Introduction
Acute Lung Injury (ALI) and acute respiratory
distress syndrome (ARDS) are two types of common and complex
inflammatory lung diseases (1). As a
kind of novel gene regulatory molecule, microRNAs play an important
role in various complicated diseases, including ALI (2).
ARDS is a common and major inflammatory lung
disease, characterized by rapid progression. It is estimated that
there are 190,000 newly diagnosed cases of ALI in the United States
alone each year, 75,000 of whom die annually (3). In China, the incidence of ALI/ARDS is
higher, with mortality rate as high as 52% (2). In recent years, it has been noted that
miRNAs play an important role in multiple biological processes and
signal transduction pathways of ALI/ARDS (4).
The expression changes of miRNAs are associated with
immune response, inflammatory signaling pathways and pathogenesis
of inflammatory lung diseases, including ALI, hence, miRNAs can be
considered as new therapeutic target (4). Although miRNAs change the expression of
related genes moderately, it may greatly affect the expression of a
large number of downstream genes, thereby influencing a variety of
biological processes. Therefore, miRNAs might be potentially used
as one of the markers (5,6).
Toll-like receptor (TLR) family serves both as
signal molecules and receptors of lipopolysaccharide (LPS)
(7). TLR4, a member of TLR family,
is mainly distributed on the surface of mononuclear macrophage,
which mediates the intracellular conduction of LPS inflammation
signals, and is the preferential receptor of LPS (8). Nuclear factor (NF)-κB collectively
refers to many DNA binding proteins which specifically bind to κB
site of promoters of a variety of genes, and promotes
transcription, which plays an important role in immune response,
inflammatory response and cell growth regulation (7). NF-κB signal pathway is the most
important downstream pathway among all signal transduction pathways
mediated by LPS, suggesting that TLR4/NF-κB pathway may be the key
target to trigger inflammatory response and organ injury (9). Zhang and Xu (10) has indicated that miRNA-140-5p
regulates hypoxia-mediated human pulmonary artery smooth muscle
cell proliferation. Herein, the aim of the present study was to
investigate the function of miRNA-140-5p in ts ALI and its
regulatory mechanism.
Materials and methods
Animals and ethics statement
C57/BL6 mice, 6–8 weeks of age, were purchased from
Guangdong Medical Laboratory Animal Centre (Guangzhou, China). The
mice were randomly divided into two groups, control group (n=6) and
Model group (n=8). In Model group, mice were inhaled with 1,000
µg/ml LPS for 1 h at 3 h intervals over a period of 8 h. In control
group, mice were inhaled with normal saline for 1 h at 3 h
intervals over a period of 8 h. The experimental procedures
conformed to the Guide for the Care and Use of Laboratory Animal,
and all of the procedures were approved by the Institutional Ethics
Committee of Sichuan Cancer Hospital (Sichuan, China).
Enzyme-linked immunosorbent assay
(ELISA) kits
Lung tissue or transfection cells were extracted in
lysis buffer (Beyotime Institute of Biotechnology, Nanjing, China)
and Protein concentration was measured by the Pierce™ BCA protein
assay kit Beyotime Institute of Biotechnology, Nanjing, China). 10
µg extracts was used to analyze tumor necrosis factor (TNF)-α,
interleukin (IL)-1β, IL-6 and MPO levels using ELISA kits.
Histopathology
Lung tissue samples were fixed in 4%
paraformaldehyde for 24 h, embedded in paraffin, and cut into 5-µm
thick sections. Lung tissue samples was stained with hematoxylin
and eosin assay for 5 min and observed using a phase fluorescence
microscope (Axio Observer; Carl Zeiss AG, Oberkochen, Germany).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from serum was extracted using TRIzol
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. cDNA was reverse transcribed, which was
performed as described using the PrimeScript™ RT reagent kit
(Takara Bio, Inc., Otsu, Japan). Relative gene expression was
quantified using ABI 7900HT Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) with SYBR®
Premix Ex Taq™ II kit (Takara Bio, Inc.). The thermocycling
conditions were as follows: 94°C for 10 min, followed by 40 cycles
of 94°C for 15 sec, 55°C for 30 sec and 72°C for 30 sec. The
relative expression levels were analyzed using the
2−ΔΔCq method (11).
Cell culture and treatment
Human lung A549 cells were maintained in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc.) containing 10% fetal calf serum (FCS; Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin
(Gibco; Thermo Fisher Scientific, Inc.) at 5% CO2 at
37°C.
Transfection
miRNA-140-5p mimic and mimic control (mimic-NC) were
purchased from Sangon Biotech Co., Ltd. (Shanghai, China). A549
cell was co-transfected with 20 nM miRNA-140-5p mimic and mimic
control (mimic-NC) using Lipofectamine 3000 (Invitrogen, Guangzhou,
China). After transfection for 4 h, old medium was removed and new
medium was added into cell. After transfection for 48 h, A549 cell
was treated by 100 ng/ml of LPS for 4 h, and then analyzed other
study.
Dual luciferase assays
293 cells were cotransfected with TLR4 luciferase
reporter construct with miR-140-5p using Lipofectamine 2000
transfection reagent (Thermo Fisher Scientific, Inc.) for 48 h.
Luciferase activity was assayed using a Dual-Luciferase Reporter
Assay System (Promega Corporation, Madison, WI, USA).
Western blotting
Transfection cells were extracted in lysis buffer
(Beyotime Institute of Biotechnology, Nanjing, China) and Protein
concentration was measured by the Pierce™ BCA protein assay kit
(Beyotime Institute of Biotechnology, Nanjing, China). Equal amount
of the extracts (50 µg) were loaded and separated in a 8–10%
polyacrylamide gel, then proteins were transferred onto a PVDF
membrane (EMD Millipore, Billerica, MA, USA). Membranes were
incubated with TLR4, myeloid differentiation primary response 88
(MyD88), NF-κB, and GADPH (Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) at 4°C over-night. Membranes were incubated with goat
anti-rabbit HRP (Santa Cruz Biotechnology, Inc.; 1:2,000
dilution).
