Introduction
Gastric cancer is one of the most common human
malignancies and is the second most common cause of
cancer-associated mortality in the world (1–3). A high
risk of metastasis of gastric cancer cells and the resistance of
cancer cells to apoptosis in patients with gastric cancer has been
reported in the previous reports (4–6).
Patients with gastric cancer present with higher morbidity and
mortality rates, compared with other types of carcinoma of the
digestive system (7–9). Therefore, investigating efficient
antitumor treatments are required in the field of cancer research
and treatments.
Currently, neoadjuvant chemoradiotherapy (CRT) is
widely used for the treatment of locally advanced gastric cancer to
inhibit tumor growth, making it more amenable to resection, and to
decrease the risk of tumor cell metastasis (10–13).
Paclitaxel chemotherapy is an effective drug for advanced gastric
cancer with acceptable toxic side-effects, and has been considered
as an effective anticancer agent due to the smooth transition to
the subsequent regimen (14).
Carboplatin radiotherapy in combination with
2-(8-hydroxy-6-methoxy-1-oxo-1H-2-benzopyran-3-yl) propionic acid
can inhibit gastric tumor growth, which may be an effective drug in
the treatment of gastric cancer (15). However, the combined therapeutic
effects of paclitaxel and carboplatin CRT in the CRT response in
gastric cancer remain to be elucidated.
The presence of tumor-infiltrating lymphocytes
(TILs) is associated with improved clinical outcome in gastric
cancer, and TILs can suppress further invasion and/or metastasis of
gastric carcinoma (16). A previous
study indicated that the density of tumor-infiltrating T cells is a
notable prognostic indicator for gastric cancer following treatment
with CRT (17). TILs in gastric
adenocarcinoma also present with marked compartmentalization with
high numbers of lymphocytes in the stroma and low intraepithelial
lymphocyte counts, which underline the importance of inflammation
for tumor therapy (18). Another
study showed that immunoscoring involves the assessment of T-cell
density in the central tumor and invasive margin based on
expression of pairs of T-cell markers (CD3 and CD8, and CD3 and
CD45), which can be used to evaluate the prognostic and
histopathologic tumor-node-metastasis stage for patients with
cancer (19). Therefore, evaluating
the association between therapeutic effects of antitumor agents and
the pathologic complete response (pCR) to neoadjuvant CRT is
crucial for the treatment of patients with gastric cancer.
In the present study, the T
regulatory-Treg-lymphocyte density and cytotoxic T lymphocyte
density were analyzed in surgically resected samples from 68
patients with locally advanced gastric cancer treated with CRT
(paclitaxel and carboplatin). The results showed that regulatory
T-cell density and cytotoxic T lymphocyte density were associated
with pCR to neoadjuvant CRT (paclitaxel and carboplatin) in the
gastric cancer response to CRT.
Materials and methods
Patients, treatment and follow-up
A total of 68 patients with gastric cancer were
recruited at The First Affiliated Hospital of Hebei North
University (Hebei, China) between February 2010 and May 2015.
Clinical gastric tumor stage was determined by computed tomography
and magnetic resonance imaging prior to treatment with CRT.
Neoadjuvant radiotherapy, comprising paclitaxel and carboplatin,
was administered for 4 weeks. Surgical resection (20), involving curative (R0) resection and
D2 lymphadenectomy, of the gastric tumor was performed following
the completion of neoadjuvant treatment. The follow-up period in
the present study was a total of 60 months, every 5 months. The
survival rate of patients with gastric cancer was recorded and
cancer-specific survival (CSS) was analyzed as the time between the
date of surgery and the date the patient succumbed to mortality
from gastric cancer. Recurrence-free survival (RFS) was evaluated
as the time between the date of surgery and the date of first
gastric cancer recurrence.
CRT administration
The patients received paclitaxel (80
mg/m2 per day intravenously on days 1, 8, and 15;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in combination with
carboplatin (75 mg/m2 per day intravenously on days 1,
8, and 15, Sigma-Aldrich; Merck KGaA) in 4-week treatment
cycles.
Histopathologic assessment
Tumor regression grading was analyzed as part of
routine pathological review using the Dworak system (21) and defined as grade 4 corresponding to
pCR and grade 0 corresponding to no regression.
Distinguishing regulatory T cells and
cytotoxic T lymphocytes
Regulatory T cells were distinguished using typical
markers CD4+CD25+Foxp3+, as
described previously (22).
Cytotoxic T lymphocytes were distinguished using typical markers
CD3+CD4−CD8+, as described
previously (23).
Immunohistochemistry
The fresh tissue specimens were deparaffinized in
xylene and dehydrated through a graduated alcohol series. The
paraffin-embedded tissue sections (4-µm) were prepared and epitope
retrieval was performed using Tris-EDTA buffer solution (pH 9.0,
Sigma-Aldrich; Merck KGaA; cat. no. SRE0063) for 60 min at 65°C.
The paraffin sections were subjected with hydrogen peroxide (3%)
for 15 min at 37°C and then blocked with 5% BSA (Sigma-Aldrich;
Merck KGaA) for 2 h at 37°C. The sections were incubated with
anti-CD3 (1:1,000, cat. no. ab16669; Abcam, Cambridge, UK),
anti-CD4 (1:1,000, cat. no. ab133616; Abcam), and anti-CD8
(1:1,000, cat. no. ab4055; Abcam), respectively, for 12 h at 4°C.
