Introduction
Coronary artery disease (CAD) is typically caused by
the development of atherosclerotic lesions and results in
myocardial ischemia (1,2). CAD is associated with inflammation and
thrombosis, which can cause luminal stenosis or occlusion (3). Prospective trials have indicated that
the morbidity and mortality of myocardial infarction is increasing
worldwide since 1990 (2,4). A review of the available clinical data
has suggested that cardiovascular interventions, including stent
implantation and radiofrequency ablation may help to reduce the
mortality of myocardial infarction (5). A large number of proteins have been
reported to protect the host against myocardial ischemia and
reperfusion injury by modulating myocardial apoptosis and
inflammation (6,7). Research has demonstrated that early
apoptotic myocardial vascular disease may increase the incidence
rate of myocardial infarction (8,9).
The potential use of stem cell transplantation to
treat human diseases has been widely investigated (10–12).
Currently, stem cell transplantation is a therapeutic protocol used
to treat CAD, as stem cells are able to migrate to damaged cardiac
tissue, repair the myocardial infarction area and ultimately reduce
infarct-associated mortality (13).
A previous study reported that bone marrow stem cell mobilization
is able to decrease left ventricular ejection fraction, stroke
volume and cardiac output for patients with coronary heart disease
(14). Ghem et al
(15) demonstrated that endothelial
stem/progenitor cells (EPCs), mesenchymal stem/progenitor cells
(MSCs), and hematopoietic stem/progenitor cells (HSCs) decreased
left ventricular dysfunction, diabetes and intermediate risk in
patients with cardiovascular diseases. However, the role and
potential mechanism of endothelial stem cells (ESCs) in cardiac
cells is not well understood.
In the present study, the therapeutic effects of
ESCs in a rat model of CAD were assessed, as well as the underlying
mechanism. The effects of ESCs on serum cytokine levels and
circulating proatherogenic cells were also investigated.
Materials and methods
Animal study
The present study was approved by the Ethics
Committee of Shenzhen Nanshan People's Hospital (Shenzhen, China).
A total of 20 female Sprague-Dawley rats (6–8 weeks of age, body
weight: 200–220 g) were purchased from the Chinese Academy of
Sciences (Shanghai, China). All rats were housed at 23°C with 50%
humidity and a 12 h light/dark cycle. Rats were provided with free
access to food and water. Rats were injected with vitamin D3 once
every 30 days (2×106 U/kg) and received a high-fat diet
containing 2% cholesterol, 3% lard oil, 0.5% sodium cholate, 0.2%
propylthiouracil and 94.3% basic diet supplemented with vitamin D3
(1.25×106 U/kg) to establish acute myocardial
infarction. Rats were then divided into the experimental (n=10) and
negative control groups (n=10). Rats were anesthetised with 35
mg/kg pentobarbital intravenously, following which they received an
intra-myocardial injection of 1×104 ESCs (Tehran Heart
Center, Tehran University of Medical Sciences, Tehran, Iran) in 100
µl PBS (experimental group) or an equal volume of PBS (control
group). At 10 days after treatment, rats were sacrificed and heart
tissues were harvested. Tissues were either snap-frozen in liquid
nitrogen or fixed in 10% formalin solution (Ph 7.4) for 15 min at
37°C for further analysis. The total experimental period was 42
days.
Cardiac function analysis
Rats were fed with the aforementioned diet for 30
days, injected with PBS or ESCs, left for 10 days and then
sacrificed. Rats were fed with the specified diet for 30 days.
Systolic blood pressure (SBP) and diastolic blood pressure (DBP)
were measured using Blood Pressure Monitors (BP786N; Omron, Kyoto,
Japan) for experimental rats between ESCs and control groups on day
42. Heart rate of experimental rats was measured using a heart rate
detector (Prince 180D; Heal Force Bio-meditech Holdings, Ltd.,
Shanghai, China).
ELISA
Central venous blood samples (5 ml) were collected
from rats in each group on day 42. Following centrifugation at
3,000 × g for 15 min at 4°C, serum levels of aspartate transaminase
(AST; E-EL-M0160), lactate dehydrogenase (LDH; cat. no. TWp001252;
Shanghai Jianglai Biological Technology Co., Ltd., Shanghai,
China), Troponin T (TnT; cat. no. 0-113685, JiangLai Biology,
Shanghai Jianglai Biological Technology Co., Ltd.), TnI (cat. no.