Immunofluorescence
Cell was washed with PBS and fixed with 4%
paraformaldehyde for 15 min, and blocked with 0.5 ml PBS containing
0.1% Triton X-100 and 1% BSA at room temperature for 1 h. Cell were
incubated with TLR4 (Santa Cruz Biotechnology, Inc.) at 4°C
over-night. Cell were then washed with PBS and incubated with Alexa
555 labelled Rabbit Anti-Goat secondary antibody (Santa Cruz
Biotechnology, Inc.) in the dark for 1 h at room temperature and
stained with DAPI for 15 min in the dark. Images cells were
obtained by using a phase fluorescence microscope (Axio Observer;
Carl Zeiss AG).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Data were analyzed using SPSS 17.0 software (SPSS, Inc.,
Chicago, IL, USA). Independent-samples t-tests between two groups
and one-way analysis of variance for multiple comparisons with
Dunnett's post hoc test were performed. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-140-5p expression in mice with
ALI
The expression of miR-140-5p was examined in ALI and
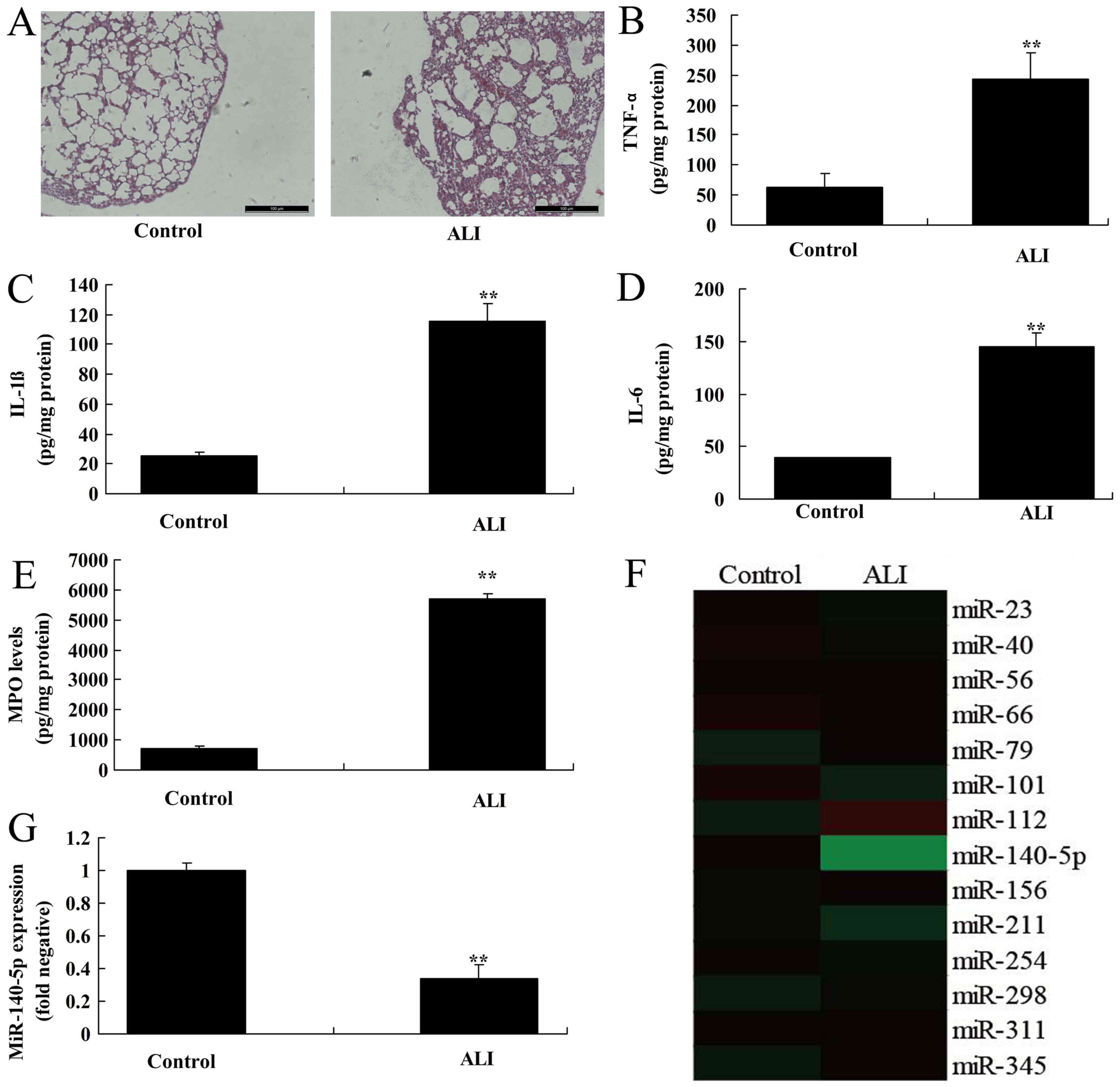
control mice. Firstly, HE staining showed there was diffuse
alveolar damage in ALI group, compared with control group (Fig. 1A). Then, the levels of TNF-α, IL-1β,
IL-6 and MPO were increased in ALI group in comparison to control
group (Fig. 1B-E). As shown in
Fig. 1G, miR-140-5p expression in
mice with ALI was suppressed, compared with normal control group.
Therefore, miR-140-5p may be a key factor for ALI.
miR-140-5p regulated inflammation in
in vitro model of ALI
To explore whether miR-140-5p regulated inflammation
of ALI, the levels of TNF-α, IL-1β, IL-6 and MPO were measured in
this study. As shown in Fig. 2A and
B, anti-miR-140-5p mimics or miR-140-5p mimics inhibited or
increased the expression of miR-140-5p in in vitro model of
ALI, compared with that in the control group. The levels of TNF-α,
IL-1β, IL-6 and MPO were significantly elevated in in vitro
model of ALI following miR-140-5p down-regulation, compared with
control group (Fig. 2C-F).
