Introduction
The worldwide incidence of skin cancer has increased
rapidly with 2.75 million new cases in the worldwide annually
(1). This is due to damage to the
ozonosphere and a lack of risk awareness (1,2).
Non-melanoma skin cancer (NMSC) is one of the most common types of
skin cancer and has a number of subtypes, including basal cell
carcinoma, cutaneous squamous cell carcinoma (cSCC), merkel cell
carcinoma and microcystic adnexal carcinoma (3–5). cSCC is
the most frequent cutaneous carcinoma, following basal cell
carcinomas and accounts for >20% of all skin cancer cases all
over the world (5–7). The initiation and development of cSCC
is caused by complex interactions between various signaling
molecules associated with genetics, infection, chemistry and
immunity (8). Tumor recurrence and
metastasis are thought to be the leading causes of mortality for
patients with cSCC (9,10). A previous study demonstrated that
patients with cSCC recurrence and metastasis have a poor long-term
prognosis, with a one-year survival rate of ~50% (11). Therefore, developing effective,
novel, molecular targets for the detection and treatment of cSCC is
of great importance.
MicroRNAs (miRNAs or miRs) are small non-coding RNAs
(2–24 nucleotides in length) that regulate gene expression by
interacting with target genes at a post-transcriptional level
(12,13). miRNA-186 (miR-186) is an important
member of the miRNA family and accumulating evidence has revealed
that miR-186 may serve a critical role in various biological
processes, including cell development, proliferation and apoptosis
(14–16). Previous evidence also indicates that
miR-186 may serve a role in various types of human cancer,
including bladder, pancreatic and liver cancer (17–19).
However, whether miR-186 is associated with the pathogenesis of
cSCC remains undetermined.
Apoptotic protease activating factor 1 (APAF1) is a
critical component of the apoptosome and previous studies have
demonstrated that it may be activated by various cellular stimuli,
including DNA damage and oncogene activation (20,21).
APAF1 inactivation is a common event in human tumors, which
suggests that it may serve as a tumor suppressor in healthy
individuals (22). Recently, APAF1
was identified as a target gene of miR-23a and miR-221 in
colorectal and ovarian cancer, respectively (23,24).
However, whether APAF1 serves a role in the pathogenesis of cSCC
remains unclear.
In the present study, the expression of miR-186 and
APAF1 was examined in cSCC cells and tissues. The correlation
between miR-186 and APAF1 was subsequently investigated using
bioinformatics analysis and a dual-luciferase activity assay. A-431
cell lines with knocked down miR-186, overexpressed miR-186,
knocked down APAF1 or overexpressed miR-186 and knocked down APAF1
were established to explore the role of miR-186 and APAF1 in
cSCC.
Materials and methods
Cell lines and tissues
The human cSCC cell line A-431 and the 293-T cell
line were purchased from the American Type Culture Collection
(Manassas, VA, USA) and cultured in Dulbecco's modified Eagle's
medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The cells were
maintained in a humidified incubator with 5% CO2 at
37°C.
A total of 15 paired tumor and adjacent normal
tissues (5 cm away from each tumor) were obtained at the same
locations from patients diagnosed with cSCC during surgery between
August 2015 and March 2017 at the First Affiliated Hospital of
Jinan University (Guangzhou, China). These cSCC cases included 8
male patients and 7 female patients with a mean age of 62.7 years
(range, 38–87 years). All tissue samples were collected, immersed
in liquid nitrogen and maintained at −80°C for further experiments.
Written informed consent was obtained from each patient prior to
the current study. The study protocol was approved by the Research
Ethics Committee at the First Affiliated Hospital of Jinan
University (Guangzhou, China).
Overexpression and knockdown
experiments
For the overexpression and knockdown experiments of
miR-186, mimic (3′-CAAAGAAUUCUCCUUUUGGGCU-5′), inhibitor
(3′-AGCCCAAAAGGAGAAGGCUUUG-5′) and negative control (NC;
3′-UUCUCCGAACGUGUCACGUTT-5′) sequences were designed by Shanghai
GenePharma Co., Ltd. (Shanghai, China). A-431 cells were
transfected with the miRNA NC, miR-186 mimic or miR-186 inhibitor
(all 100 nM) using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according the manufacturer's
protocol for 24 h.
For APAF1 knockdown, APAF1 siRNA (si-APAF1;
3′-GUGCCUACAAAGGUGUUAUUU-5′) and NC siRNA for APAF1 (NC-siRNA;
3′-UUCUCCGAACGUGUCACGUUU-5′) were designed by Shanghai GenePharma
Co., Ltd. The A-431 cells were transfected with si-APAF1 or
NC-siRNA (both 25 nM) with or without miR-186 inhibitor (100 nM)
using Lipofectamine 2000 according to the manufacturer's protocol
for 24 h.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cSCC and control
tissues, and miR-NC-, miR-186 mimic- or miR-186
inhibitor-transfected A-431 cells using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). mRNA was reverse transcribed into
cDNA using the Revert Aid First Strand cDNA Synthesis kit (cat. no.
K1622; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol, and the thermocycling conditions were 25°C
for 5 min, 42°C for 60 min and 70°C for 10 min. qPCR was performed
using SYBR-Green Real-Time Master mix (Toyobo Life Science, Osaka,
Japan) following the manufacturer's protocol. The thermocycling
conditions of qPCR were as follows: 95°C for 10 min, followed by 40
cycles of 95°C for 15 sec and 60°C for 1 min. GAPDH and U6 were
used as internal controls for APAF1 and miR-186, respectively. The
primers used for the detection of miR-186 and APAF1 were as
follows: miR-186, sense 5′-GCGGCGCAAAGAATTCTCCT-3′ and antisense
5′-GTGCAGGGTCCGAGGT-3′; APAF1, sense 5′-ATGGACACCTTCTTGGACGACAG-3′
and antisense 5′-TGTGGGGGCGGACAACTAA-3′; GAPDH, sense
5′-TGTTCGTCATGGGTGTGAAC-3′ and antisense
5′-ATGGCATGGACTGTGGTCAT-3′; U6, sense 5′-CGCTTCGGCAGCACATATACTA-3′
and antisense 5′-CGCTTCACGAATTTGCGTGTCA-3′. Primers were designed
by Sangon Biotech Co., Ltd. (Shanghai, China). The relative
expression of miR-186 and APAF1 were calculated and normalized
using the 2−ΔΔCq method (25).
Western blot analysis
Total protein was extracted from the tissue samples
and miR-NC-, miR-186 mimic-, miR-186 inhibitor, si-APAF1+miR-186
inhibitor or NC-siRNA+miR-186 inhibitor-transfected A-431 cells
using an SDS lysis buffer (cat. no. P0013G; Beyotime Institute of
Biotechnology, Haimen, China) on ice for 30 min. The concentration
of total protein was measured using a bicinchoninic acid protein
assay kit (Pierce; Thermo Fisher Scientific, Inc.). A total of 50
µg/lane protein was separated by 10% SDS-PAGE and transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). Membranes were blocked with 10% low fat dried milk at 25°C
for a minimum of 1 h, followed by incubation with primary
antibodies against APAF1 (1:1,000; cat. no. ab32372), light chain
3B (LC3-B; 1:500; cat. no. ab48394) or Beclin1 (1:1,000; cat. no.
ab62557; all Abcam, Cambridge, MA, USA) for 1 h at room temperature
(RT). Membranes were subsequently incubated with horseradish
peroxidase-conjugated secondary antibodies (cat. no. BA1054; Wuhan
Boster Biological Technology, Ltd., Wuhan, China) for 2 h at RT.