All sections were washed three times with PBS for 15 min at 37°C
and then incubated with goat anti-rabbit IgG H&L secondary
antibodies (Alexa Fluor® 488, 1:1,000, cat. no.
ab150077; Abcam) for 2 h at 37°C. The tumor sections were observed
in three-random views under a fluorescence microscope (YS100; Nikon
Corporation, Tokyo, Japan). Densitometric quantification of the
immunohistochemistry data was performed by using Quantity-One 1.0
software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Assessment of T-cell density
Images of the gastric tumor sections (4-µm) were
captured using a high-resolution digital scanner (Aperio Scanscope
XT; Leica Biosystems, North Ryde, NSW, Australia) at ×40
magnification. The T-cell density (cells per mm2 tissue)
was analyzed using software (Aperio Imagescope version 11; Leica
Biosystems).
Statistical analysis
Data are presented as the mean and standard error of
the mean and statistical analyses were performed using SAS version
9.3 (SAS Institute, Inc., Cary, NC, USA). Differences in T-cell
density in each group were analyzed using general linear models.
Associations of clinical variables and pCR were analyzed using
logistic regression. The Kaplan-Meier method with and the log-rank
test was used to analyze time-to-event outcomes (CSS and RFS). The
prognostic value of CD3+, CD4+ and
CD8+ for cancer-specific survival rate was analyzed
using the Kaplan-Meier method and multivariate Cox proportional
hazard model analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics and treatment
response
A total of 68 patients with gastric cancer were
identified and tumor samples from these 68 patients were included
in the tissue microarrays. The clinical characteristics of the 68
patients with gastric cancer are shown in Table I. There were 48 (70.6%) patients with
clinical T3 stage, 12 (17.6%) patients with clinical T2 stage 2,
four (5.9%) patients with clinical T4 stage and four (5.9%)
patients with metastatic disease (Table
II).
 | Table I.Characteristics of patients with
gastric cancer. |
Table I.
Characteristics of patients with
gastric cancer.
| Characteristic | n |
|---|
| Patients | 68 |
| Male | 34 |
| Female | 34 |
| Age in years, mean
(SD) | 63
(12.3)a |
| Weeks between end
of CRT and surgery, median (IQR) | 6
(5,7)b |
| Pretreatment
clinical T stage, n (%) |
|
| T2 | 12 (17.6) |
| T3 | 48 (70.6) |
| T4 | 4 (5.9) |
 | Table II.Clinical and pathological response of
patients with gastric cancer to neoadjuvant chemoradiotherapy
paclitaxel and carboplatin. |
Table II.
Clinical and pathological response of
patients with gastric cancer to neoadjuvant chemoradiotherapy
paclitaxel and carboplatin.
| Stage | n (%) |
|---|
| T2 | 12 (17.6) |
| T3 | 48 (70.6) |
| T4 | 4 (5.9) |
| Metastatic | 4 (5.9) |
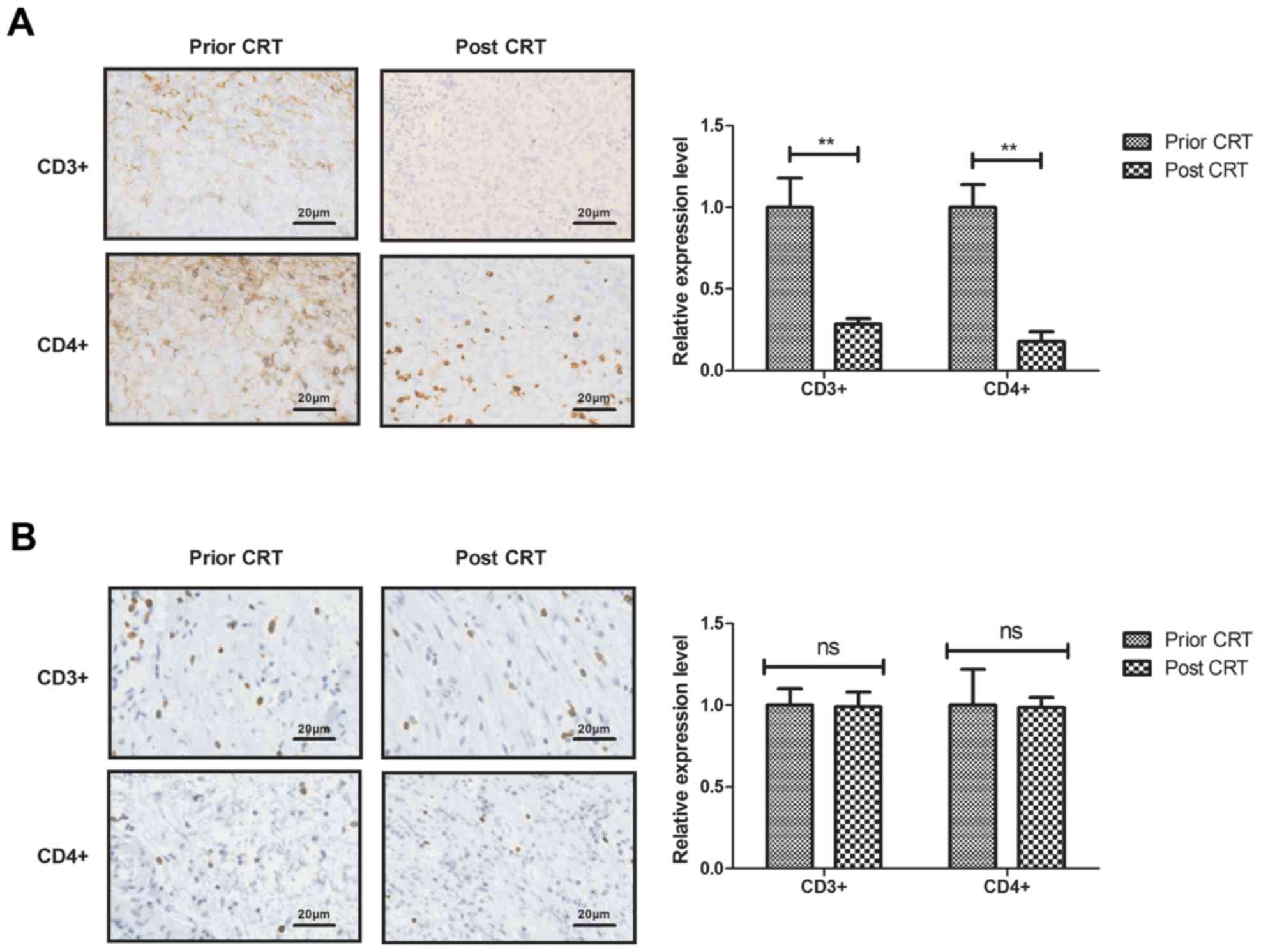
Regulatory T-cell density in the
gastric tumor microenvironment following CRT
The present study analyzed the regulatory T-cell
density in the gastric tumor microenvironment following CRT. The
density of CD3+ and CD4+ T cells were
decreased in the T4 stage gastric tumor tissue (Fig. 1A). The T3 stage gastric tumor tissue
showed a similar distribution of CD3+ and
CD4+ T cells (Fig. 1B).