YBE-0070Q; Shanghai Jianglai Biological Technology Co., Ltd.)
interleukin (IL)-1β (RLB00; R&D Systems, Inc., Minneapolis, MN,
USA), IL-17a (M1700; R&D Systems, Inc.) and tumor necrosis
factor (TNF)-α (RTA00; R&D Systems, Inc.) were measured using
commercial ELISA kits according to the manufacturer's protocol. The
results were read using an ELISA microplate reader at 450 nm
(Spectra Max 190, Molecular Devices LLC, Sunnyvale, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from heart tissues (1.0 g)
using an RNAeasy Mini kit (Qiagen AB, Sollentuna, Sweden). The
concentration of RNA samples were measured using a Nanodrop ND-2000
spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Total RNA (1 µg) was reverse-transcribed to cDNA using a
Transcriptor First Strand cDNA Synthesis kit (Invitrogen; Thermo
Fisher Scientific, Inc.). Micro (mi)RNA expression was assessed
using an miRNA microarray analysis with the miRCURY LNA™
miRNA Array (Qiagen AB) according to the manufacturer's protocol.
All miRNA primers were synthesized by Invitrogen (Thermo Fisher
Scientific, Inc.) Primers used were as follows: miRNA-146a forward,
AUGCCAUUGCGAGGGAUUUCG-3′; and reverse,
5′-ATACCTTCAGAGCCTGAGACTCTGCC-3′; RNU6B (U6) forward,
5′-CGCAAGGAUGACACGCAAAUUCGUGAAGCGUUCCAUAUUUUU-3′ and reverse,
5′-GATATCCCAGCTGAAGAACTGAATTTGAC-3′. RT-qPCR reaction was performed
using a TaqMan™ MicroRNA Reverse Transcription kit (cat. no.
4366596; Invitrogen; Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: 2 min at 95°C; followed
by 40 cycles of 10 sec at 95°C, annealing for 20 sec at 55°C and a
primer extension for 30 sec at 72°C and then for 10 min at 72°C.
The microarray data was validated using real-time PCR with TaqMan
probes and relative miRNA expression levels were normalized using
the RNU6B (U6) small non-coding RNA as control. Expression was
calculated using the 2−ΔΔCq method (16).
MiRNA-146a overexpression
Myocardial cells (1×105 cells/well) from
experimental rats were cultured in 6-well plates until they reached
90% confluence, following which the medium was discarded. Cells
were subsequently transfected with 10 pmol plentivirus-miRNA-146a
(pmiRNA-146a, 5′-UACGCCCUUUUAACAUUGCAUCG-3′) or 10 pmol
miRNA-146a-mimic control (pControl, 5′-ACGUACUUUUGUGUAGUACC-3′)
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) in Opti-MEM medium according to the
manufacturer's protocol. Cells were used for further analysis
following 72 h transfection.
Knockdown of miRNA-146a
Myocardial cells (1×105 cells/well) were
seeded in 6-well plates for 12 h at 37°C. Cells were washed with
PBS three times. Small interfering (si)RNR-miRNA-146a
(miRNA-146aKN) and siRNR-mimic (Control) were synthesized by
Guangzhou RiboBio Co., Ltd. (Guangzhou, China). Sequences were as
follows: miRNA-146aKN, 5′-CCUGCUGGAUUGAGCUACACCUGAA-3′ and
siRNR-mimic, 5′-CUCGUCUCAUUGATGACAGTT-3′. SiRNAs (100 pmol) were
transfected into myocardial cells using RNAi MAX (Thermo Fisher
Scientific, Inc.) according to the manufacturers' protocol. Further
experiments were performed at 72 following transfection.
Viability of myocardial cells
Myocardial cells (2×103 cells/well) were
seeded in 96-well plates and cultured at 37°C for 12 h. A CCK-8
detection kit (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was
used to measure cell viability according to the manufacturer's
protocol.