GAPDH was used as the internal control and signals were detected
using enhanced chemiluminescent reagents (cat. no. SW2030; Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China). The
band net optical density was analysed using Image-Pro Plus software
(version 6.0; Media Cybernetics, Inc., Rockville, MD, USA).
Luciferase reporter transfection and
dual-luciferase reporter assay
The TargetScan database (targetscan.org) predicted one binding site for miR-186
on APAF1. A wild-type (WT) APAF1 [WT-3′-untranslated region
(UTR)-APAF1] with the predicted miR-186 target binding sequence and
a mutant APAF1 (MUT-3′-UTR-APAF1) with a mutation in the binding
site, were synthesized and cloned into the psi-CHECK2 vector (cat.
no. C8021; Promega Corporation, Madison, WI, USA). The 293-T cells
were seeded in 96-well plates at a density of 1×104
cells/well. Following overnight incubation at 37°C, the 293-T cells
were transfected with the reconstructed plasmid containing
WT-3′-UTR-APAF1 or MUT-3′-UTR-APAF1 in the presence of miR-186
mimics or the negative control (NC) by Lipofectamine®
2000. Cells were harvested and the luciferase activity was measured
using a Dual-Luciferase Reporter assay system (Promega Corporation,
Madison, WI, USA) 48 h following transfection. Renilla
luciferase activity was used for normalization of the firefly
luciferase activity.
Immunofluorescence
miR-NC-, miR-186 mimic- or miR-186
inhibitor-transfected A-431 cells were seeded at a density of
1×105 cells/ml on a coverslip pre-coated with
poly-L-lysine. They were subsequently fixed with cold 4%
formaldehyde at 4°C overnight. After washing three times with PBS
containing 0.1% Triton X-100, cells were blocked with 10% bovine
serum albumin (cat. no. FA016-50G; Amresco, LLC, Solon, OH, USA)
for 2 h at RT followed by incubation with primary antibodies
against APAF1 (1:1,000; cat. no. ab2001; Abcam) and DAPI (1:2,000;
cat. no. ab104139; Abcam) at 4°C overnight. Cells were then
incubated with Alexa Fluor 488 donkey anti-mouse immunoglobulin G
(1:200; ab150105; Abcam) secondary antibodies for 1 h at RT and
visualized using a confocal laser-scanning microscope.
Magnification at ×200.
Hoechst staining
si-APAF1+miR-186 inhibitor or NC-siRNA+miR-186
inhibitor-transfected A-431 cells were seeded into 6-well plates
and incubated overnight at 37°C. Cells were fixed with 50 µl cold
4% formaldehyde for 30 min at RT. Then the cells were washed twice
with cold PBS. Hoechst 33258 was added to the wells at a
concentration of 20 µg/ml (Sigma-Aldrich; Merck KGaA) and incubated
for a minimum of 20 min at RT. Following washing with PBS, the
cells were visualized using a Leica confocal laser-scanning
microscope (TCS SP8; Leica Microsystems GmbH, Wetzlar, Germany) at
365 nm. Magnification at ×400.
EdU staining
The Click-iT Plus EdU Alexa Fluor 1594 Imaging kit
(Invitrogen; Thermo Fisher Scientific, Inc.) was used according to
the manufacturer's protocol, to determine the effects of miR-186
mimics or inhibitor on cell proliferation. miR-NC-, miR-186 mimic-
or miR-186 inhibitor-transfected A-431 cells were fixed with 50 µl
cold 4% formaldehyde for 30 min at RT. DAPI (1:2,000) was used to
stain the cell nucleus for 30 min at RT and signals were detected
using an Olympus FLUOVIEW FV1000 confocal laser-scanning microscope
(Olympus Corporation, Tokyo, Japan) at a magnification of ×100.
Colony formation assay
A-431 cells transfected with miR-186 NC, mimic or
inhibitor were seeded onto glass dishes at a density of
1×103 cells/ml and incubated in an atmosphere containing
5% CO2 at 37°C for 2 weeks. The cells were fixed with 50
µl cold 4% formaldehyde for 30 min at RT and subsequently stained
with 0.1% crystal violet for 15 min at RT. Local cloning morphology
was photographed with an inverted microscope. The colonies were
counted and each of the experimental conditions was performed by
using a Nikon Eclipse Ti inverted microscope (Nikon Corporation,
Tokyo, Japan) in triplicate. Magnification at ×100.
Matrigel invasion assay
To evaluate the effects of miR-186 on the invasive
ability of cSCC cells, a Matrigel assay was performed. miR-NC-,
miR-186 mimic-, miR-186 inhibitor, si-APAF1+miR-186 inhibitor or
NC-siRNA+miR-186 inhibitor-transfected A-431 cells were suspended
in 100 µl DMEM at a concentration of 1×105 cells/ml and
seeded into the upper Transwell chamber with an 8-µm pore size
coated with Matrigel (Corning Inc., Corning, NY, USA). A total of
200 µl DMEM containing 15% FBS was added to the lower Transwell
chamber and cells were cultured at 37°C for 24 h. Cells in the
upper chamber were removed and those in the lower chamber were
fixed with 4% paraformaldehyde for 30 min at RT stained with 0.1%
crystal violet for 5 min at RT and observed using a Nikon Eclipse
Ti inverted microscope in triplicate Magnification at ×200.
Wound-healing assay
A wound-healing assay was used to assess the effects
of miR-186 on cell migration ability. miR-NC-, miR-186 mimic- or
miR-186 inhibitor-transfected A-431 cells were seeded in 6-well
plates at a concentration of 1×104 cells/well. A
parallel wound was made using a pipette tip once the A-431 cells
reached 100% confluence. Cells were then cultured at 37°C in a 5%
CO2 atmosphere and images were captured using an
inverted microscope at 0 and 48 h. Magnification at ×100.