However, the density of CD3+ and CD4+ T cells
were increased in the T2 gastric tumor tissue (Fig. 1C).
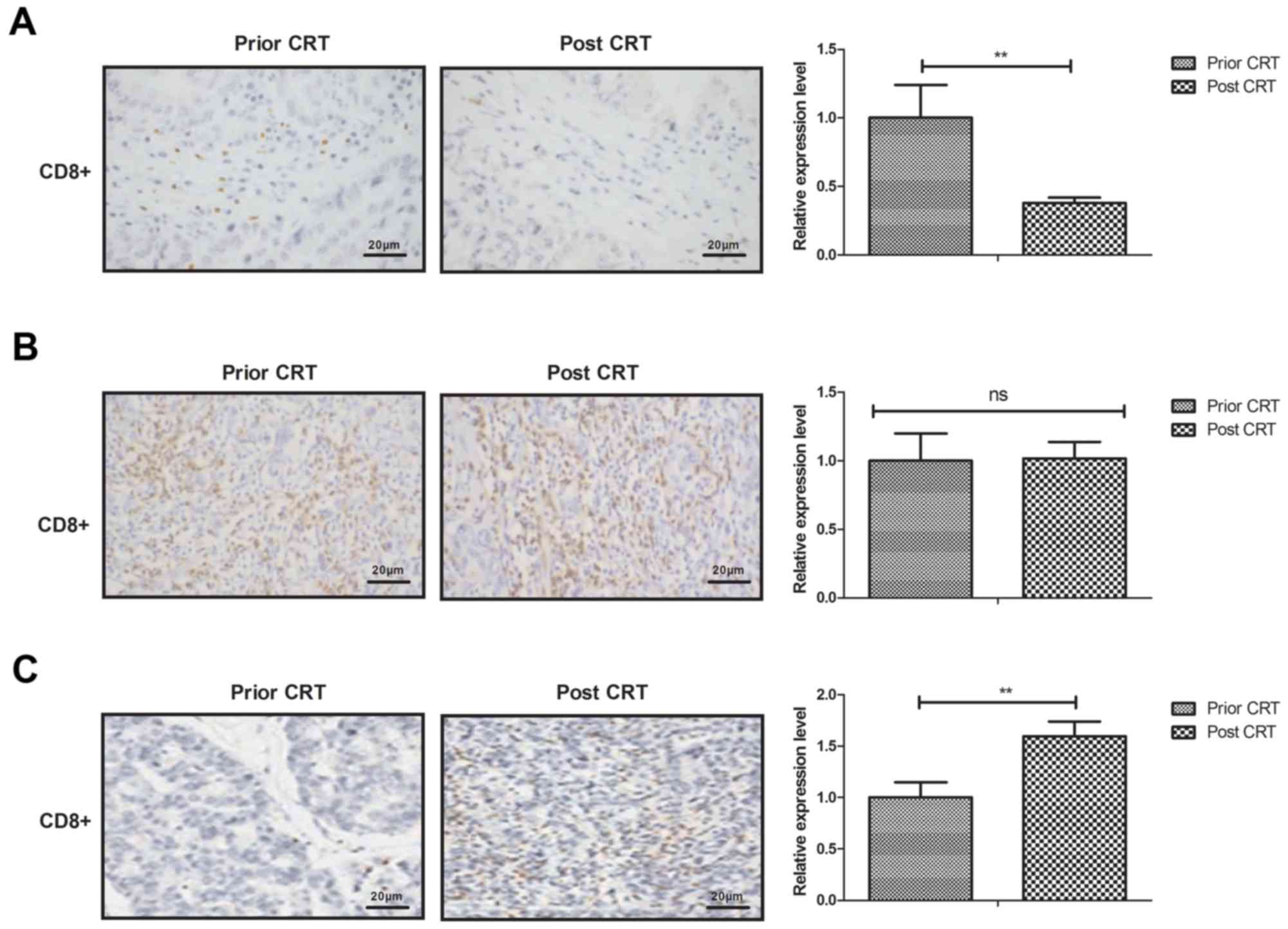
Cytotoxic T lymphocyte in the gastric
tumor microenvironment following CRT
The present study analyzed the cytotoxic T
lymphocyte density in the gastric tumor microenvironment following
CRT. The T4 stage gastric tumor tissue exhibited a lower density of
CD8+ cells compared with prior to CRT (Fig. 2A). The density of CD8+
cells in T3 stage gastric tumor tissue did not differ significantly
between pre-CRT and post CRT (Fig.