Over-expression of miR-140-5p inhibited the levels of TNF-α, IL-1β,
IL-6 and MPO in in vitro model of ALI, in comparison with
control group (Fig. 2G-J). Hence,
miR-140-5p may regulate the inflammation of ALI.
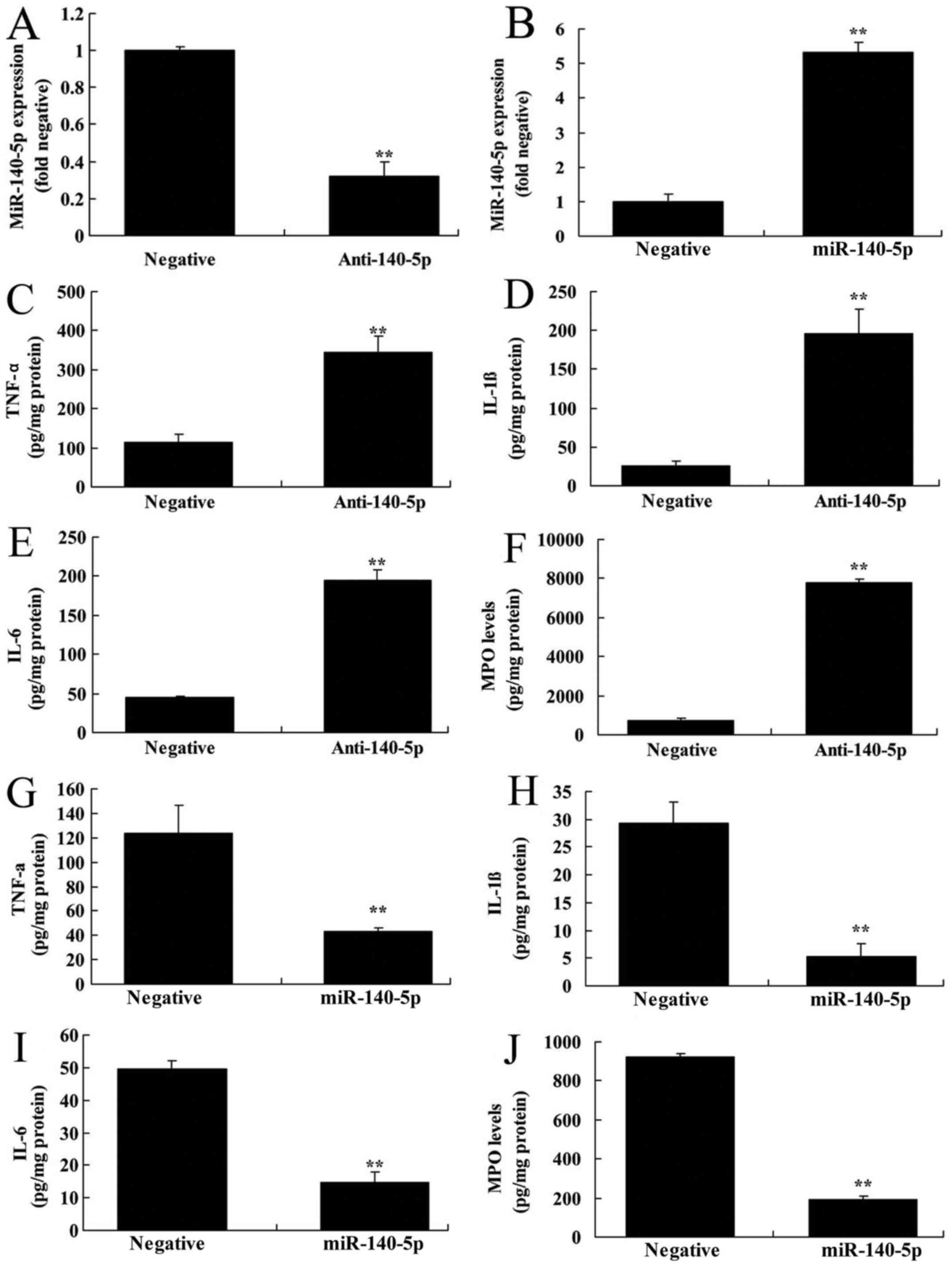 | Figure 2.miR-140-5p regulates inflammation in
an in vitro model of acute lung injury. Reverse
transcription-quantitative polymerase chain reaction was performed
to determine the expression of (A and B) miR-140-5p in the
anti-140-5p and miR-140-5p groups, as well as that of (C) TNF-α,
(D) IL-1β, (E) IL-6 and (F) MPO levels following of miR-140-5p
downregulation; and also (G) TNF-α, (H) IL-1β, (I) IL-6 and (J) MPO
levels following miR-140-5p overexpression. **P<0.01 vs.