Cell apoptosis and cell cycle
distribution analyses
To assess cell apoptosis and perform cell cycle
analysis, miR-NC-, miR-186 mimic- or miR-186 inhibitor-transfected
A-431 cells were seeded in 24-well plates at a density of
1×105 cells/well and cultured in DMEM with 10% FBS at
37°C for 24 h. For the cell cycle distribution analysis, the cells
were digested by trypsin (Gibco; Thermo Fisher Scientific, Inc.),
washed with PBS three times and fixed with 80% ethanol for 5 min at
4°C. They were then incubated with 0.25 mg/ml Ribonuclease A
(Sigma-Aldrich; Merck KGaA) for 30 min at 37°C and 20 µg/ml
propidium iodide (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China)
for 20 min at room temperature. For the cell apoptosis analysis,
the cells were digested with trypsin, centrifuged at 111.8 × g for
5 min at 4°C, washed with PBS, re-suspended in 100 µl 1X binding
buffer [cat. no. 70-AP101-100-BB; Hangzhou Multi Sciences (Lianke)
Biotech Co., Ltd., Hangzhou, China]. Then the cells were double
stained with an Annexin V-FITC/PI apoptosis detection kit (Bestbio
Company, Shanghai China) in the dark for 15 min at room
temperature. Following staining, the apoptosis rate and cell cycle
distribution was analyzed using a BD Accuri™ C6 Plus flow cytometer
and equipped with CellQuest software (version 6.1×; both BD
Bioscience, San Jose, CA, USA) according to the manufacturer's
protocol.
Statistical analysis
Data are presented as the mean ± standard deviation
and all statistical analyses were performed using SPSS 20.0
software (IBM Corp., Armonk, NY, USA). Cell experiments had >3
biological replicates. One-way analysis of variance and the least
significant difference post hoc multiple comparison test were used
to compare the differences among multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
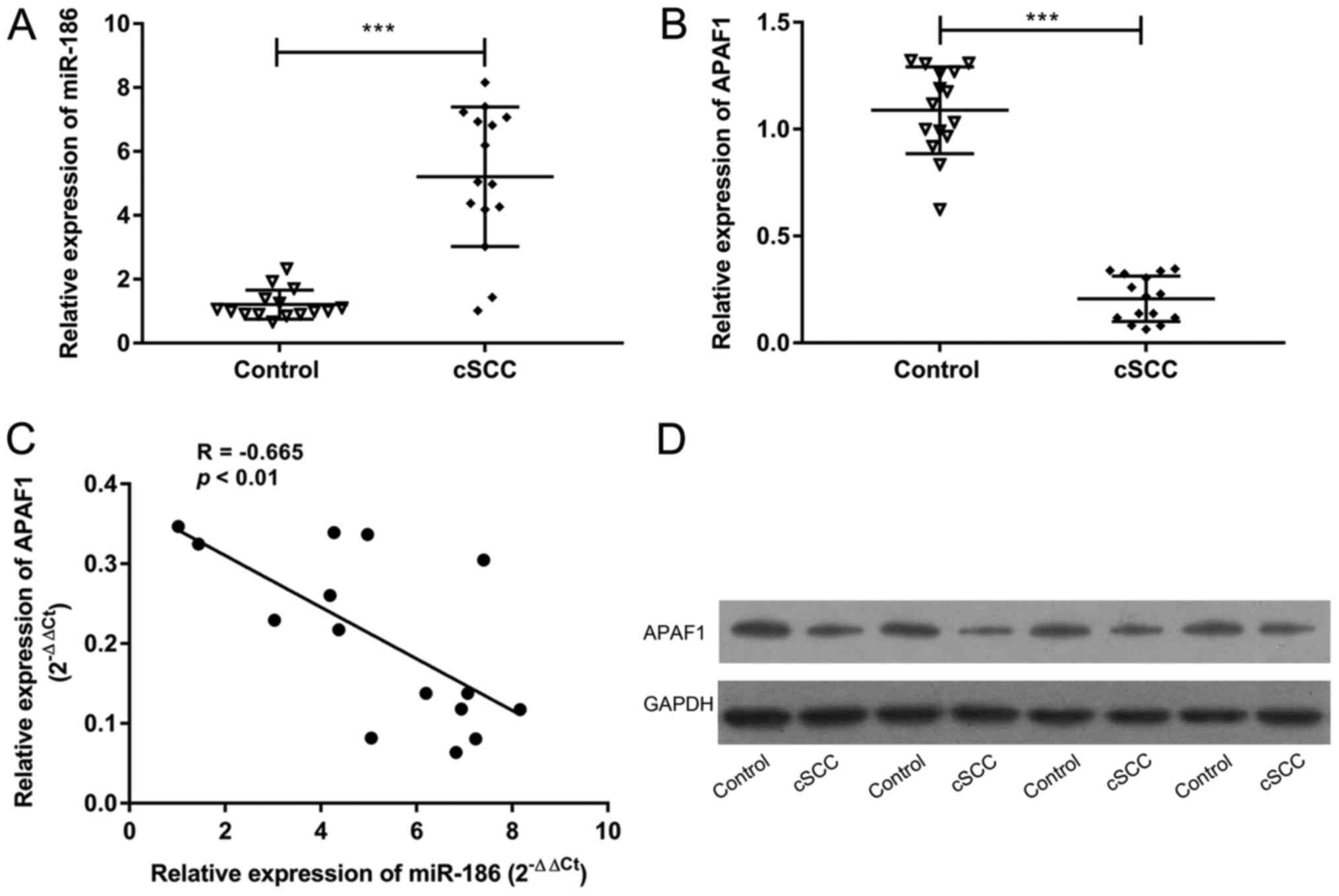
miR-186 expression is upregulated and
APAF1 expression is downregulated in cSCC tissues
To investigate the precise roles of miR-186 and
APAF1 in the tumorigenesis of cSCC, their expression in cSCC
tissues was compared with corresponding normal tissues. RT-qPCR
results revealed that miR-186 expression was significantly higher
in cSCC tissues compared with the corresponding normal tissues
(P<0.001, Fig. 1A). Conversely,
APAF1 expression was significantly reduced in cSCC tissues compared
with the control samples (P<0.001, Fig. 1B). In addition, the relative
expression of APAF1 was negatively correlated with the expression
of miR-186 in cSCC tissues (R=−0.665, P<0.01; Fig. 1C). Western blotting revealed that the
expression of APAF1 protein was reduced in cSCC tissues compared
with the controls (Fig. 1D). These
results suggest that miR-186 and APAF1 may serve a critical role in
the pathogenesis of cSCC.