Western blotting
Myocardial tissues (5 µg) were homogenized in lysate
buffer containing protease-inhibitor (Sigma-Aldrich; Merck KGaA)
and centrifuged at 8,000 × g for 10 min at 4°C. The protein
concentration was measured using a BCA protein assay kit (Thermo
Fisher Scientific, Inc.). Protein samples (20 µg) were separated by
15% SDS-PAGE and transferred onto a polyvinylidene fluoride
membrane. After blocking for 1 h at 37°C with 10% Bovine Serum
Albumin (cat. no. 10735108001; Roche Applied Science, Penzburg,
Germany), the membrane was incubated with primary antibodies
against the following: Vascular endothelial growth factor (VEGF;
1:1,000; ab32152; Abcam, Cambridge, UK), IL-6 (1:1,000; ab9324;
Abcam), intercellular adhesion molecule 1 (ICAM-1; 1:1,000;
sc-7891, Santa Cruz Biotechnology, Inc., Dallas, TX, USA), FAS
(1:1,000; ab82419; Abcam), TNF-α (1:1,000; ab6671, Abcam) and
β-actin (1:2,000; cat. no. ab8226; Abcam) for 12 h at 4°C. The
membrane was next incubated with horseradish peroxidase-conjugated
anti-rabbit IgG antibody (1:2,000; PV-6001, OriGene Technologies,
Inc., Rockville, MD, USA) for 24 h at 4°C. The results were
visualized by using a chemiluminescence kit (Pierce; Thermo Fisher
Scientific, Inc.).
Immunological staining
Immunohistochemistry was performed as previously
described (17). Cardiac tissues
were prepared, fixed in 4% paraformaldehyde for 2 h at 37°C,
embedded in paraffin and cut into 4 µm sections. Epitope retrieval
was performed using Tris-HCl buffer (AP-9005-050; Thermo Fisher
Scientific, Inc.). The paraffin sections were quenched with
hydrogen peroxide (3%) for 15 min at 37°C, and subsequently blocked
with 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA) for 15 min
at 37°C. Cardiac tissue sections were rinsed with PBS and incubated
with rabbit anti-rat primary antibodies against VEGF (1:1,000),
sFAS (1:1,000) and TNF-α (1:1,000) for 12 h at 4°C. Sections were
washed with PBS for 30 min at room temperature and incubated with
Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488; 1:2,000;
ab150077; Abcam) for 2 h at 37°C. The results were visualized by
using an enhanced chemiluminescence kit (Pierce; Thermo Fisher
Scientific, Inc.). Densitometry was performed using Quantity-One
software 1.2 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
TUNEL analysis
A TUNEL assay was performed to assess the apoptosis
of myocardial cells in rats. Cardiac tissue sections were stained
using TUNEL with an In Situ Cell Death Detection kit (Roche
Applied Science) according to the manufacturer's protocol. Images
of tissue sections were captured using a ZEISS LSM 510 confocal
microscope at 488 nm (ZEISS AB, Oberkochen, Germany). Images were
analyzed using ImageJ software 2.0 (National Institutes of Health,
Bethesda, MA, USA).
Statistical analysis
All data are expressed as the mean ± standard
deviaton of three independent experiments. Data were cmpared using
Student's t test with SPSS version 19. (IBM, Corp., Armonk, NY,
USA) and P<0.05 was considered to indicate a statistically
significant difference.
Results
ESCs therapy improves cardiac function
in a rat model of CAD
The therapeutic effects of ESCs on cardiac function
were investigated in a rat model of CAD. The results revealed that
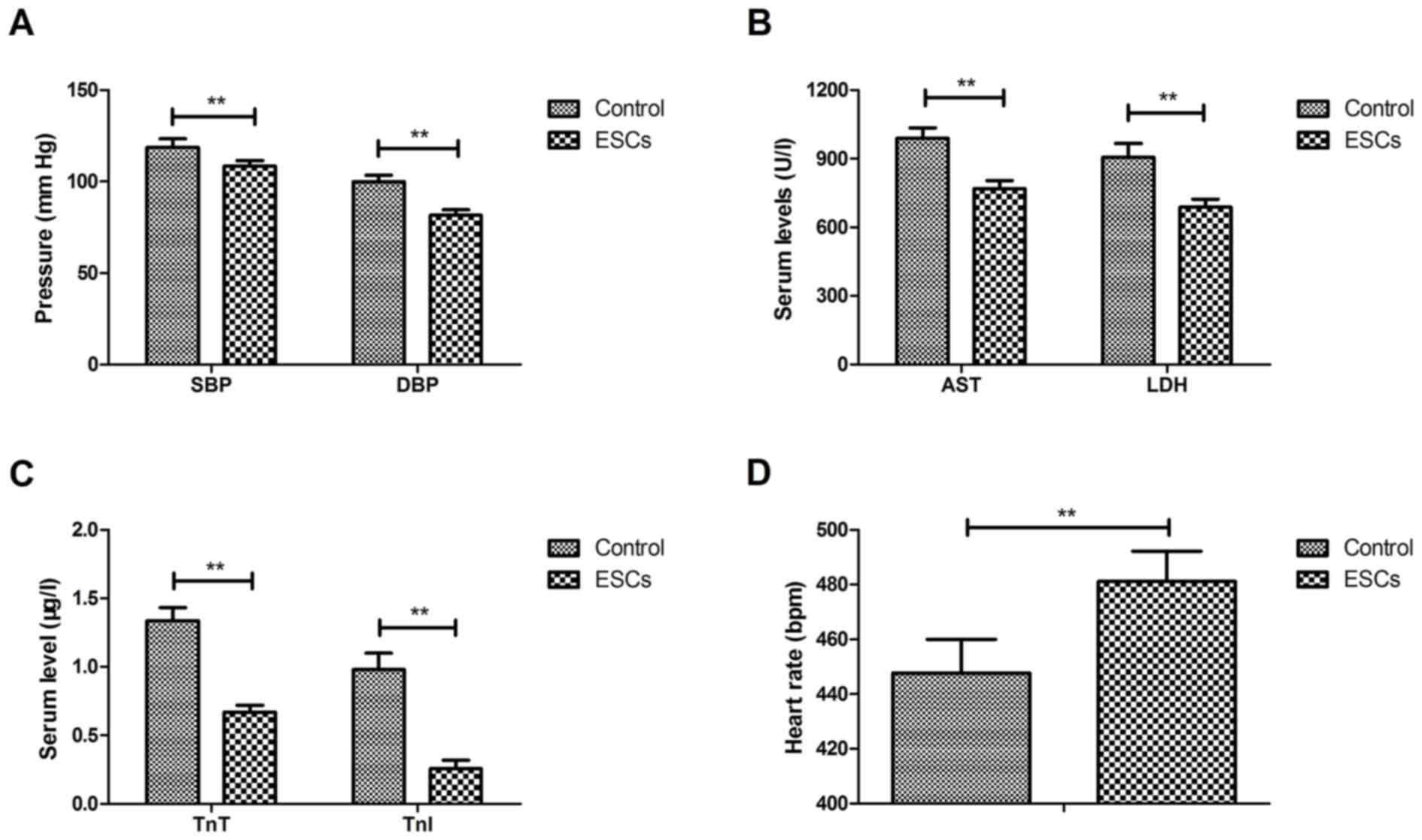
ESCs therapy decreased SBP and DBP in CAD rats compared with the
control group (Fig. 1A). ESCs
therapy also decreased serum AST, LDH (Fig. 1B), TnT and TnI (Fig. 1C) compared with the control group.
The results also demonstrated that ESCs therapy improved rats'
heart rate compared with the control group (Fig. 1D). ESCs therapy improved the
atherosclerosis indices compared with the control group (Table I). These data suggest that ESCs
therapy is able to improve cardiac function in a rat model of
CAD.
 | Table I.Plasma total cholesterol,
HDL-cholesterol, LDL-cholesterol and triacylglycerides indices. |
Table I.
Plasma total cholesterol,
HDL-cholesterol, LDL-cholesterol and triacylglycerides indices.