2B). The density of CD8+ cells were increased in T2
stage gastric tumor tissue (Fig.
2C).
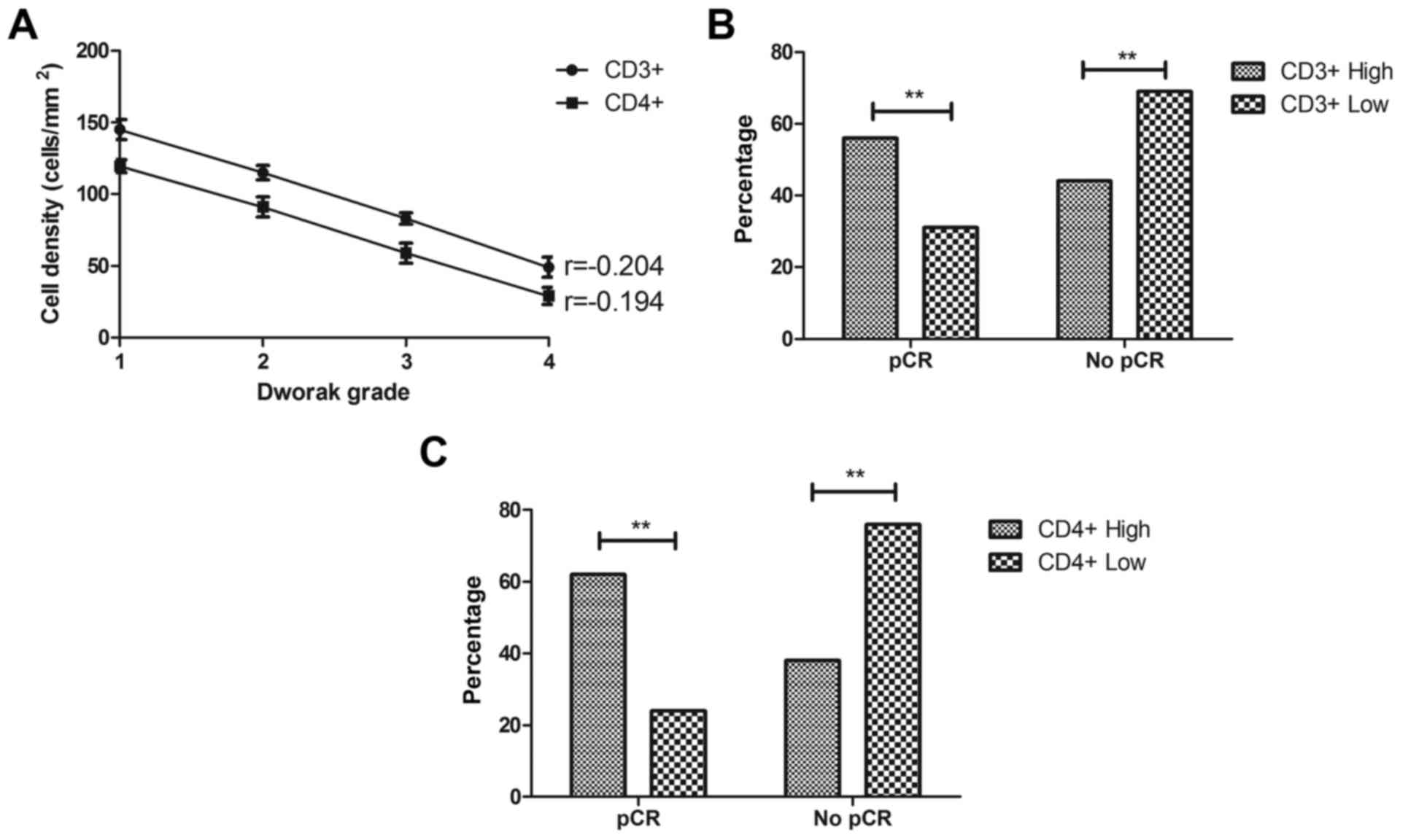
Regulatory T-cell density is
associated with the response to CRT
Analysis of the regulatory T-cell density revealed
an inverse correlation between CD3+, CD4+
cell density in gastric cancer regression (r=−0.204 and −0.194,
respectively, Fig. 3A). Among the
patients who had pCR, 31% had a low density of CD3+
cells, which was lower than 56% of those with pCR with a high
CD3+ cell density (Fig.
3B). The results also demonstrated that, of patients with a
high CD4+ cell density, 62% had a pCR, which was
significantly higher than that in patients with a low
CD4+ cell density (Fig.
3C).