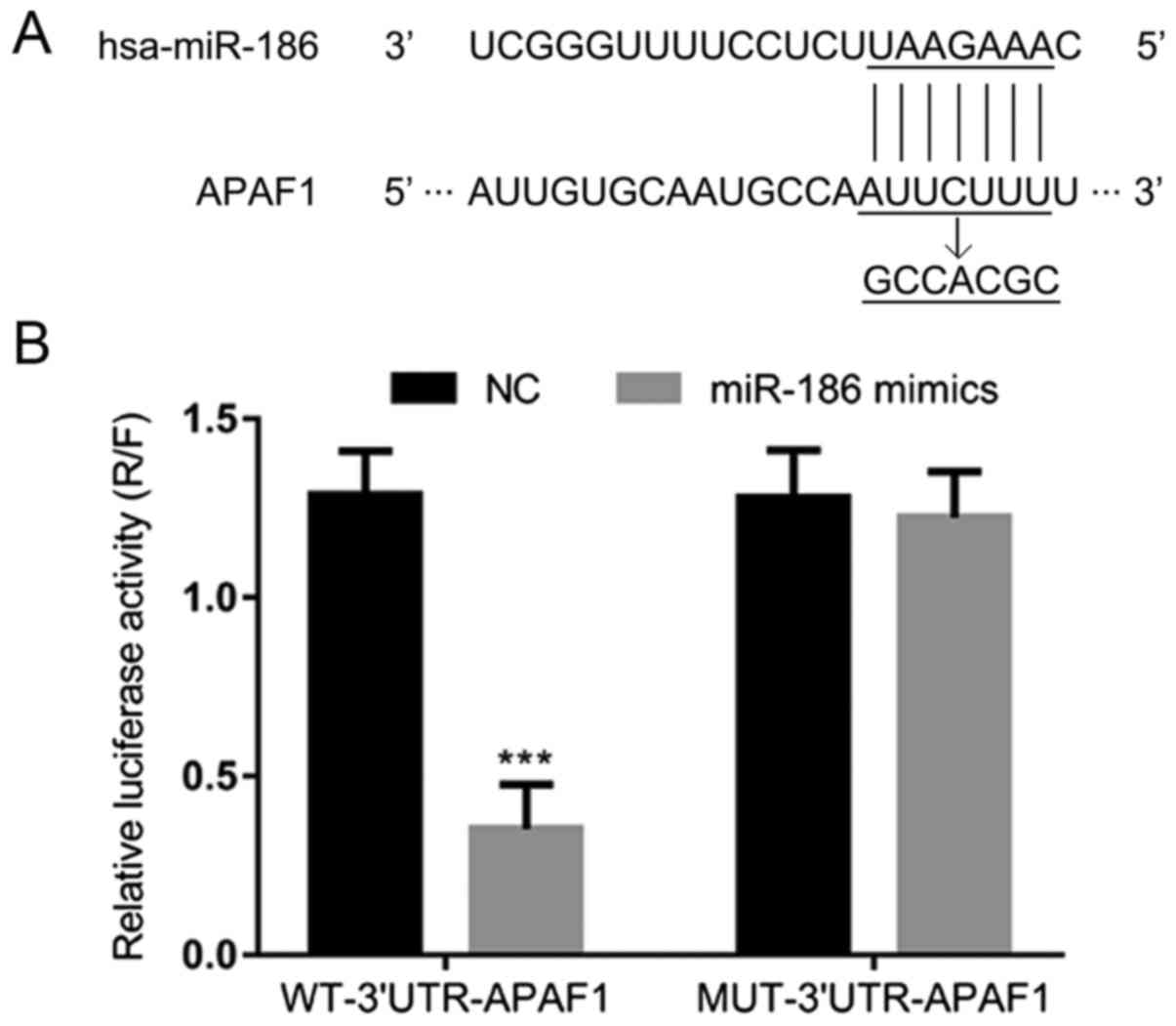
APAF1 is a potential target gene of
miR-186
To further explore the correlation between APAF1 and
miR-186, the TargetScan miRNA target predication database was used.
One predictive target site for miR-186 was identified in APAF1
(Fig. 2A). A dual-luciferase
reporter assay revealed that the relative luciferase activity was
significantly attenuated by miR-186 mimics in the WT-3′-UTR-APAF1
system, whereas it was not significantly altered by the application
of miR-186 mimics in the MUT-3′-UTR-APAF1 system (P<0.001;
Fig. 2B). These results suggest that
the APAF1 gene is a direct target of miR-186.
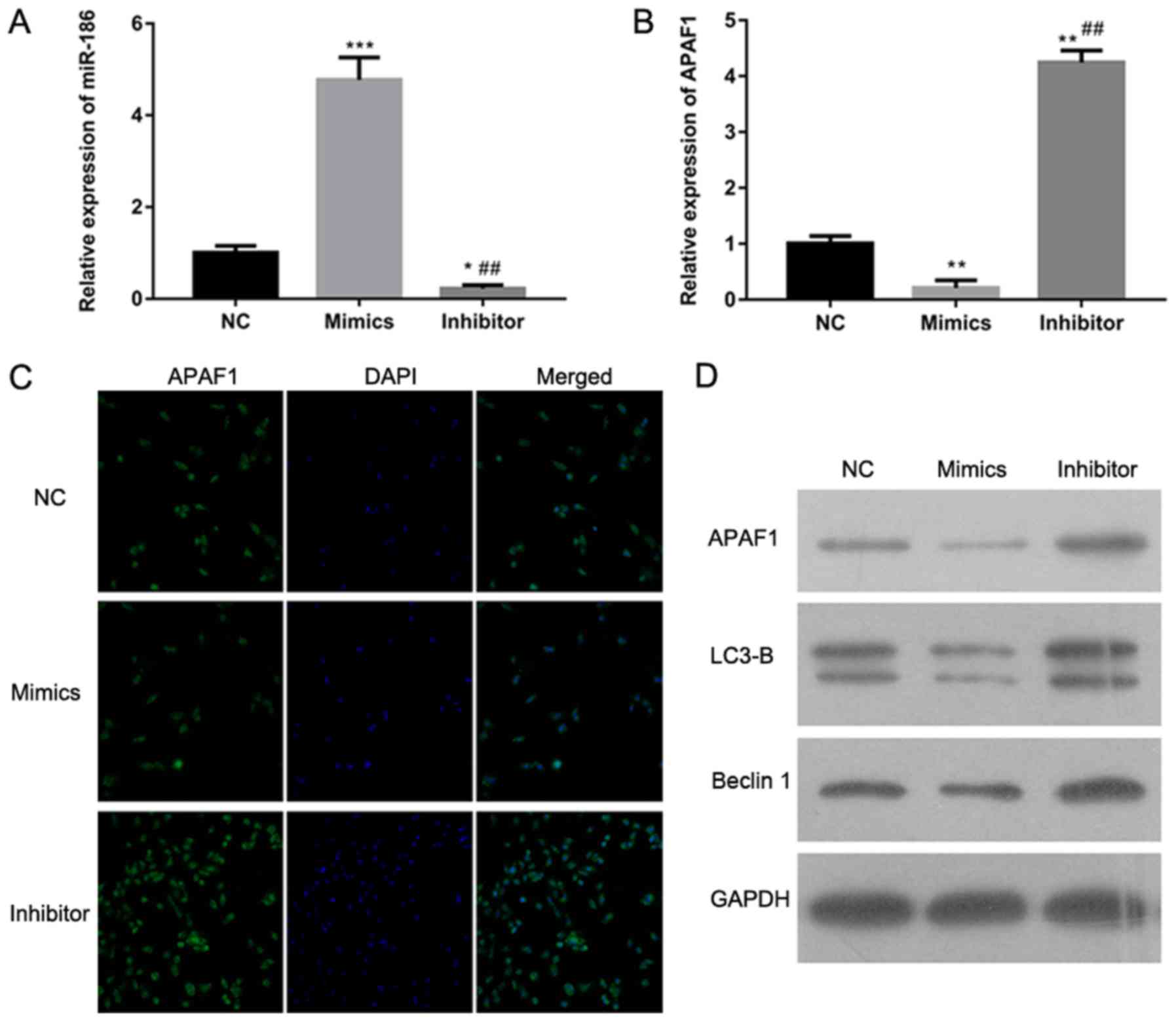
APAF1 expression is regulated by
miR-186
To determine how miR-186 regulates the expression of
APAF1, RT-qPCR, immunohistochemistry and western blotting were
performed to detect APAF1 expression in A-431 cells with miR-186
overexpression or knockdown. The RT-qPCR results revealed that
miR-186 expression was significantly increased in A-431 cells
transfected with miR-186 mimics and significantly decreased in
A-431 cells transfected with miR-186 inhibitors compared with the
NC group (P<0.001 and P<0.05, respectively; Fig. 3A). The RT-qPCR assay also
demonstrated that APAF1 expression was significantly downregulated
in A-431 cells transfected with miR-186 mimics and significantly
upregulated in those transfected with miR-186 inhibitors
(P<0.01; Fig. 3B).