|
| Concentration
(mg/dl) |
|---|
|
|
|
|---|
| Plasma component | Baseline | Control group | ESCs group |
|---|
| Total
cholesterol | 172.25±8.82 | 163.58±7.54 |
81.10±5.42a,b |
| HDL-cholesterol | 64.66±6.28 | 62.46±5.12 |
41.36±3.06a,b |
| LDL-cholesterol | 110.24±7.20 | 112.85±6.85 |
70.54±6.40a,b |
|
Triacylglycerides | 42.15±4.32 | 43.25±5.24 |
30.22±3.06a,b |
ESCs therapy decreases serum cytokine
levels and circulating proatherogenic cells in a rat model of
CAD
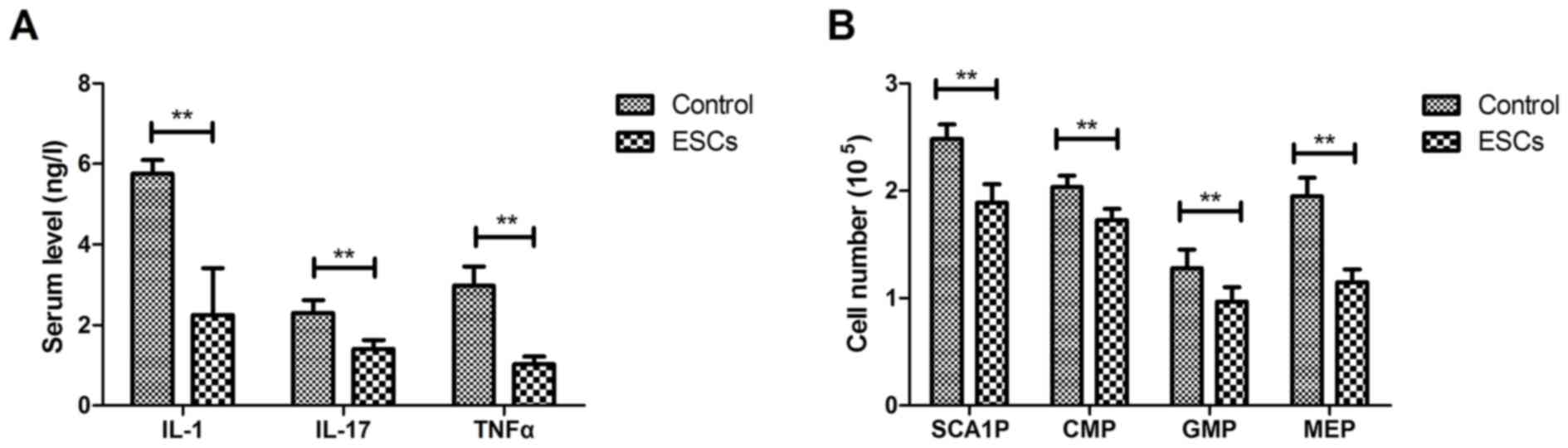
The anti-inflammatory effects of ESCs were
investigated in a rat model of CAD. The results demonstrated that
ESCs treatment decreased IL-1, IL-17 and TNFα serum levels compared
with the control group (Fig. 2A).
Rats treated with ESCs had fewer progenitor cells, including stem
cells antigen-1-negative progenitors (SCA1P), common myeloid
progenitors (CMP), granulocyte-macrophage progenitors (GMP) and
megakaryocyte-erythroid progenitors (MEP) (Fig. 2B). These results suggest that ESCs
therapy could decrease serum cytokine levels and circulating
proatherogenic cells in rats with CAD.
ESCs therapy decreases myocardial
infarction in a rat model of CAD
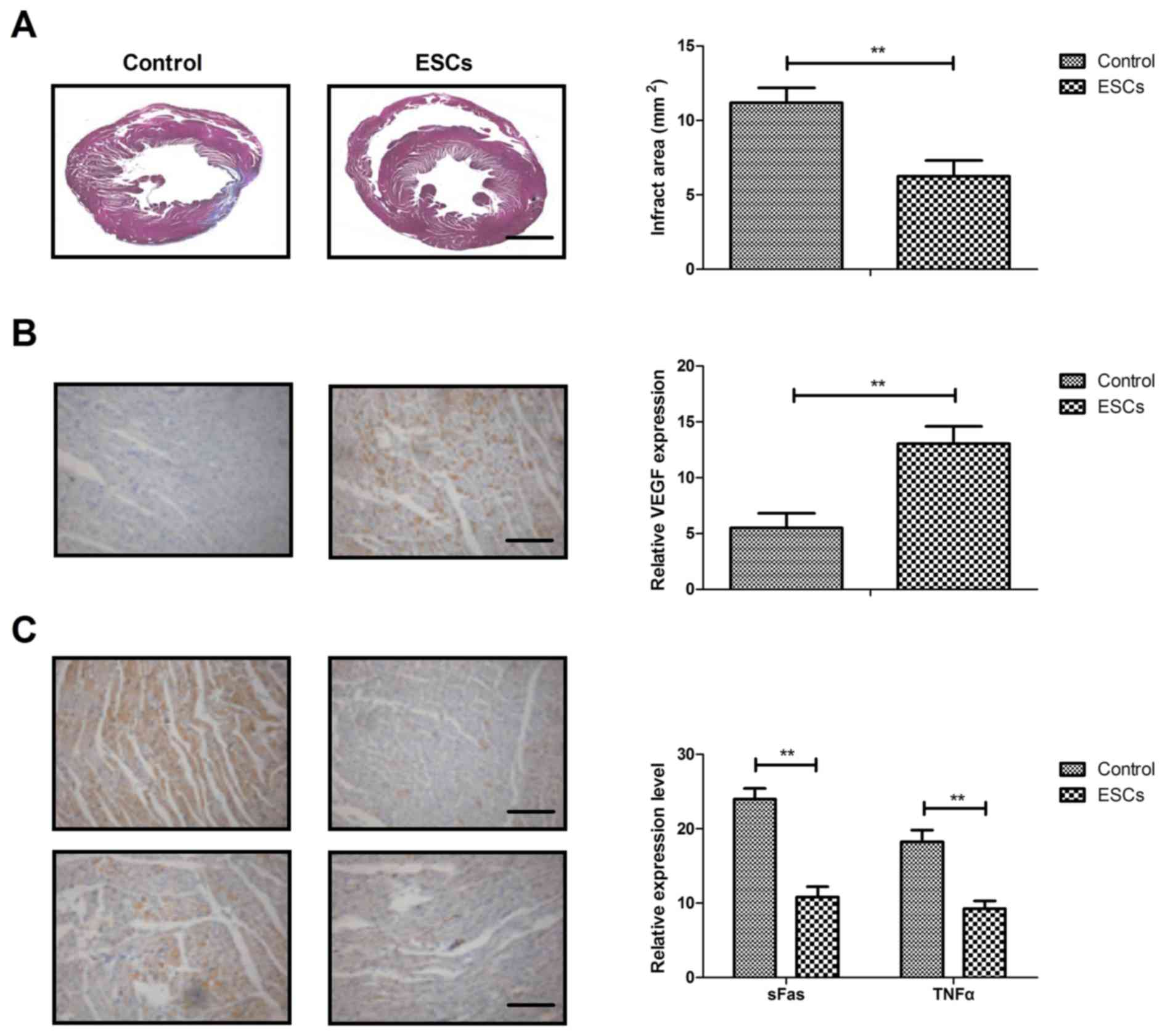
Myocardial infartctions and cardiac apoptosis were
analyzed on day 30 after ESCs therapy. Rats treated with ESCs
therapy exhibited smaller infarct areas in the border zone compared
