
identified that Tan-IIA could improve insulin resistance in type 2
DM mice via the NF-κB-induced AMPK signaling pathway.
Inflammation is regarded as a central
pathophysiological process in the development of type 2 DM
(22). A previous study demonstrated
that circulating IL-8 is associated with reduced insulin-like
growth factor 1 and poor metabolism in adolescents with DM
(23). A systematic review and
meta-analysis indicated that serum TNF-α levels are upregulated in
type 2 DM patients, which is regarded as an elevated inflammatory
burden in type 2 DM patients (24).
In addition, CRP is a biomarker for patients with DM (25). Furthermore, a previous study revealed
that IL-6 levels are higher in patients with type 2 DM compared
with healthy individuals (26). In
the present study, it was observed that Tan-IIA treatment
significantly decreased inflammatory factors TNF-α, IL-6, CRP and
Il-8 in a type 2 DM rat model. However, long-term experiments are
required to verify these findings.
DM significantly affects blood glucose levels due to
insufficient insulin concentration (27). It was observed in the current study
that Tan-IIA treatment decreased blood glucose and reduced insulin
resistance in DM rats. A previous report identified that serum
levels of BUN and creatinine are higher in DM patients compared
with healthy individuals (28). In
the present study, it was demonstrated that Tan-IIA downregulated
serum levels of BUN and creatinine, and increased KW and BW for
type 2 DM rats. Additionally, lipid metabolism disorder frequently
occurs in DM patients (29). It was
observed that Tan-IIA treatment significantly reduced TC, NEFAs, TG
and LDL-C compared with the control group in type 2 DM rats.
NF-κB is involved in inflammation and insulin
resistance in adipose cells in type 2 DM, and contributes to
inhibition of inflammation and improves insulin resistance
(30). In the present study, it was
observed that NF-κB expression levels were increased in type 2 DM
rats and downregulated by Tan-IIA treatment. A previous report
demonstrated that targeting of the IRS-1 pathway may regulate
insulin resistance in type 2 DM rats (31). In the present study, Tan-IIA elevated
expression levels of IRS-1 in renal cells in type 2 DM rats.
Upregulation of AMPK levels following Tan-IIA treatment was first
reported in renal cells, and may further reduce production and
activity of glucose-6-phosphatase in type 2 DM rats (32). However, a limitation of the present
study was that only the number of immunocytes and expression of
inflammatory cytokine levels were measured in renal cells. Further
studies should be performed to analyze immunocytes and inflammatory
cytokine expression in liver or adipose tissues in type 2 DM.
In conclusion, the present study indicates that
Tan-IIA may have beneficial effects for treating type 2 DM rats.
The findings suggest that Tan-IIA treatment improves type 2 DM via
regulation of the NF-κB-induced AMPK signaling pathway. However,
further studies are required to identify the efficacy of Tan-IIA in
patients with type 2 DM.
Acknowledgements
Not applicable.
Funding
The present study was supported by Cadre Health Care
Program of Sichuan Province as part of the following study, ‘Study
on the role of islet alfa cell function in the initiation and
development of type 2 diabetes mellitus and the mechanisms of
related drug intervention’ (grant no. 2012–202, 30305030325).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
FYY performed the experiments. MZ designed the
experiments. PX, DX, PC, MR, QS, JYS, JD and XLT prepared the
investigations and analyzed data.