Immunohistochemistry and western blotting also supported these
results (Fig. 3C and D). Western
blotting indicated that the expression of LC3-B and Beclin1 was
lower in A-431 cells transfected with miR-186 mimics and markedly
higher in A-431 cells transfected with miR-186 inhibitors compared
with the NC group (Fig. 3D). These
results indicate that APAF1 expression is directly regulated by
miR-186 in the A-431 cSCC cell line.
miR-186 promotes cell proliferation,
invasion and migration and inhibits cell apoptosis in the A-431
cell line
To understand the role of miR-186 in the
tumorigenesis of cSCC, proliferation, invasion, migration and
apoptosis were investigated in A-431 cells transfected with miR-186
mimics or inhibitors. EdU staining and colony formation assays were
used to examine the effects of miR-186 on cell proliferation. The
results revealed the proliferation ability was increased in A-431
cells transfected with miR-186 mimics compared with the
NC-transfected cells, while proliferation was decreased in A-431
cells transfected with miR-186 inhibitors (Fig. 4A and B). Transwell and wound-healing
assays demonstrated that invasion and migration were significantly
enhanced in A-431 cells transfected with miR-186 mimics and
significantly attenuated in A-431 cells transfected with the
miR-186 inhibitor compared with the NC-treated cells (P<0.01;
Fig. 4C and D). Cell apoptosis was
not significantly affected in A-431 cells transfected with miR-186
mimics compared with the NC group; however, it was significantly
upregulated in A-431 cells transfected with miR-186 inhibitors
(P<0.01; Fig. 4E). Flow cytometry
was performed to assess the effects of miR-186 on cell cycle
distribution. The results revealed that miR-186 overexpression
significantly decreased the percentage of A-431 cells in the G0/G1
phase and significantly increased the percentage of A-431 cells in
S phase compared with the NC group (P<0.05 and P<0.01,
Fig. 4F). These results suggest that
miR-186 may act as an oncogene in the cSCC A-431 cell line.
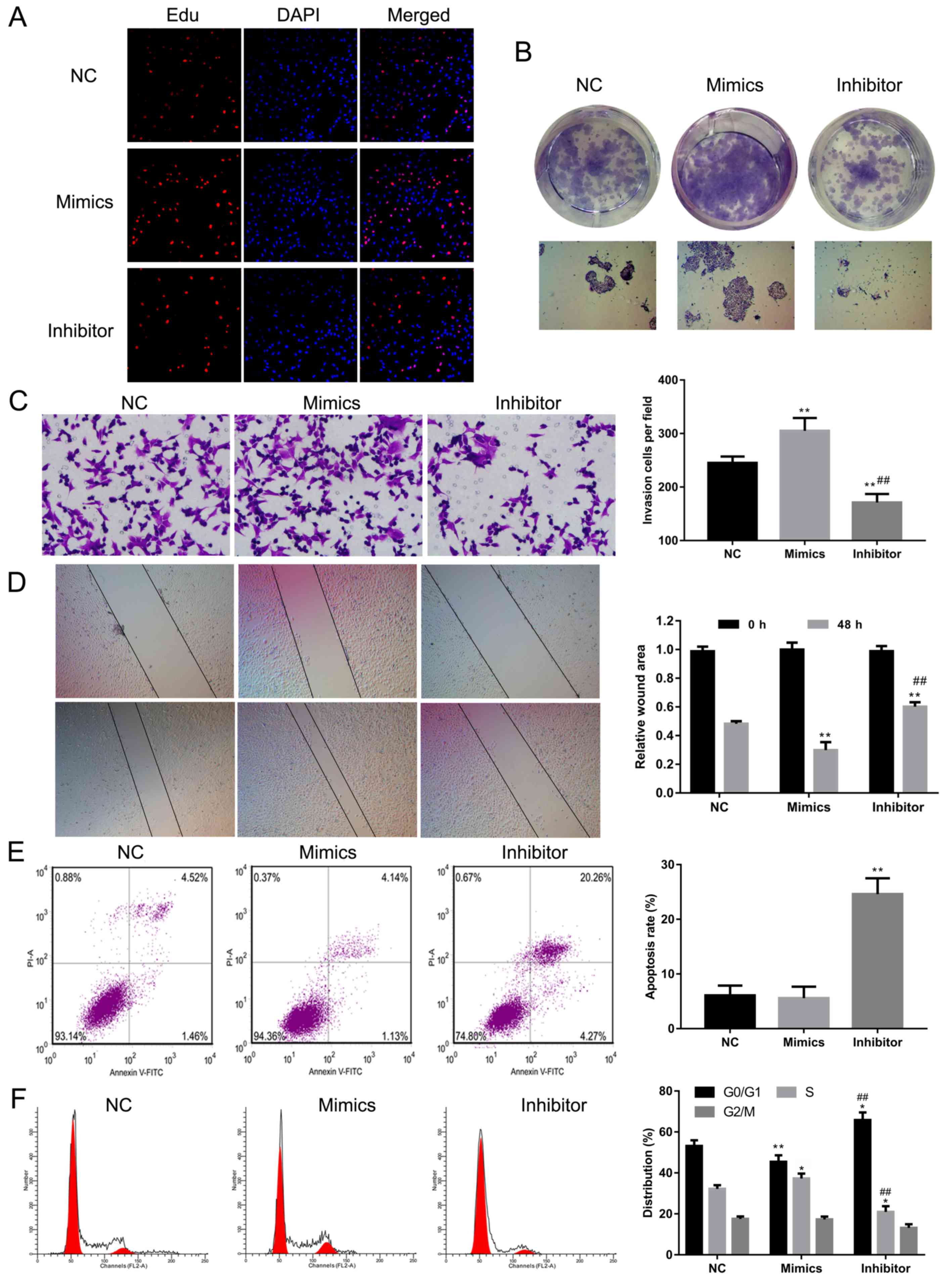 | Figure 4.Effect of miR-186 overexpression or
knockdown on cell proliferation, apoptosis, invasion and cell cycle
progression in A-431 cells. (A) EdU staining was performed in the
NC, miR-186 mimics and miR-186 inhibitors groups. Red indicates EdU
staining and blue indicates DAPI. Magnification, ×100. (B) A colony
formation assay was performed to assess the effect of miR-186 on
cell proliferation in the NC, mimics and inhibitor groups.