negative control group. miR, microRNA; Anti-140-5p, downregulation
of miR-140-5p group; miR-140-5p, overexpression of miR-140-5p
group; TNF-α, tumor necrosis factor-α; IL, interleukin; MPO,
myeloperoxidase. |
miR-140-5p regulated inflammation in
in vitro model of ALI by MyD88/NF-KB signaling pathway by targeting
TLR4
Next TLR4/MyD88/NF-κB signaling pathway was measured
in this study to determine the mechanism function of miR-140-5p in
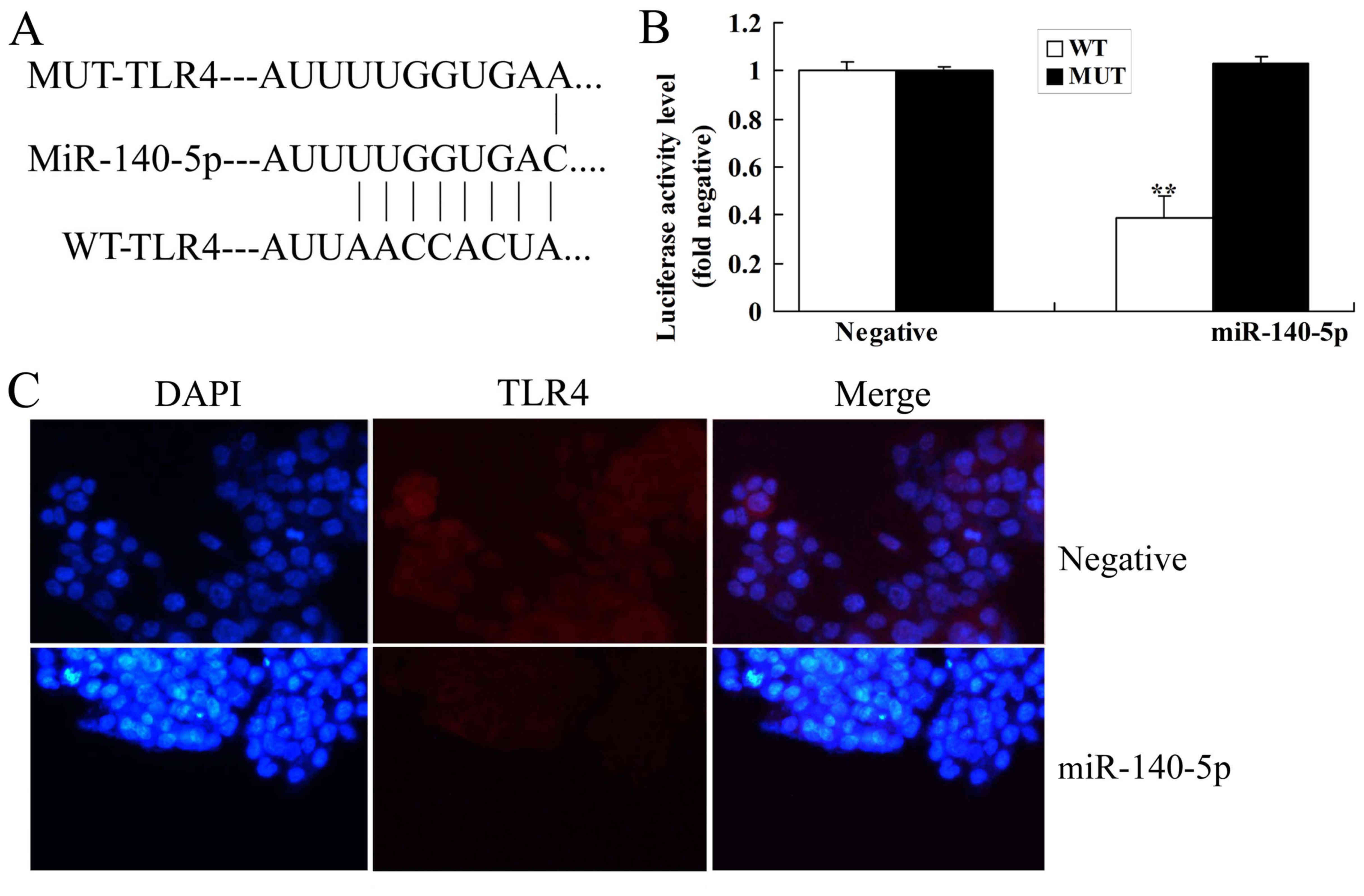
ALI. As shown in Fig. 3A and B,
putative binding sequences of miR-140-5p in the 3′UTR of TLR4, and
the luciferase assay of miR-140-5p was reduced in WT group,
compared with that in the control group. Immunofluorescence showed
that over-expression of miR-140-5p suppressed TLR4 protein
expression in in vitro model of ALI, compared with control
group (Fig. 3C). Down-regulation of
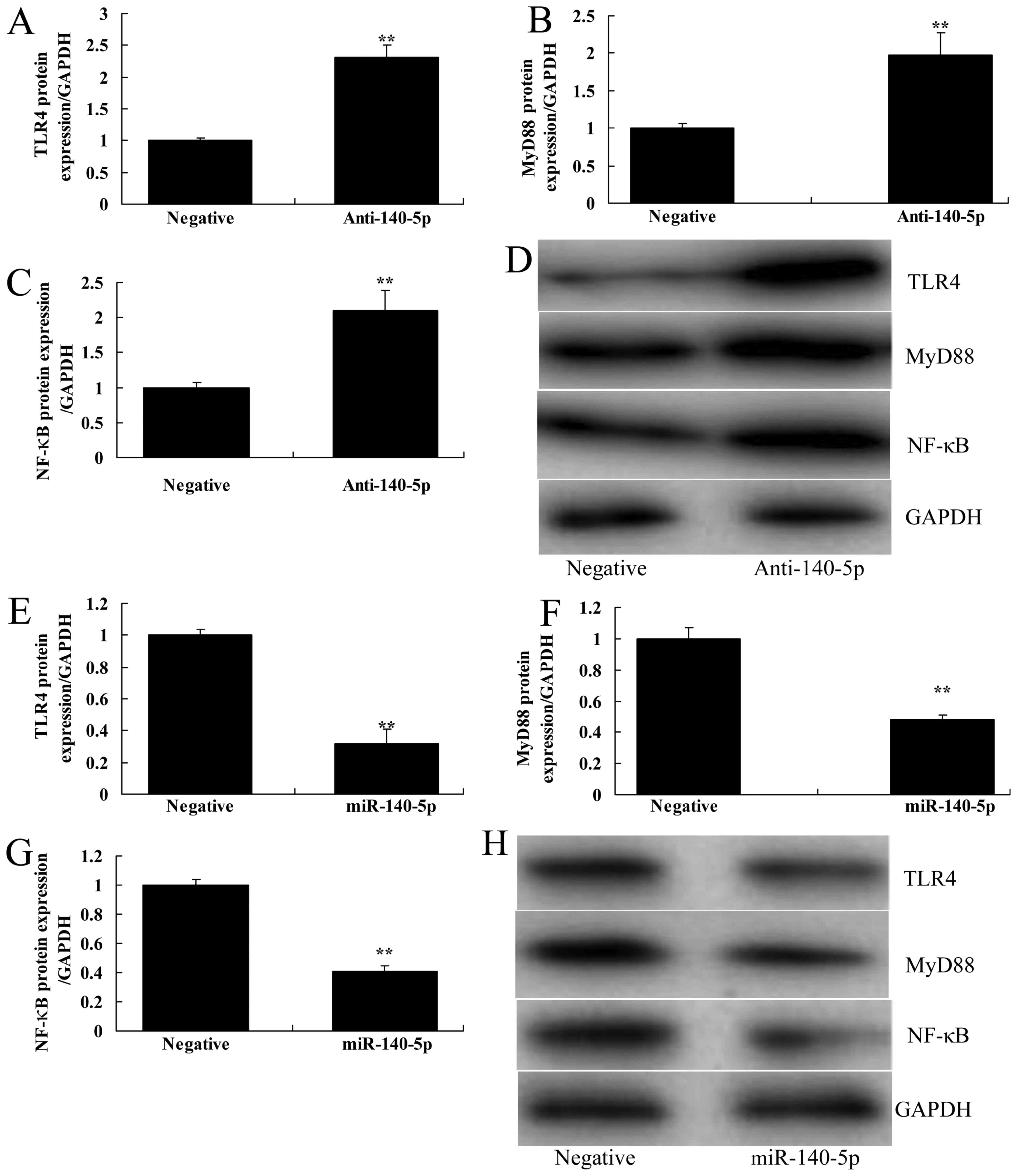
miR-140-5p induced TLR4/MyD88/NF-κB signaling pathway in in
vitro model of ALI, in comparison to control group (Fig. 4A-D). Over-expression of miR-140-5p
suppressed TLR4/MyD88/NF-κB signaling pathway in in vitro
model of ALI, compared with control group (Fig. 4E-H). Hence, miR-140-5p may reduce the