with control rats (Fig. 3A). The
results demonstrated that ESCs therapy increased VEGF expression
compared with the control group (Fig.
3B). Markers of myocardial apoptosis, sFas and TNFα, were
decreased by ESCs therapy compared with the control group (Fig. 3C). These results suggest that ESCs
therapy may decrease myocardial infarction size in a rat model of
CAD.
ESCs therapy regulates cardiac
apoptosis via downregulating miRNA-146a
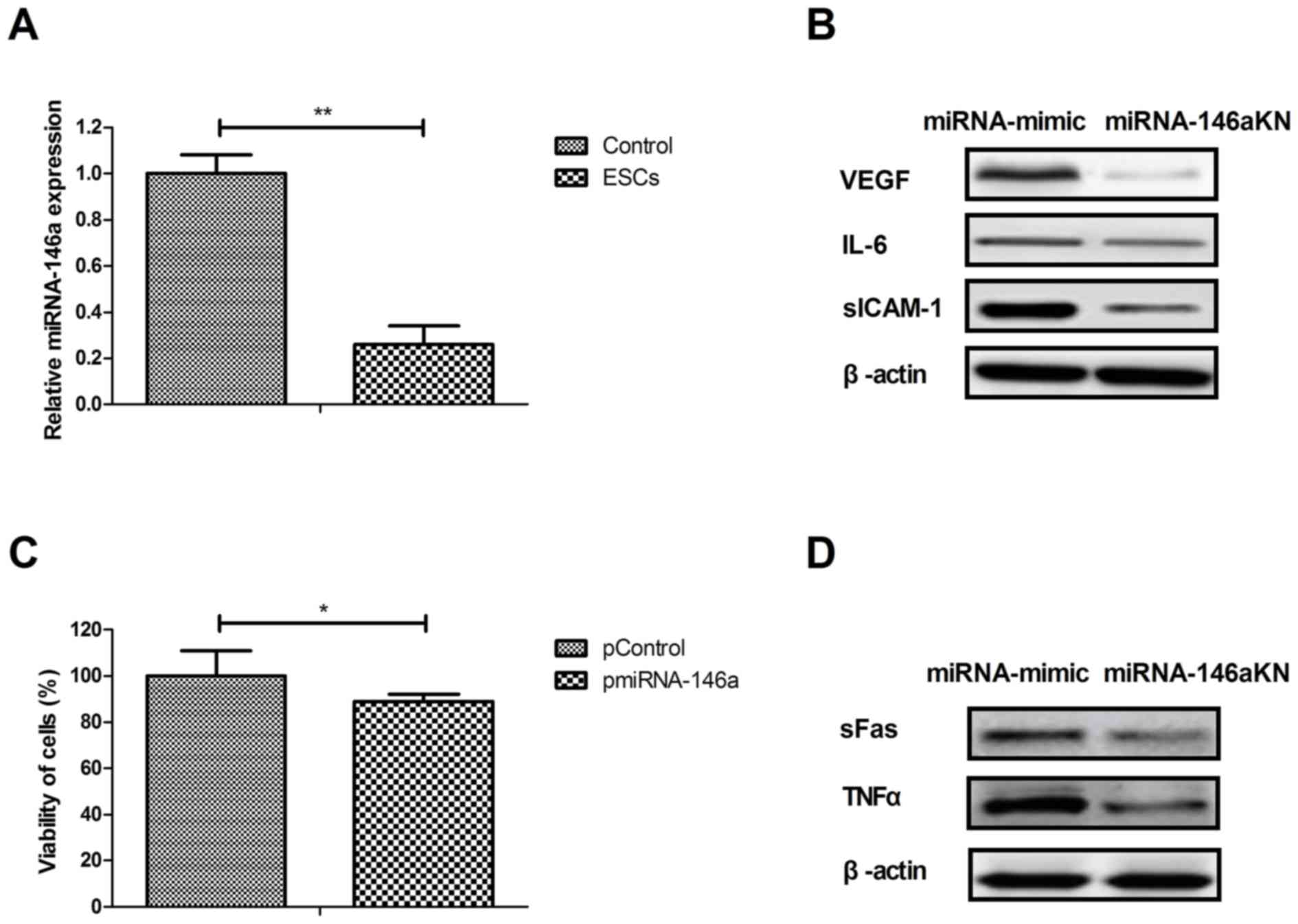
As presented in Fig.
4A, miRNA-146a expression was decreased in myocardial cells
isolated from rats treated with ESCs compared to those from the
control group. Transfection with miRNA-146aKN decreased VEGF, IL-6
and sICAM-1 protein expression in myocardial cells compared with
cells transfected with miRNA-mimic (Fig.
4B). Transfection with pmiRNA-146a the decreased viability of
myocardial compared with cells transfected with miRNA-mimic
(Fig. 4C). sFas and TNFα expression
was also downregulated by miRNA-146a knockdown compared with the
miRNA-mimic group (Fig. 4D). These
results suggest that ESCs therapy regulates cardiac apoptosis by
downregulating miRNA-146a expression.
Discussion
CAD is associated with an increase in the expression
of inflammatory cytokines (18). It
has previously been reported that miR-146a serves an essential role