Magnification, ×100. (C) A Transwell assay was performed to detect
the invasive ability of A-431 cells transfected with NC, mimics or
inhibitor. Magnification, ×200. (D) A wound-healing assay was used
to assess the migratory ability of A-431 cells treated with NC,
mimics or inhibitor. Magnification, ×100. (E and F) Annexin
V-FITC/PI staining and flow cytometry analysis were performed to
evaluate the effects of miR-186 on (E) cell apoptosis and (F) cell
cycle distribution in miR-186 overexpressed or inhibited A-431
cells, respectively. *P<0.05, **P<0.01 vs. NC group.
##P<0.01 vs. mimics group. NC, negative control; miR,
microRNA; APAF1, apoptotic protease activating factor 1; FITC,
fluorescein isothiocyanate; PI, propidium iodide. |
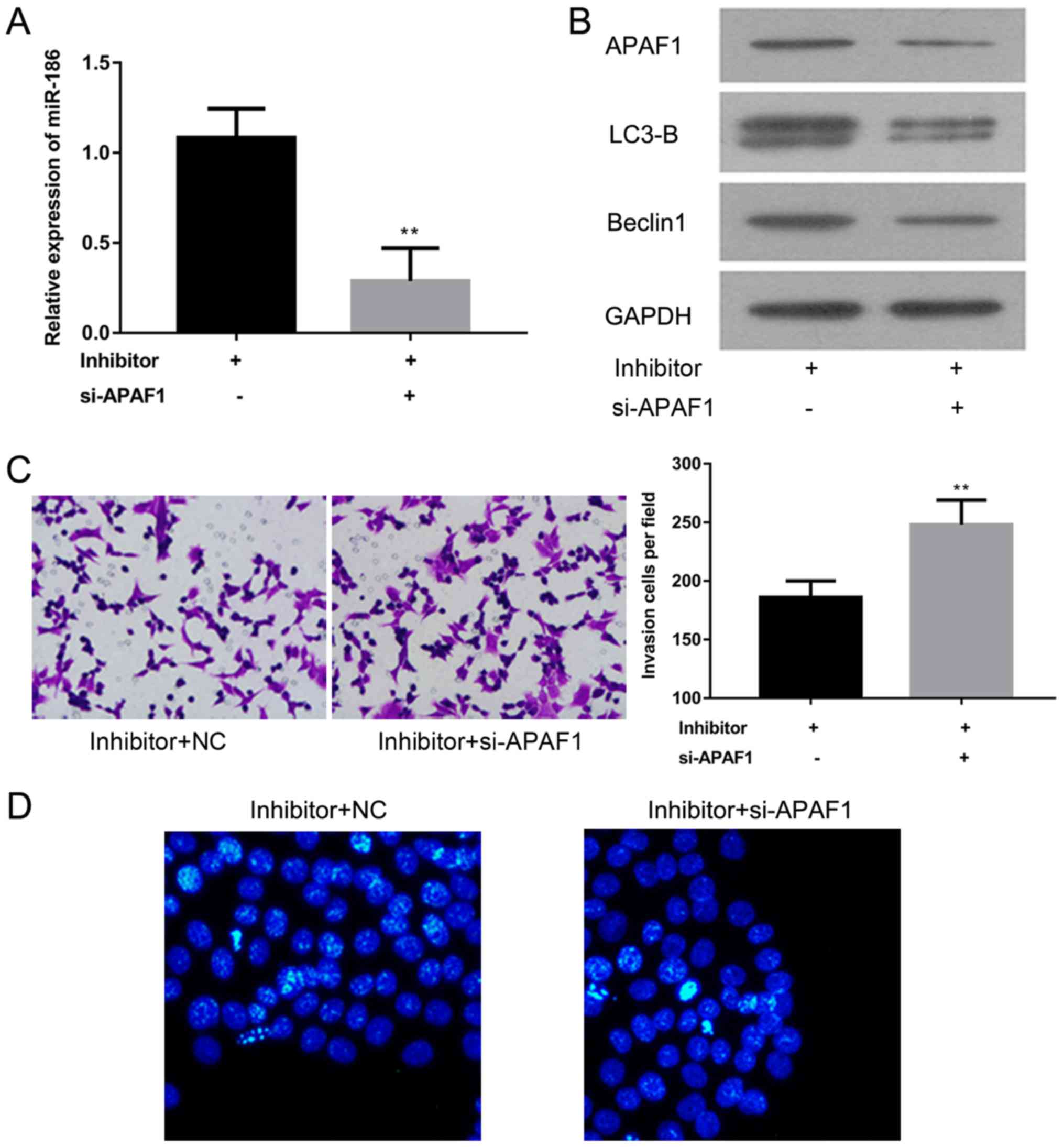
APAF1 knockdown promotes invasion and
inhibits apoptosis in A-431 cells transfected with miR-186
inhibitors
To further explore the role of APAF1 in cSCC,
miR-186 expression was assessed using RT-qPCR and the expression of
APAF1, LC3-B and Beclin1 proteins was analyzed using western
blotting in A-431 cells transfected with miR-186 inhibitors with or
without si-APAF1. The results revealed that miR-186 expression was
significantly decreased in the si-APAF1 transfected group compared
with the control group (P<0.01; Fig.
5A). Western blotting demonstrated that APAF1, LC3-B and
Beclin1 protein expression was notably decreased in A-431 cells
transfected with miR-186 inhibitors and si-APAF1, compared with the
A-431 cells transfected with miR-186 inhibitors alone (Fig. 5B). A Matrigel assay and Hoechst
staining were performed to evaluate the effect of APAF1 on cell
invasion and apoptosis in A-431 cells transfected with miR-186
inhibitors. The results suggest that cell invasion is significantly
enhanced, while migration is markedly attenuated in A-431 cells
transfected with miR-186 inhibitors and si-APAF1 compared with
A-431 cells transfected with the miR-186 inhibitor alone
(P<0.01; Fig. 5C and D). These
results suggest that APAF1 may act as a tumor suppressor in the
A-431 cSCC cell line.
Discussion
Despite advances in the prevention, diagnosis and
treatment of cSCC worldwide, the exact molecular basis of cSCC
remains unclear (5,26,27).
miRNAs are considered to be important genetic regulators of various
biological processes, including cell proliferation, development,
invasion and apoptosis (28–30). A number of previous studies have
demonstrated that miRNAs are involved in the pathogenesis of a
number of human diseases, particularly different types of cancer
(12,31,32).
Aberrant miRNA expression is frequently observed in cancer,
including pancreatic, breast and various types of skin cancer
(33–35). Increasing numbers of miRNAs have been
identified as critical regulators in the initiation and progression
of cSCC, including miR-1, miR-34a, miR-124 and miR-125b (36–39).