Ethics approval and consent to
participate
The protocols were approved by the Ethics Committee
of Sichuan Province People's Hospital & Sichuan Academy of
Medical Sciences (Chengdu, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bgeginski R, Ribeiro PAB, Mottola MF and
Ramos JGL: Effects of weekly supervised exercise or physical
activity counseling on fasting blood glucose in women diagnosed
with gestational diabetes mellitus: A systematic review and
meta-analysis of randomized trials. J Diabetes. 9:1023–1032. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moosazadeh M, Asemi Z, Lankarani KB,
Tabrizi R, Maharlouei N, Naghibzadeh-Tahami A, Yousefzadeh G,
Sadeghi R, Khatibi SR, Afshari M, et al: Family history of diabetes
and the risk of gestational diabetes mellitus in Iran: A systematic
review and meta-analysis. Diabetes Metab Syndr. 11 Suppl
1:S99–S104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gyawali B, Ferrario A, van Teijlingen E
and Kallestrup P: Challenges in diabetes mellitus type 2 management
in Nepal: A literature review. Glob Health Action. 9:317042016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mustafa SB, Mehmood Z, Akhter N, Kauser A,
Hussain I, Rashid A, Akram M, Tahir IM, Munir N, Riaz M, et al:
Review-medicinal plants and management of diabetes mellitus: A
review. Pak J Pharm Sci. 29 Suppl 5:S1885–S1891. 2016.
|
|
5
|
Gomes JMG, Costa JA and Alfenas RCG:
Metabolic endotoxemia and diabetes mellitus: A systematic review.
Metabolism. 68:133–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mizamtsidi M, Paschou SA, Grapsa J and
Vryonidou A: Diabetic cardiomyopathy: A clinical entity or a
cluster of molecular heart changes? Eur J Clin Invest. 46:947–953.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shang Y, Zhang X, Chen L, Leng W, Lei X,
Yang Q, Liang Z and Wang J: Assessment of left ventricular
structural remodelling in patients with diabetic cardiomyopathy by
cardiovascular magnetic resonance. J Diabetes Res.
2016:47869252016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng J, Li S and Chen H: Tanshinone IIA
inhibits myocardial remodeling induced by pressure overload via
suppressing oxidative stress and inflammation: Possible role of
silent information regulator 1. Eur J Pharmacol. 791:632–639. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shu M, Hu XR, Hung ZA, Huang DD and Zhang
S: Effects of tanshinone IIA on fibrosis in a rat model of
cirrhosis through heme oxygenase-1, inflammation, oxidative stress
and apoptosis. Mol Med Rep. 13:3036–3042. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu Q, Zhang P, Zhang X and Chen J:
Experimental study of the anti-cancer mechanism of tanshinone IIA
against human breast cancer. Int J Mol Med. 24:773–780. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu M, Cao FL, Zhang YF, Shan L, Jiang XL,
An XJ, Xu W, Liu XZ and Wang XY: Tanshinone IIA therapeutically
reduces LPS-induced acute lung injury by inhibiting inflammation
and apoptosis in mice. Acta Pharmacol Sin. 36:179–187. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang DT, Huang RH, Cheng X, Zhang ZH, Yang
YJ and Lin X: Tanshinone IIA attenuates renal fibrosis and
inflammation via altering expression of TGF-β/Smad and NF-kappaB
signaling pathway in 5/6 nephrectomized rats. Int Immunopharmacol.
26:4–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han F, Sheng L, Jian Q and Weng Q:
Therapeutic effects of Tanshinone IIA towards early renal damage in
patients with type 2 diabetes. West Indian Med J. 2016. View Article : Google Scholar
|
|
14
|
Karabulut A, Akyer SP, Mete Abban G and
Sahin B: Effects of menopause, diabetes mellitus and steroid use on
type I mesh-induced tissue reaction in a rat model. Eur J Obstet
Gynecol Reprod Biol. 179:27–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Galea R, Wells RG, Ross CK, Lockwood J,
Moore K, Harvey JT and Isensee GH: A comparison of rat SPECT images
obtained using 99mTc derived from 99Mo
produced by an electron accelerator with that from a reactor. Phys
Med Biol. 58:2737–2750. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arbillaga L, Vettorazzi A, Gil AG, van
Delft JH, García-Jalón JA and de Cerain López A: Gene expression
changes induced by ochratoxin A in renal and hepatic tissues of
male F344 rat after oral repeated administration. Toxicol Appl
Pharmacol. 230:197–207. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu H, Xu S, Hu S, Gao Y and Shui H: Effect
of 1,25(OH)2D3 on transdifferentiation of rat
renal tubular epithelial cells induced by high glucose. Biomed Rep.