inflammation of ALI by regulating TLR4/MyD88/NF-κB signaling
pathway in vitro.
The inhibition of TLR4 suppressed the
effects of TLR4/MyD88/NF-κB signaling pathway on anti-miR-140-5p in
ALI in vitro
To confirm the mechanism function of TLR4 in
miR-140-5p in ALI in vitro, TLR4 inhibitor (TAK-242, 0.5 nM,
48 h) was used to inhibit the expression of TLR4. As shown in
Fig. 5A-D, TLR4 inhibitor suppressed
TLR4/MyD88/NF-κB signaling pathway in in vitro model of ALI
following miR-140-5p down-regulation, compared with miR-140-5p
down-regulation group. In addition, TLR4 inhibitor also reduced the
levels of TNF-α, IL-1β, IL-6 and MPO in in vitro model of
ALI following miR-140-5p down-regulation, compared with miR-140-5p
down-regulation group (Fig.
5E-H).
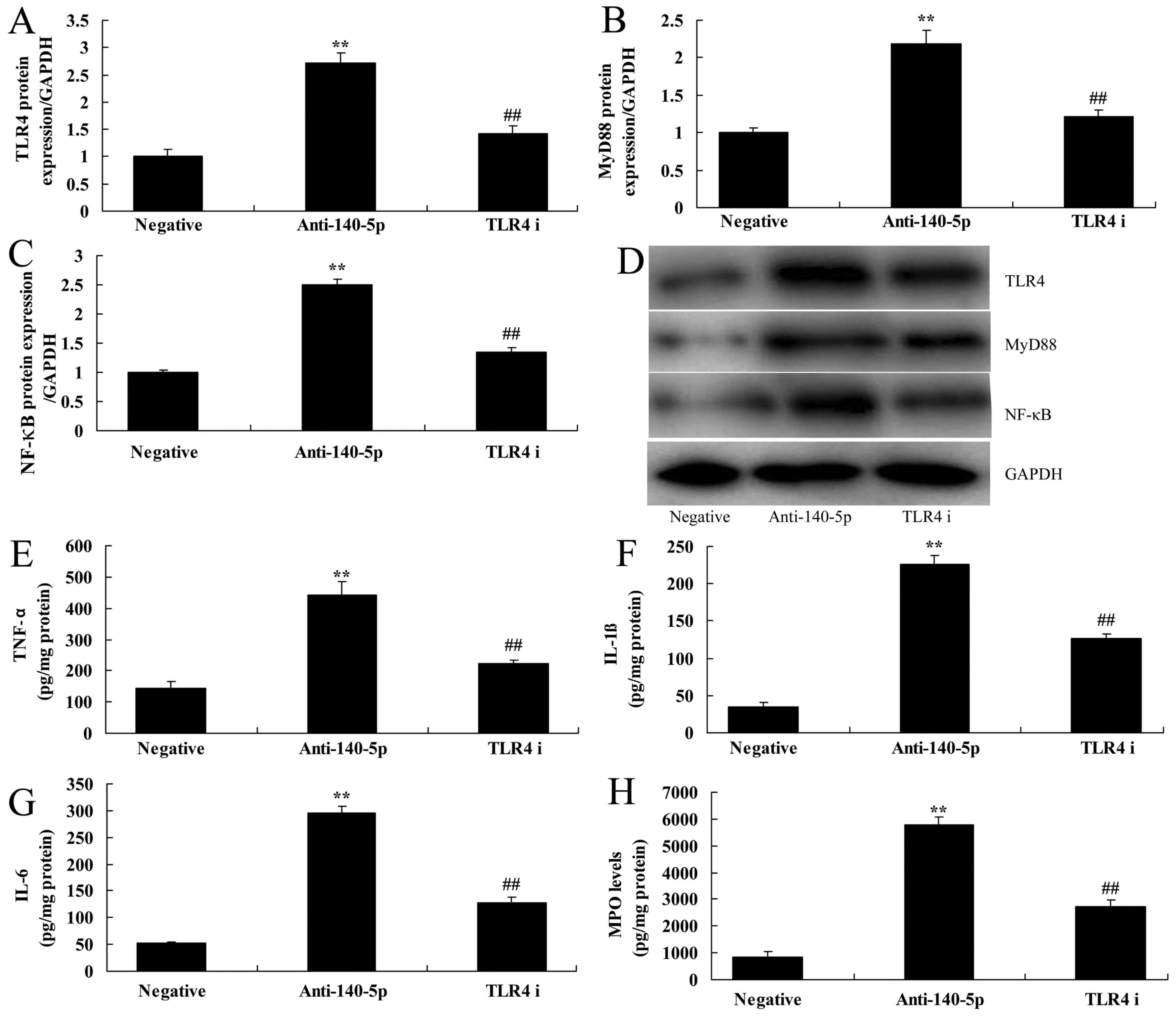 | Figure 5.Inhibition of TLR4 suppresses the
TLR4/MyD88/NF-κB signaling pathway and the effects of
anti-miR-140-5p on the in vitro acute lung injury model. The
protein expression of (A) TLR4, (B) MyD88 and (C) NF-κB was
determined by (D) western blotting assays. The protein expression
of (E) TNF-α, (F) IL-1β, (G) IL-6 and (H) MPO was also evaluated.