Fleming et al (36) reported
that miR-1 expression was reduced in cSCC cell lines, while the
results of functional assays indicated that miR-1 inhibits cell
proliferation and promotes cell apoptosis by targeting various
genes. It has been reported that miR-125 is a tumor suppressor in
cSCC, while matrix metallopeptidase 13 was identified as its gene
target (39). It was demonstrated
that miR-186 was associated with a number of different types of
human cancer, including bladder cancer, hepatocellular carcinoma
and gastric cancer; however, its involvement in the tumorigenesis
of cSCC remains unclear (17,40,41).
The present study revealed that miR-186 expression was upregulated
and cell proliferation, invasion and migration were promoted in
A-431 cells, suggesting that miR-186 is an oncogene in cSCC.
APAF1 serves a critical role as a regulator of the
mitochondrial apoptotic signaling pathway, inducing cell apoptosis
by binding with cytochrome C and activating caspase-9 in the
cytosol (21). Previous studies have
demonstrated that APAF1 functions as a tumor suppressor by
interacting with various miRNAs (40–42). Li
et al (24) reported that
miR-221 expression was significantly increased in ovarian tumor
tissues and that it promoted cell proliferation by inhibiting APAF1
expression. Zang et al (43)
observed that miR-155 expression was significantly increased and
APAF1 expression was notably reduced in lung cancer tissues. They
also reported that miR-155 attenuated the sensitivity of lung
cancer cell lines to cisplatin by decreasing APAF1 expression. In
the present study, a negative correlation between miR-186 and APAF1
was observed in cSCC tissues. It was revealed that APAF1 is a
direct target gene of miR-186, which suggests that APAF1 may serve
as a downstream signaling molecule of miR-186. Consistent with
previous studies, further functional assays have demonstrated that
APAF1 knockdown in A-431 cSCC cells may enhance their invasive
ability and attenuate migration (24,43,44).
LC3-B and Beclin1 are considered to be critical
autophagy-associated genes and their expression levels are often
used to assess autophagic activity (45–47). In
the present study, it was revealed that LC3-B and Beclin1
expression was significantly downregulated in A-431 cells
transfected with miR-186 mimics or inhibitors co-incubated with
APAF1 siRNA. This suggests that LC3-B and Beclin1 may be potential
therapeutic targets for patients with cSCC.
In conclusion, the results of the present study
demonstrate that APAF1 acts as a target gene of miR-186 in A-431
cSCC cells, while miR-186 upregulation promotes cell proliferation,
invasion and migration and inhibits cellular apoptosis. APAF1
knockdown promoted cell growth and inhibited cell apoptosis. These
results suggest that therapeutic agents targeting the miR-186/APAF1
axis may have potential clinical applications as a treatment option
for cSCC.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during the current
study are included in this published article.
Authors' contributions
JT and LHD made substantial contributions to
conception and design, and analysis of data. RS and YZY helped in
drafting the manuscript. JT and LHD revised the manuscript. All
authors read and approved the final version.
Ethics approval and consent to
participate
The study protocol was approved by the Research
Ethics Committee at the First Affiliated Hospital of Jinan
University and written informed consent was obtained from each
participant prior to their inclusion within the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gordon R: Skin cancer: An overview of
epidemiology and risk factors. Semin Oncol Nurs. 29:160–169. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Linares MA, Zakaria A and Nizran P: Skin
cancer. Prim Care. 42:645–659. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Glass AG and Hoover RN: The emerging
epidemic of melanoma and squamous cell skin cancer. JAMA.
262:2097–2100. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dubas LE and Ingraffea A: Nonmelanoma skin
cancer. Facial Plast Surg Clin North Am. 21:43–53. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parekh V and Seykora JT: Cutaneous
squamous cell carcinoma. Clin Lab Med. 37:503–525. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Green AC and Olsen CM: Cutaneous squamous
cell carcinoma: An epidemiological review. Br J Dermatol.
177:373–381. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leiter U, Gutzmer R, Alter M, Ulrich C,
Lonsdorf AS, Sachse MM and Hillen U: Cutaneous squamous cell
carcinoma. Hautarzt. 67:857–866. 2016.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prieto-Granada C and Rodriguez-Waitkus P:
Cutaneous squamous cell carcinoma and related entities:
Epidemiology, clinical and histological features, and basic science
overview. Curr Probl Cancer. 39:206–215. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thompson AK, Kelley BF, Prokop LJ, Murad
MH and Baum CL: Risk factors for cutaneous squamous cell carcinoma
recurrence, metastasis and disease-specific death: A systematic
review and meta-analysis. JAMA Dermatol. 152:419–428. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trodello C, Pepper JP, Wong M and Wysong
A: Cisplatin and cetuximab treatment for metastatic cutaneous
squamous cell carcinoma: A systematic review. Dermatol Surg.
43:40–49. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wells JL III and Shirai K: Systemic
therapy for squamous cell carcinoma of the skin in organ transplant
recipients. Am J Clin Oncol. 35:498–503. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han C, Seebacher NA, Hornicek FJ, Kan Q
and Duan Z: Regulation of microRNAs function by circular RNAs in
human cancer. Oncotarget. 8:64622–64637. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Self-Fordham JB, Naqvi AR, Uttamani JR,
Kulkarni V and Nares S: MicroRNA: Dynamic regulators of macrophage
polarization and plasticity. Front Immunol. 8:10622017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dang RY, Liu FL and Li Y: Circular RNA
hsa_circ_0010729 regulates vascular endothelial cell proliferation
and apoptosis by targeting the miR-186/HIF-1α axis. Biochem Biophys
Res Commun. 490:104–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu C, Wang J, Hu Y, Xie H, Liu M and Tang
H: Upregulation of kazrin F by miR-186 suppresses apoptosis but
promotes epithelial-mesenchymal transition to contribute to
malignancy in human cervical cancer cells. Chin J Cancer Res.
29:45–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang JJ, Wang DD, Du CX and Wang Y: Long
noncoding RNA ANRIL promotes cervical cancer development by acting
as a sponge of miR-186. Oncol Res. May 22–2017.(Epub ahead of
print). View Article : Google Scholar
|
|
17
|
He X, Ping J and Wen D: MicroRNA-186
regulates the invasion and metastasis of bladder cancer via
vascular endothelial growth factor C. Exp Ther Med. 14:3253–3258.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Niu Q, Li X, Xia D, Jiang Y, Tian Z, Bian
C, Zhang C, Liu P, Zhang F, Yang Y and Wang G: MicroRNA-186 affects
the proliferation of tumor cells via yes-associated protein 1 in
the occurrence and development of pancreatic cancer. Exp Ther Med.
14:2094–2100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Chen F, Zhao M, Yang Z, Li J,
Zhang S, Zhang W, Ye L and Zhang X: The long noncoding RNA HULC
promotes liver cancer by increasing the expression of the HMGA2
oncogene via sequestration of the microRNA-186. J Biol Chem.