5:699–704. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Myers J: Antigen retrieval: A review of
commonly used methods and devices. MLO Med Lab Obs. 38(10): 12–15;
quiz 16–17. 2006.
|
|
19
|
Barami K, Lyon L and Conell C: Type 2
diabetes mellitus and glioblastoma multiforme-assessing risk and
survival: Results of a large retrospective study and systematic
review of the literature. World Neurosurg. 106:300–307. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li YH, Xu Q, Xu WH, Guo XH, Zhang S and
Chen YD: Mechanisms of protection against diabetes-induced
impairment of endothelium-dependent vasorelaxation by Tanshinone
IIA. Biochim Biophys Acta. 1850:813–823. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang P, Li C, Xiang Z and Jiao B:
Tanshinone IIA reduces the risk of Alzheimer's disease by
inhibiting iNOS, MMP2 and NFkappaBp65 transcription and translation
in the temporal lobes of rat models of Alzheimer's disease. Mol Med
Rep. 10:689–694. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hamar P: Role of regulatory micro RNAs in
type 2 diabetes mellitus-related inflammation. Nucleic Acid Ther.
22:289–294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Van Sickle BJ, Simmons J, Hall R, Raines
M, Ness K and Spagnoli A: Increased circulating IL-8 is associated
with reduced IGF-1 and related to poor metabolic control in
adolescents with type 1 diabetes mellitus. Cytokine. 48:290–294.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen YL, Qiao YC, Xu Y, Ling W, Pan YH,
Huang YC, Geng LJ, Zhao HL and Zhang XX: Serum TNF-α concentrations
in type 2 diabetes mellitus patients and diabetic nephropathy
patients: A systematic review and meta-analysis. Immunol Lett.
186:52–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kawada T: C-reactive protein, depressive
symptoms and incident diabetes mellitus with special emphasis on
physical activity. J Psychosom Res. 78:4072015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Darko SN, Yar DD, Owusu-Dabo E, Awuah AA,
Dapaah W, Addofoh N, Salifu SP, Awua-Boateng NY and Adomako-Boateng
F: Variations in levels of IL-6 and TNF-α in type 2 diabetes
mellitus between rural and urban Ashanti Region of Ghana. BMC
Endocr Disord. 15:502015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Khosrozadeh M, Heydari N and Abootalebi M:
The effect of Abelmoschus Esculentus on blood levels of glucose in
diabetes mellitus. Iran J Med Sci. 41:S632016.
|
|
28
|
Javadi S, Asri-Rezaei S and
Allahverdizadeh M: Interrelationship of beta-2 microglobulin, blood
urea nitrogen and creatinine in streptozotocin-induced diabetes
mellitus in rabbits. Vet Res Forum. 5:7–11. 2014.PubMed/NCBI
|
|
29
|
van Olden C, Groen AK and Nieuwdorp M:
Role of intestinal microbiome in lipid and glucose metabolism in
diabetes mellitus. Clin Ther. 37:1172–1177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhong L, Luo Y, Huang C and Liu L: Effect
of NF-kB decoy on insulin resistance of adipocytes from patients
with type 2 diabetes mellitus. Diabetes Metabol. 37:520–526. 2011.
View Article : Google Scholar
|
|
31
|
Cai S, Sun W, Fan Y, Guo X, Xu G, Xu T,
Hou Y, Zhao B, Feng X and Liu T: Effect of mulberry leaf (Folium
Mori) on insulin resistance via IRS-1/PI3K/Glut-4 signalling
pathway in type 2 diabetes mellitus rats. Pharm Biol. 54:2685–2691.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yao L, Wan J, Li H, Ding J, Wang Y, Wang X
and Li M: Resveratrol relieves gestational diabetes mellitus in
mice through activating AMPK. Reprod Biol Endocrinol. 13:1182015.
View Article : Google Scholar : PubMed/NCBI
|