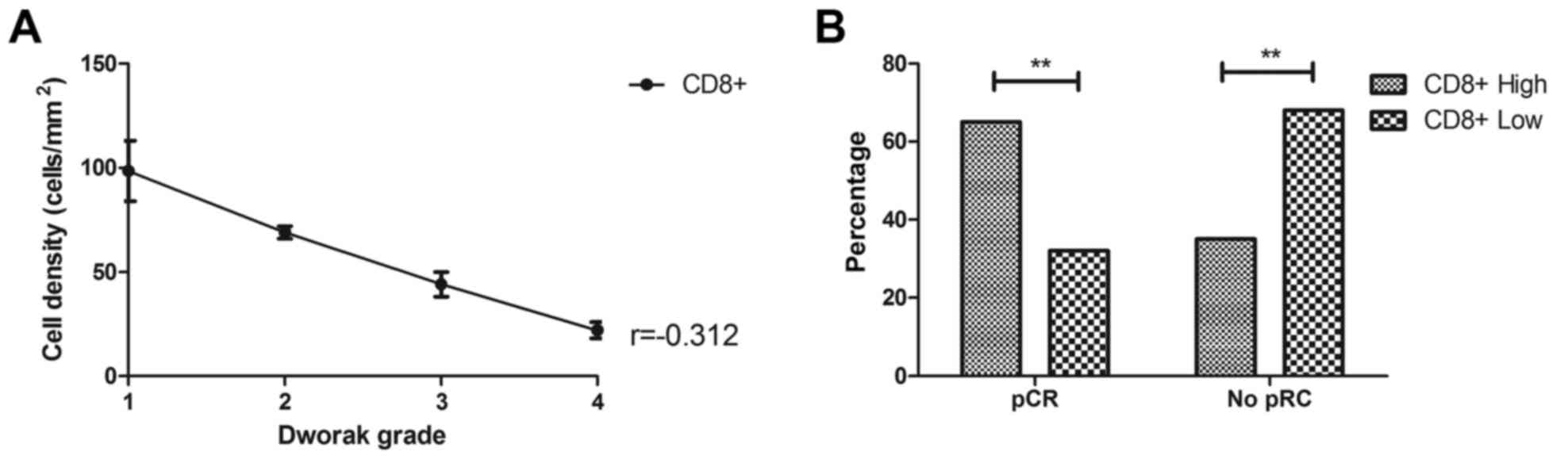
Cytotoxic T lymphocyte density is
associated with the response to CRT
The association between cytotoxic T lymphocytes and
pCR was also analyzed in the present study. As shown in Fig. 3A, cytotoxic T lymphocyte
CD8+ cells had an inverse correlation with gastric
cancer regression (r=−0.312, Fig.
4A). It was observed that 65% patients with a high
CD8+ cell density showed pCR, whereas 32% patients with
a low CD8+ cell density showed pCR (Fig. 4B).
Regulatory T-cell and cytotoxic T
lymphocyte density as a prognostic marker in neoadjuvantly treated
gastric cancer
A total of 56 patients (82%) survived the 60-month
follow-up from the date of surgery, 12 (21%) patients succumbed to
mortality during the follow-up period, 28 patients (50%) survived
without cancer recurrence, 12 patients (21%) showed local
recurrence, and four patients (8%) showed colon metastases
(Table III). It was shown that
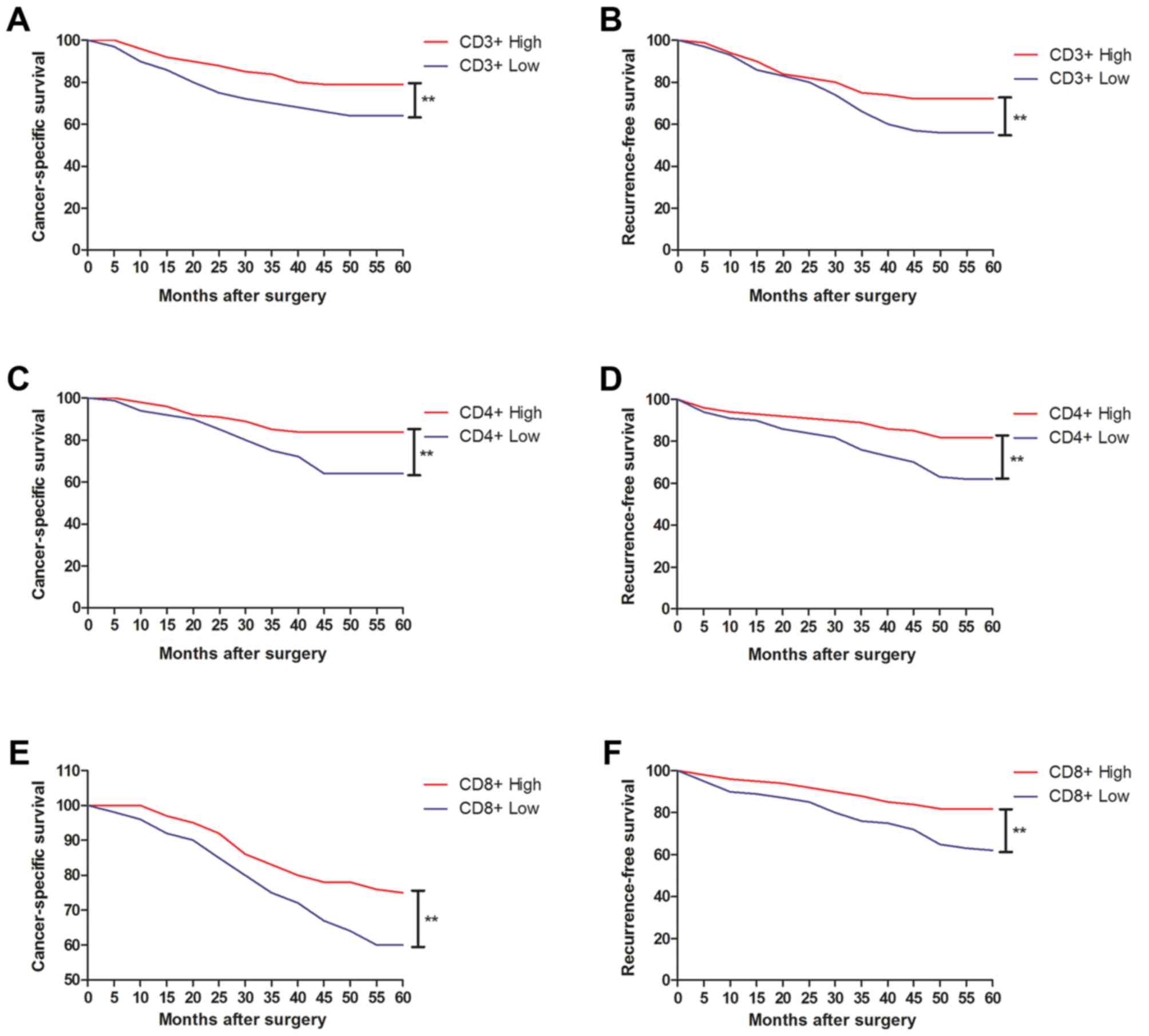
CD3+ and CD4+ cell density were associated
with the survival rate of patients with gastric cancer (Fig. 5A-D). The probabilities of 5-year CSS
and RFS were 79, vs. 64% and 72, vs. 56% in the CD3+
high, vs. low groups, respectively (Fig.