**P<0.01 vs. negative control group; ##P<0.01 vs.
anti-140-5p group. miR, microRNA; Anti-140-5p, downregulation of
miR-140-5p group; TLR4 i, downregulation of miR-140-5p and TLR4
inhibitor group; TLR4, Toll-like receptor 4; MyD88, myeloid
differentiation primary response 88; NF-κB, nuclear factor-κB;
TNF-α, tumor necrosis factor-α; IL, interleukin; MPO,
myeloperoxidase. |
The inhibition of NF-κB suppressed the
effects of NF-κB signaling pathway on anti-miR-140-5p in ALI in
vitro
Meanwhile, NF-κB inhibitor (JSH-23, 2 µM, 48 h) was
utilized to suppress the protein expression of NF-κB in ALI in
vitro following miR-140-5p down-regulation, compared with
miR-140-5p down-regulation group (Fig.
6A and B). NF-κB inhibitor also inhibited the levels of TNF-α,
IL-1β, IL-6 and MPO in ALI in vitro following miR-140-5p
down-regulation, in comparison with miR-140-5p down-regulation
group (Fig. 6C-F).
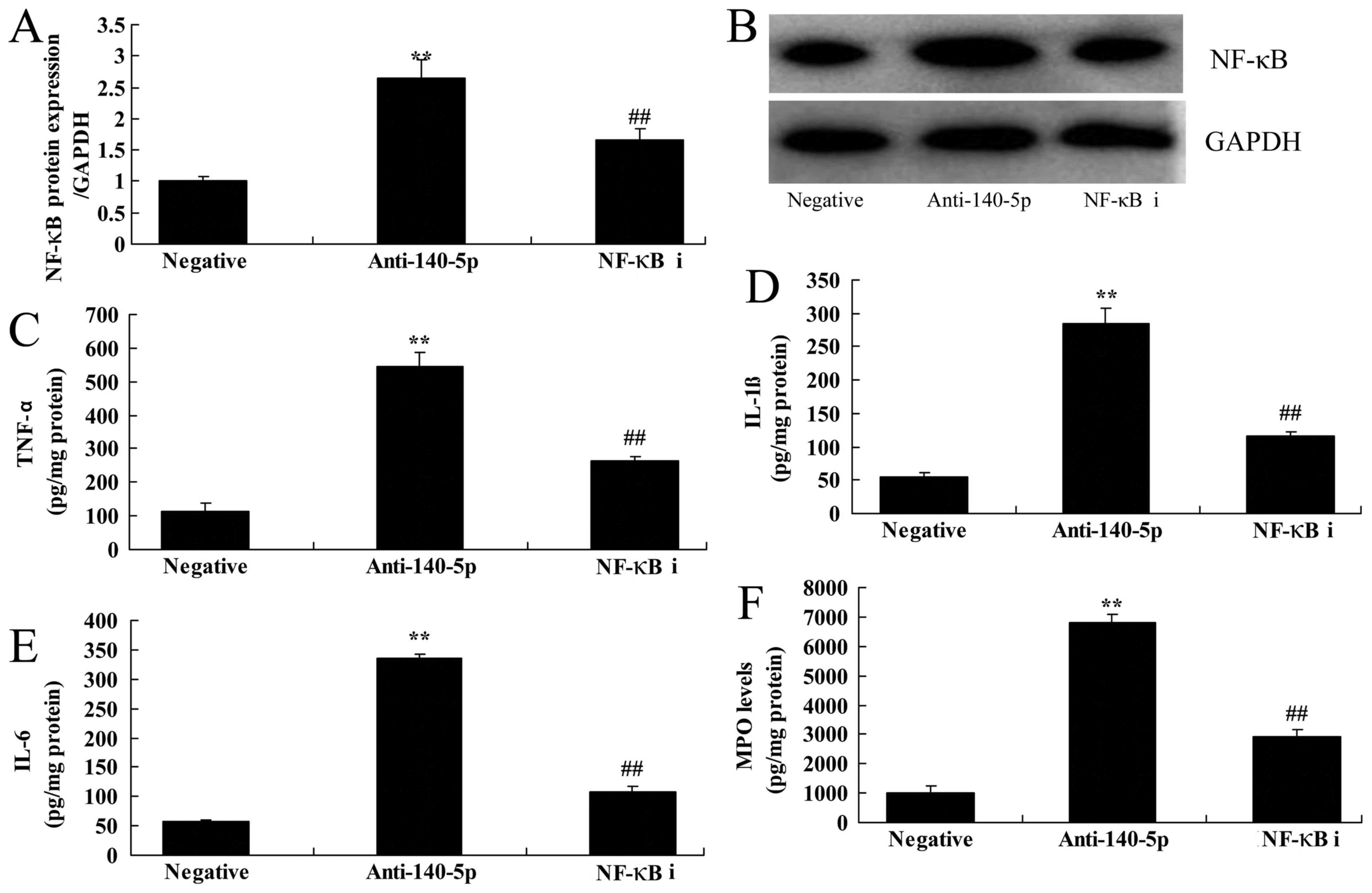 | Figure 6.Inhibition of NF-κB suppresses the
NF-κB signaling pathway and the effects of anti-miR-140-5p on the
in vitro acute lung injury model. (A) NF-κB protein
expression was determined by (B) western blot assays, as well as
(C) TNF-α, (D) IL-1β, (E) IL-6 and (F) MPO levels. **P<0.01 vs.