in inflammatory signaling pathways and the formation of
atherosclerotic plaques (19).
Advancements in stem cell technology have provided novel strategies
for the treatment of heart disease (20). In the present study, the therapeutic
effects of ESCs transplantation in a rat model of CAD were
assessed. The results revealed that ESCs therapy decreased serum
IL-1, IL-17 and TNFα levels and apoptosis in the myocardium of rats
with CAD. ESCs therapy was demonstrated to regulate cardiac
apoptosis via downregulating miRNA-146a expression in myocardial
cells.
The current understanding of atherosclerosis
pathophysiology emphasizes the role of inflammatory mediators in
the development of CAD (21). IL-1
signaling may be regarded as an essential mediator in the
pathogenesis of heart failure as it inhibits cardiac contractility,
promotes myocardial hypertrophy and induces cardiomyocyte apoptosis
(22). The results of the present
study suggest that ESCs therapy decreases serum IL-1 levels in a
CAD rat model. Bujak et al (22) reported that serum IL-17 levels may be
associated with predictive or prognostic values of ischemic heart
disease. Li et al (23) revealed that serum TNFα levels
transiently declined after 24 h of cardiopulmonary bypass and may
be an important biological indicator for monitoring the efficacy of
cardiopulmonary bypass in children with congenital heart disease.