5A and B). The probabilities of 5-year CSS and RFS were 84, vs.
64% and 82, vs. 62% in the CD4+ high, vs. low groups,
respectively (Fig. 5C and D). It was
also shown that CD8+ cell density was associated with
the survival rates of patients with gastric cancer (Fig. 5E and F). The probabilities of 5-year
CSS and RFS were 75%, vs. 60% and 81, vs. 62% in the
CD8+ high, vs. low groups, respectively (Fig. 5E and F). The densities of
CD3+, CD4+ and CD8+ cells were
associated with CSS of patients with gastric cancer (Table IV). In addition, tumor size and
concomitant diseases markedly affected the CSS of patients with
gastric cancer.
 | Table III.Clinical outcomes of patients with
gastric cancer following neoadjuvant chemoradiotherapy with
paclitaxel and carboplatin. |
Table III.
Clinical outcomes of patients with
gastric cancer following neoadjuvant chemoradiotherapy with
paclitaxel and carboplatin.
| Outcome | n (%) |
|---|
| Mortality | 12 (21) |
| Survival without
cancer recurrence | 28 (50) |
| Local
recurrence | 12 (21) |
| Colon
metastases | 4 (8) |
 | Table IV.Univariate and multivariable analyses
of prognostic factors for CSS following curative gastric cancer
resection. |
Table IV.
Univariate and multivariable analyses
of prognostic factors for CSS following curative gastric cancer
resection.
|
| CSS |
|---|
|
|
|
|---|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Prognostic
factor | Log-rank | P-value | HR | 95% CI | P-value |
|---|
| CD3+
cell density |
|
|
|
|
|
|
Interaction |
|
|
|
|
|
| No
interaction | 1.193 | 0.026a | 1.704 | 1.104–2.824 | 0.038a |
| CD4+
cell density |
|
|
|
|
|
|
Interaction |
|
|
|
|
|
| No
interaction | 1.826 | 0.058 | 0.886 | 0.914–2.320 | 0.026a |
| CD8+
cell density |
|
|
|
|
|
|
Interaction |
|
|
|
|
|
| No
interaction | 2.520 | 0.037a | 2.158 | 1.104–2.824 | 0.025a |
| Concomitant
disease |
|
|
|
|
|
|
Interaction |
|
|
|
|
|
| No
interaction | 4.726 | 0.016a | 1.862 | 1.426–3.026 | 0.018a |
| Tumor size
(mm) |
|
|
|
|
|
|
<50 | 3.746 | 0.014a | 1.638 | 1.242–2.858 | 0.0163a |
|
≥50 |
|
|
|
|
|
Discussion
Although previous reports have shown that CRT
treatment can inhibit gastric cancer growth in the process of
cancer progression (24–26), the associations between regulatory
T-cell density, cytotoxic T lymphocyte density and pCR to
paclitaxel and carboplatin have not reported in gastric cancer. In
the present study, the densities of regulatory T-cell density and
cytotoxic T lymphocytes were analyzed in gastric cancer tissues
from a total of 68 patients. An association was found between
regulatory T-cell density and cytotoxic T lymphocyte density in the
local tumor microenvironment following neoadjuvant CRT of
paclitaxel and carboplatin in locally gastric cancer. Owing to the
evidence of an immune-mediated component to chemoradiotherapy, it
was hypothesized that regulatory T-cells inhibited the response of
gastric carcinoma to neoadjuvant CRT and cytotoxic T lymphocytes
promoted the response of gastric carcinoma to neoadjuvant CRT.
Currently, paclitaxel is one of the most widely used
clinical anticancer drugs in chemotherapy (27). The combined administration of
paclitaxel has been reported to markedly enhance tumor necrosis
factor-related apoptosis-inducing ligand-induced apoptosis in
resistant cancer cells in vitro and in vivo (28), and Zhu et al indicated that
CD4+Foxp3+ regulatory T-cell impairment by
paclitaxel is independent of Toll-like receptor 4 (29). In the present study, the results
revealed that the regulatory T-cell density of CD3+ and
CD4+ T cells were decreased in T4 stage gastric tumor
and increased in T2 gastric tumor following CRT. However, T3 stage
gastric tumor showed similar distribution of CD3+ and
CD4+ T cells between pre-CRT and post-CRT gastric cancer
tissues. The findings also indicated that regulatory T-cell
CD3+, CD4+ cell density was inversely
correlated with gastric cancer regression. However, further
analysis is required in larger gastric cancer populations.