negative control group; ##P<0.01 vs. anti-140-5p
group. miR, microRNA; NF-κB, nuclear factor-κB; Anti-140-5p,
downregulation of miR-140-5p group; NF-κB i, downregulation of
miR-140-5p and NF-κB inhibitor group; TNF-α, tumor necrosis
factor-α; IL, interleukin; MPO, myeloperoxidase. |
Discussion
ALI and ARDS are acute respiratory distress
syndromes caused by pulmonary capillary diffuse injury and enhanced
permeability due to internal and external factors, mainly
characterized by pulmonary edema, hyalinosis and atelectasis, with
respiratory distress and refractory hypoxemia as clinical features
(3). Internal pulmonary factors
include direct injury to the lung, such as injury by stomach
contents, lung injury, radiation injury, severe pneumonia, etc.
External pulmonary factors consist of severe shock, endotoxemia,
severe non-thoracic trauma, large area burns, large blood
transfusion, acute pancreatitis and drug poisoning (12). In spite of recent great progress
concerning the studies on ALI/ARDS, there is still a lack of
effective clinical treatments in addition to protective mechanical
ventilation (13). Therefore, it is
urgent to explore a novel therapeutic strategy to alleviate
ALI/ARDS (13). To the best of our
knowledge, this is the first report demonstrating the suppressive
expression of miR-140-5p in mice with ALI, compared with normal
control group. Nagy et al (14) has showed that miR-140-5p regulates
inflammation in cystic fibrosis.
At present, researches concerning the relationship
between ALI/ARDS and miRNAs are still in early stage, and the study
on human lung tissues and organs will provide direct and reliable
evidence about the role of miRNA in ALI/ARDS (5). Our study showed that down-regulation of
miR-140-5p significantly induced the levels of TNF-α, IL-1β, IL-6
and MPO in ALI in vitro. Li et al (15) reported that miR-140-5p inhibited
inflammatory cytokines secretion through targeting TLR4.
ALI, a kind of severe lung infectious disease, is
mainly caused by bacterial infection-induced sepsis. LPS, the main
component of the cell wall of gram negative bacteria, is the major
pathogenic factor of ALI (9). LPS
interacts with the receptor on the effector cell membrane to
initiate an intracellular signal transduction system, which causes
the activation of nuclear transcription factor NF-κB, to initiate
gene transcription and to produce a variety of proinflammatory
cytokines. TLR4 is considered to be an important receptor mediating
LPS signal transduction (16).
MyD88, a key adaptor molecule in TLR4 signaling pathway, plays an
important role in the transmission of upstream signaling as well as
the incidence and progression of diseases (17). TLR4-mediated MyD88 signal pathway
regulates the expression of multiple genes related to inflammation
during immune response to pathogen invasion (18). Consistent with this finding, our
studies showed that over-expression of miR-140-5p significantly
suppressed TLR4/MyD88/NF-κB signaling pathway in in vitro
model of ALI. Sun et al (19)
suggest that miR-140-5p mediated the inflammation of human dental
pulp stem cells through TLR4 signaling pathway.
NF-κB, one of the downstream molecules of TLR4, is a
heterodimer composed of p65 and p50 subunits (20). In the inactive state, NF-κB binds to
inhibitive protein IκB and exists in the cytoplasm in an inactive
form (21). Some studies have showed
that in microglia, LPS activates TLR4, releases the inhibition of
NF-κB by IκB, promotes NF-κB nuclear translocation, and stimulates
the expression of genes related to inflammation, thereby promoting
the synthesis and release of IL-1 and TNF-α (21,22). We
found that the inhibition of TLR4 using TLR4 inhibitor or NF-κB
inhibitor reduced the proinflammation of miR-140-5p in ALI in
vitro through TLR4/MyD88/ NF-κB signaling pathway. Jude et
al (23) indicated that
miR-140-3p regulated TNF-α-induced CD38 expression in human airway
smooth muscle cells through regulating the protein expression of
p38 MAPK and NF-κB.
Results from our current study suggest that
miR-140-5p inhibits the inflammation of ALI via TLR4/MyD88/ NF-κB
signaling pathway. Our findings may provide a novel strategy of
miR-140-5p for the treatment of ALI and protection against
inflammation in further clinical research.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
JjL designed the experiments. YY, DL, YX, JL and BL
performed the experiments. YY and JjL analyzed the data. JjL wrote
the manuscript.