292:15395–15407. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shakeri R, Kheirollahi A and Davoodi J:
Apaf-1: Regulation and function in cell death. Biochimie.
135:111–125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
De Zio D, Maiani E and Cecconi F: Apaf1 in
embryonic development-shaping life by death and more. Int J Dev
Biol. 59:33–39. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hickman ES and Helin K: The regulation of
APAF1 expression during development and tumourigenesis. Apoptosis.
7:167–171. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu N, Sun YY, Zhang XW, Chen S, Wang Y,
Zhang ZX, Song SW, Qiu GB and Fu WN: Oncogenic miR-23a in
pancreatic ductal adenocarcinogenesis via inhibiting APAF1. Dig Dis
Sci. 60:2000–2008. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li J, Li Q, Huang H, Li Y, Li L, Hou W and
You Z: Overexpression of miRNA-221 promotes cell proliferation by
targeting the apoptotic protease activating factor-1 and indicates
a poor prognosis in ovarian cancer. Int J Oncol. Mar 7–2017.(Epub
ahead of print).
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yesantharao P, Wang W, Ioannidis NM,
Demehri S, Whittemore AS and Asgari MM: Cutaneous squamous cell
cancer (cSCC) risk and the human leukocyte antigen (HLA) system.
Hum Immunol. 78:327–335. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Karia PS, Morgan FC, Ruiz ES and Schmults
CD: Clinical and incidental perineural invasion of cutaneous
squamous cell carcinoma: A systematic review and pooled analysis of
outcomes data. JAMA Dermatol. 153:781–788. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grossi I, Salvi A, Abeni E, Marchina E and
De Petro G: Biological function of MicroRNA193a-3p in health and
disease. Int J Genomics. 2017:59131952017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mulholland EJ, Dunne N and McCarthy HO:
MicroRNA as therapeutic targets for chronic wound healing. Mol Ther
Nucleic Acids. 8:46–55. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei F, Yang S and Wang S: MicroRNAs: A
critical regulator under mechanical force. Histol Histopathol.
33:335–342. 2018.PubMed/NCBI
|
|
31
|
Celano M, Rosignolo F, Maggisano V, Pecce
V, Iannone M, Russo D and Bulotta S: MicroRNAs as biomarkers in
thyroid carcinoma. Int J Genomics. 2017:64965702017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shomali N, Mansoori B, Mohammadi A,
Shirafkan N, Ghasabi M and Baradaran B: MiR-146a functions as a
small silent player in gastric cancer. Biomed Pharmacother.
96:238–245. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Negoi I, Hostiuc S, Sartelli M, Negoi RI
and Beuran M: MicroRNA-21 as a prognostic biomarker in patients
with pancreatic cancer-A systematic review and meta-analysis. Am J
Surg. 214:515–524. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jang MH, Kim HJ, Gwak JM, Chung YR and
Park SY: Prognostic value of microRNA-9 and microRNA-155 expression
in triple-negative breast cancer. Hum Pathol. 68:69–78. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Neu J, Dziunycz PJ, Dzung A, Lefort K,
Falke M, Denzler R, Freiberger SN, Iotzova-Weiss G, Kuzmanov A,
Levesque MP, et al: miR-181a decelerates proliferation in cutaneous
squamous cell carcinoma by targeting the proto-oncogene KRAS. PLoS
One. 12:e01850282017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fleming JL, Gable DL, Samadzadeh-Tarighat
S, Cheng L, Yu L, Gillespie JL and Toland AE: Differential
expression of miR-1, a putative tumor suppressing microRNA, in
cancer resistant and cancer susceptible mice. PeerJ. 1:e682013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lefort K, Brooks Y, Ostano P, Cario-André
M, Calpini V, Guinea-Viniegra J, Albinger-Hegyi A, Hoetzenecker W,
Kolfschoten I, Wagner EF, et al: A miR-34a-SIRT6 axis in the
squamous cell differentiation network. EMBO J. 32:2248–2263. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamane K, Jinnin M, Etoh T, Kobayashi Y,
Shimozono N, Fukushima S, Masuguchi S, Maruo K, Inoue Y, Ishihara
T, et al: Down-regulation of miR-124/-214 in cutaneous squamous
cell carcinoma mediates abnormal cell proliferation via the
induction of ERK. J Mol Med (Berl). 91:69–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu N, Zhang L, Meisgen F, Harada M,
Heilborn J, Homey B, Grandér D, Ståhle M, Sonkoly E and Pivarcsi A:
MicroRNA-125b down-regulates matrix metallopeptidase 13 and
inhibits cutaneous squamous cell carcinoma cell proliferation,
migration, and invasion. J Biol Chem. 287:29899–29908. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
He J, Feng X, Hua J, Wei L, Lu Z, Wei W,
Cai H, Wang B, Shi W, Ding N, et al: miR-300 regulates cellular
radiosensitivity through targeting p53 and apaf1 in human lung
cancer cells. Cell Cycle. 16:1943–1953. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yeung Au CL, Co NN, Tsuruga T, Yeung TL,
Kwan SY, Leung CS, Li Y, Lu ES, Kwan K, Wong KK, et al: Exosomal
transfer of stroma-derived miR21 confers paclitaxel resistance in
ovarian cancer cells through targeting APAF1. Nat Commun.
7:111502016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Song S, Seo HH, Lee SY, Lee CY, Lee J, Yoo
KJ, Yoon C, Choi E, Hwang KC and Lee S: MicroRNA-17-mediated
down-regulation of apoptotic protease activating factor 1
attenuates apoptosome formation and subsequent apoptosis of
cardiomyocytes. Biochem Biophys Res Commun. 465:299–304. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zang YS, Zhong YF, Fang Z, Li B and An J:
MiR-155 inhibits the sensitivity of lung cancer cells to cisplatin
via negative regulation of Apaf-1 expression. Cancer Gene Ther.
19:773–778. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shang J, Yang F, Wang Y, Wang Y, Xue G,
Mei Q, Wang F and Sun S: MicroRNA-23a antisense enhances
5-fluorouracil chemosensitivity through APAF-1/caspase-9 apoptotic
pathway in colorectal cancer cells. J Cell Biochem. 115:772–784.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zou M, Lu N, Hu C, Liu W, Sun Y, Wang X,
You Q, Gu C, Xi T and Guo Q: Beclin 1-mediated autophagy in
hepatocellular carcinoma cells: Implication in anticancer
efficiency of oroxylin A via inhibition of mTOR signaling. Cell
Signal. 24:1722–1732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ravikumar B, Sarkar S, Davies JE, Futter
M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M,
Korolchuk VI, Lichtenberg M, Luo S, et al: Regulation of mammalian
autophagy in physiology and pathophysiology. Physiol Rev.
90:1383–1435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Klionsky DJ, Abeliovich H, Agostinis P,
Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA,
Ballabio A, et al: Guidelines for the use and interpretation of
assays for monitoring autophagy in higher eukaryotes. Autophagy.
4:151–175. 2008. View Article : Google Scholar : PubMed/NCBI
|