Carboplatin is active in advanced gastric and
esophageal cancer (30). A report
found that carboplatin and paclitaxel added to radiation and
fluoropyrimidine analogs is a well-tolerated regimen in the
adjuvant setting, which is a safe and feasible regimen in this
relatively high-risk group of patients with gastric cancer and is
of interest for future development (31). The density of tumor-infiltrating T
cells (CD8+ T cells), is an indicator of improved
survival rate in patients with gastric cancer, and the CD8 T-cell
density predicts the likelihood of tumor regression following CRT
(32). In the present study, it was
found that cytotoxic T lymphocyte CD38+ CD8+
density was decreased in T4 stage gastric tumor tissue and
increased in T2 stage gastric tumor tissue. Cytotoxic T lymphocyte
CD3+ CD8+ density was also inversely
correlated with gastric cancer regression.
In conclusion, the results of the present study
indicated that regulatory T-cell and cytotoxic T lymphocyte density
may be considered as prognostic markers in neoadjuvantly treated
gastric cancer. Notably, regulatory T-cell and cytotoxic T
lymphocyte density were associated with pCR and the improved
long-term outcome of patients with gastric cancer. These results
suggest that regulatory T-cells in the local microenvironment may
inhibit the response of gastric cancer to neoadjuvant CRT and
cytotoxic T lymphocytes may promote the response of gastric cancer
to CRT.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DH performed the experiments. YY, SZ, ZS, TP, XW and
YZ prepared and analysed experimental data. SL designed the
experiments.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the First Affiliated Hospital of Hebei North University. All
patients provided written informed consent prior to undergoing any
protocol-specific screening procedures or treatments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Visa L, Jimenez-Fonseca P, Martinez EA,
Hernández R, Custodio A, Garrido M, Viudez A, Buxo E, Echavarria I,
Cano JM, et al: Efficacy and safety of chemotherapy in older versus
non-older patients with advanced gastric cancer: A real-world data,
non-inferiority analysis. J Geriatr Oncol. 9:254–264. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suzuki H and Mori H: World trends for H.
pylori eradication therapy and gastric cancer prevention strategy
by H. pylori test-and-treat. J Gastroenterol. 53:354–361. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carmona-Bayonas A, Jimenez-Fonseca P,
Custodio A, Sánchez Cánovas M, Hernández R, Pericay C, Echavarria
I, Lacalle A, Visa L, Palomo Rodríguez A, et al:
Anthracycline-based triplets do not improve the efficacy of
platinum-fluoropyrimidine doublets in first-line treatment of
advanced gastric cancer: Real-world data from the AGAMEMON National
Cancer Registry. Gastric Cancer. 21:96–105. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun N, Sun Q, Liu Q, Zhang T, Zhu Q, Wang
W, Cao M and Zang QI: α-fetoprotein-producing gastric carcinoma: A
case report of a rare subtype and literature review. Oncol Lett.
11:3101–3104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tian Q, Zeng J, Tao X, Zhang Z, Zhou X and
Wang Y: Clinical pathology of metastatic gastric carcinoma to the
breast: A report of two cases and a review of literature. Oncol
Lett. 11:3081–3084. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Petrioli R, Roviello G, Zanotti L,
Roviello F, Polom K, Bottini A, Marano L, Francini E, Marrelli D
and Generali D: Epirubicin-based compared with docetaxel-based
chemotherapy for advanced gastric carcinoma: A systematic review
and meta-analysis. Crit Rev Oncol Hematol. 102:82–88. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shan B, Shan L, Morris D, Golani S and
Saxena A: Systematic review on quality of life outcomes after
gastrectomy for gastric carcinoma. J Gastrointest Oncol. 6:544–560.
2015.PubMed/NCBI
|
|
8
|
Bollschweiler E, Berlth F, Baltin C, Mönig
S and Hölscher AH: Treatment of early gastric cancer in the Western
World. World J Gastroenterol. 20:5672–5678. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoshikawa T, Aoyama T, Hayashi T, Kuwabara
H, Mikayama Y, Ogata T, Cho H and Tsuburaya A: Neoadjuvant
chemotherapy for gastric cancer-evidence from the world and future
strategy in Japan. Gan To Kagaku Ryoho. 39:866–870. 2012.(In
Japanese). PubMed/NCBI
|
|
10
|
Kocáková I, Vetchá H, Soumarová R and
Vyzula R: Role of adjuvant chemoradiotherapy in the treatment of
gastric carcinoma. Cas Lek Cesk. 142 Suppl 1:S26–S28. 2003.(In
Czech).