Ethics approval and consent to
participate
The experimental procedures conformed to the Guide
for the Care and Use of Laboratory Animals, and all of the
procedures were approved by the Institutional Ethics Committee of
Sichuan Cancer Hospital (Sichuan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stapleton RD, Dixon AE, Parsons PE, Ware
LB and Suratt BT: NHLBI Acute Respiratory Distress Syndrome
Network: The association between BMI and plasma cytokine levels in
patients with acute lung injury. Chest. 138:568–577. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Z, Chen L and Ni H: The
effectiveness of Corticosteroids on mortality in patients with
acute respiratory distress syndrome or acute lung injury: A
secondary analysis. Sci Rep. 5:176542015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Braunschweig CA, Sheean PM, Peterson SJ,
Perez Gomez S, Freels S, Lateef O, Gurka D and Fantuzzi G:
Intensive nutrition in acute lung injury: A clinical trial
(INTACT). JPEN J Parenter Enteral Nutr. 39:13–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li W, Qiu X, Jiang H, Han Y, Wei D and Liu
J: Downregulation of miR-181a protects mice from LPS-induced acute
lung injury by targeting Bcl-2. Biomed Pharmacother. 84:1375–1382.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao Y, Lyu YI, Tang J and Li Y: MicroRNAs:
Novel regulatory molecules in acute lung injury/acute respiratory
distress syndrome. Biomed Rep. 4:523–527. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang K, Gao B, Wei W, Li Z, Pan L, Zhang
J, Zhao Q, Chen W and Xu Z: Changed profile of microRNAs in acute
lung injury induced by cardio-pulmonary bypass and its mechanism
involved with SIRT1. Int J Clin Exp Pathol. 8:1104–1115.
2015.PubMed/NCBI
|
|
7
|
Wang X, Zhang L, Duan W, Liu B, Gong P,
Ding Y and Wu X: Anti-inflammatory effects of triptolide by
inhibiting the NF-kappaB signalling pathway in LPS-induced acute
lung injury in a murine model. Mol Med Rep. 10:447–452. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He X, Qian Y, Li Z, Fan EK, Li Y, Wu L,
Billiar TR, Wilson MA, Shi X and Fan J: TLR4-upregulated IL-1beta
and IL-1RI promote alveolar macrophage pyroptosis and lung
inflammation through an autocrine mechanism. Sci Rep. 6:316632016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun XJ, Li XQ, Wang XL, Tan WF and Wang
JK: Sevoflurane inhibits nuclear factor-kappaB activation in
lipopolysaccharide-induced acute inflammatory lung injury via
toll-like receptor 4 signaling. PLoS One. 10:e01227522015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y and Xu J: miR-140-5p regulates
hypoxia-mediated human pulmonary artery smooth muscle cell
proliferation, apoptosis and differentiation by targeting Dnmt1 and
promoting SOD2 expression. Biochem Biophys Res Commun. 473:342–348.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feiner JR, Gropper MA, Toy P, Lieberman J,
Twiford J and Weiskopf RB: A clinical trial to detect subclinical
transfusion-induced lung injury during surgery. Anesthesiology.
123:126–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge
M, Deng K, Zhang L, Zou B, Cheng B and Xu J: Treatment of acute
respiratory distress syndrome with allogeneic adipose-derived
mesenchymal stem cells: A randomized, placebo-controlled pilot
study. Respir Res. 15:392014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagy B Jr, Nagy B, Fila L, Clarke LA,
Gönczy F, Bede O, Nagy D, Újhelyi R, Szabó Á, Anghelyi A, et al:
Human epididymis protein 4: A novel serum inflammatory biomarker in
cystic fibrosis. Chest. 150:661–672. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Guan SB, Lu Y and Wang F: miR-140-5p
inhibits synovial fibroblasts proliferation and inflammatory
cytokines secretion through targeting TLR4. Biomed Pharmacother.
96:208–214. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chi X, Wei X, Gao W, Guan J, Yu X, Wang Y,
Li X and Cai J: Dexmedetomidine ameliorates acute lung injury
following orthotopic autologous liver transplantation in rats
probably by inhibiting Toll-like receptor 4-nuclear factor kappa B
signaling. J Transl Med. 13:1902015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu DD, Cao G, Han LK, Ye YL, Zhang Q,
Sima YH and Ge WH: Flavonoids from Radix Tetrastigmae improve
LPS-induced acute lung injury via the TLR4/MD-2-mediated pathway.
Mol Med Rep. 14:1733–1741. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu L, Xue T, Zhang J and Qu J: Knockdown
of versican V1 induces a severe inflammatory response in
LPS-induced acute lung injury via the TLR2-NF-κB signaling pathway
in C57BL/6J mice. Mol Med Rep. 13:5005–5012. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun DG, Xin BC, Wu D, Zhou L, Wu HB, Gong
W and Lv J: miR-140-5p-mediated regulation of the proliferation and
differentiation of human dental pulp stem cells occurs through the
lipopolysaccharide/toll-like receptor 4 signaling pathway. Eur J
Oral Sci. 125:419–425. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang Q, Yi M, Guo Q, Wang C, Wang H, Meng
S, Liu C, Fu Y, Ji H and Chen T: Protective effects of polydatin on
lipopolysaccharide-induced acute lung injury through
TLR4-MyD88-NF-κB pathway. Int Immunopharmacol. 29:370–376. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tianzhu Z and Shumin W: Esculin inhibits
the inflammation of LPS-induced acute lung injury in mice via
regulation of TLR/NF-κB pathways. Inflammation. 38:1529–1536. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang X, Sun CY, Zhang YB, Guo HZ, Feng
XX, Peng SZ, Yuan J, Zheng RB, Chen WP, Su ZR and Huang XD: Kegan
Liyan oral liquid ameliorates lipopolysaccharide-induced acute lung
injury through inhibition of TLR4-mediated NF-κB signaling pathway
and MMP-9 expression. J Ethnopharmacol. 186:91–102. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jude JA, Dileepan M, Subramanian S, Solway
J, Panettieri RA Jr, Walseth TF and Kannan MS: miR-140-3p
regulation of TNF-α-induced CD38 expression in human airway smooth
muscle cells. Am J Physiol Lung Cell Mol Physiol. 303:L460–L468.
2012. View Article : Google Scholar : PubMed/NCBI
|