|
|
11
|
Sher DJ: Neoadjuvant chemoradiotherapy for
stage III Non-small cell lung cancer. Front Oncol. 7:2812017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van der Sluis FJ, van Westreenen HL, van
Etten B, van Leeuwen BL and de Bock GH: Pretreatment identification
of patients likely to have pathologic complete response after
neoadjuvant chemoradiotherapy for rectal cancer. Int J Colorectal
Dis. 33:149–157. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun Y, Zhang Y, Wu X, Lin H, Lu X, Huang
Y, Xu Z, Huang S, Wang X and Chi P: Prognostic significance of
neoadjuvant rectal score in locally advanced rectal cancer after
neoadjuvant chemoradiotherapy and construction of a prediction
model. J Surg Oncol. 117:737–744. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rino Y, Yukawa N, Wada N, Suzuki M,
Murakami H, Yamada T, Nakayama H, Yamamoto N, Sato T, Yamada R, et
al: Phase II study of S-1 monotherapy as a First-line, combination
therapy of S-1 plus cisplatin as a Second-line, and weekly
paclitaxel monotherapy as a Third-line therapy in patients with
advanced gastric carcinoma: Phase II study of S-1, S-1 plus
cisplatin, and weekly paclitaxel in patients with advanced gastric
carcinoma. Clin Med Oncol. 2:375–383. 2008.PubMed/NCBI
|
|
15
|
Chen JL, Zhu JS, Hong J, Chen MX, Lu JL,
Chen WX, Shen B, Zhu ZM and Chen NW: Effect of
2-(8-hydroxy-6-methoxy-1-oxo-1H-2-benzopyran-3-yl) propionic acid
in combination with carboplatin on gastric carcinoma growth in
vivo. World J Gastroenterol. 13:509–514. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fukuda K, Tsujitani S, Maeta Y, Yamaguchi
K, Ikeguchi M and Kaibara N: The expression of RCAS1 and tumor
infiltrating lymphocytes in patients with T3 gastric carcinoma.
Gastric Cancer. 5:220–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koyama S: Differential expression of
intracellular apoptotic signaling molecules in tumor and
tumor-infiltrating lymphocytes during development of invasion
and/or metastasis of gastric carcinoma. Dig Dis Sci. 48:2290–2300.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haas M, Buttner M, Rau TT, Fietkau R,
Grabenbauer GG and Distel LV: Inflammation in gastric
adenocarcinoma of the cardia: How do EBV infection, Her2
amplification and cancer progression influence tumor-infiltrating
lymphocytes? Virchows Arch. 458:403–411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mlecnik B, Tosolini M, Kirilovsky A,
Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman
WH, Pagès F and Galon J: Histopathologic-based prognostic factors
of colorectal cancers are associated with the state of the local
immune reaction. J Clin Oncol. 29:610–618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Waddell T, Verheij M, Allum W, Cunningham
D, Cervantes A and Arnold D: Gastric cancer: ESMO-ESSO-ESTRO
clinical practice guidelines for diagnosis, treatment and
follow-up. Eur J Surg Oncol. 40:584–591. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dworak O, Keilholz L and Hoffmann A:
Pathological features of rectal cancer after preoperative
radiochemotherapy. Int J Colorectal Dis. 12:19–23. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Szurek E, Cebula A, Wojciech L, Pietrzak
M, Rempala G, Kisielow P and Ignatowicz L: Differences in
expression level of helios and Neuropilin-1 do not distinguish
thymus-derived from extrathymically-induced CD4+Foxp3+ regulatory T
cells. PLoS One. 10:e01411612015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brandt D, Sergon M, Abraham S, Mäbert K
and Hedrich CM:
TCR+CD3+CD4−CD8−
effector T cells in psoriasis. Clin Immunol. 181:51–59. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang X, Shen Y, Zhu H, Zhao Y, Li Z, Qiu
M, Li Q, Gou H, Yang Y, Cao D, et al: A phase II trial of
concurrent 3D-CRT/IMRT and oxaliplatin, 5-fluorouracil and
leucovorin (FOLFOX) in gastric cancer patients with R0 gastrectomy
and D2 lymph node dissection. Gastric Cancer. 19:245–254. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang X, Li G, Zhang Y, Bai S, Xu F, Wei Y
and Gong Y: Single-arc volumetric-modulated arc therapy (sVMAT) as
adjuvant treatment for gastric cancer: Dosimetric comparisons with
three-dimensional conformal radiotherapy (3D-CRT) and
intensity-modulated radiotherapy (IMRT). Med Dosim. 38:395–400.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Papadimitriou K, Vassiliou V, Kountourakis
P, Polyviou P, Andreopoulos D and Papamichael D: Adjuvant
chemoradiotherapy (CRT) for high-risk gastric cancer (GC) patients:
Single-center experience using infusional 5-fluorouracil (5FU) and
radiotherapy (RT). Med Oncol. 29:2716–2717. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Plosker GL and Hurst M: Paclitaxel: A
pharmacoeconomic review of its use in non-small cell lung cancer.
Pharmacoeconomics. 19:1111–1134. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li L, Wen XZ, Bu ZD, Cheng XJ, Xing XF,
Wang XH, Zhang LH, Guo T, Du H, Hu Y, et al: Paclitaxel enhances
tumoricidal potential of TRAIL via inhibition of MAPK in resistant
gastric cancer cells. Oncol Rep. 35:3009–3017. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu Y, Liu N, Xiong SD, Zheng YJ and Chu
YW: CD4+Foxp3+ regulatory T-cell impairment by paclitaxel is
independent of toll-like receptor 4. Scand J Immunol. 73:301–308.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, Pang L and Feng J: A phase II
study of etoposide, doxorubicin, and carboplatin in the treatment
of advanced gastric cancer. Am J Clin Oncol. 25:71–75. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mobayed M, Heilbrun LK, Shields AF,
Washington T, Venkatramanamoorthy R, Philip PA and El-Rayes BF:
Safety and feasibility of carboplatin and paclitaxel followed by
fluoropyrimidine analogs and radiation as adjuvant therapy for
gastric cancer. Case Rep Oncol. 2:220–228. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu X, Yang L, Yao D, Wu X, Li J, Liu X,
Deng L, Huang C, Wang Y, Li D and Liu J: Tumor antigen-specific
CD8+ T cells are negatively regulated by PD-1 and Tim-3
in human gastric cancer. Cell Immunol. 313:43–51. 2017. View Article : Google Scholar : PubMed/NCBI
|