The results of the present study suggest that IL-17 and TNFα
upregulation in CAD may be inhibited by ESCs therapy. However,
further investigation is required to confirm the anti-inflammatory
effects of ESCs in CAD.
It has previously been reported that ESCs are a
valuable source of cell therapy for angiogenesis induction as a
treatment for myocardial ischemia (23). The results of the present study
indicate that the therapeutic effects of ESCs improve the
atherosclerosis indices in a rat model of CAD compared with control
rats. Wu et al (24)
reported that miRNA-146a induced vascular smooth muscle cell
apoptosis via the NF-κB signaling pathway. In the present study,
ESCs therapy decreased miRNA-146a expression and increased VEGF
expression in the myocardium of rats with CAD. The results
indicated that miRNA-146 inhibition reduced cardiac apoptosis and
increased VEGF expression. Furthermore, it was demonstrated that
ESCs therapy decreased serum AST, LDH, TnT and TnI levels in a rat
model of CAD, as well as improving heart rate.
In conclusion, the results of the present study
demonstrate the therapeutic effects of ESCs in decreasing
inflammatory cytokines and the number of apoptotic cells in heart
tissue. It was also demonstrated that miR-146a downregulation
mediated by ESCs ameliorates the apoptosis of cardiac cells,
suggesting that ESCs may have potential applications in CAD
treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YF performed the experiments. SC, ZL, WA, XH, LW, PX
and BJ prepared and analyzed experimental data. HF designed the
experiments.
Ethics approval and consent to
participate
This experiment was approved by the Ethics Committee
of Shenzhen Nanshan People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fan FF, Xu Q, Sun Q, Zhao SJ, Wang P and
Guo XR: Assessment of the reporting quality of randomized
controlled trials on treatment of coronary heart disease with
traditional chinese medicine from the chinese journal of integrated
traditional and Western medicine: a systematic review. PLoS One.
9:e863602014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Doyle F, Rohde D, Rutkowska A, Morgan K,
Cousins G and McGee H: Systematic review and meta-analysis of the
impact of depression on subsequent smoking cessation in patients
with coronary heart disease: 1990 to 2013. Psychosom Med. 76:44–57.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tully PJ and Baumeister H: Collaborative
care for the treatment of comorbid depression and coronary heart
disease: A systematic review and meta-analysis protocol. Syst Rev.
3:1272014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li SM, Xu H and Chen KJ: Integrative
medicine on coronary heart disease: Annual academic review 2013.
Zhongguo Zhong Xi Yi Jie He Za Zhi. 34:1029–1034. 2014.(In
Chinese). PubMed/NCBI
|
|
5
|
Ray IB, Menezes AR, Malur P, Hiltbold AE,
Reilly JP and Lavie CJ: Meditation and coronary heart disease: A
review of the current clinical evidence. Ochsner J. 14:696–703.
2014.PubMed/NCBI
|
|
6
|
Niermann C, Gorressen S, Klier M, Gowert
NS, Billuart P, Kelm M, Merx MW and Elvers M: Oligophrenin1
protects mice against myocardial ischemia and reperfusion injury by
modulating inflammation and myocardial apoptosis. Cell Signal.
28:967–978. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo CX, Jiang X, Zeng XJ, Wang HX, Li HH,
Du FH and Chen BX: Soluble receptor for advanced glycation
end-products protects against ischemia/reperfusion-induced
myocardial apoptosis via regulating the ubiquitin proteasome
system. Free Radic Biol Med. 94:17–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi ZY, Liu Y, Dong L, Zhang B, Zhao M,
Liu WX, Zhang X and Yin XH: Cortistatin improves cardiac function
after acute myocardial infarction in rats by suppressing myocardial
apoptosis and endoplasmic reticulum stress. J Cardiovasc Pharmacol
Ther. 18:2016.
|
|
9
|
Nishikido T, Oyama J, Shiraki A, Komoda H
and Node K: Deletion of apoptosis inhibitor of macrophage
(AIM)/CD5L attenuates the inflammatory response and infarct size in
acute myocardial infarction. J Am Heart Assoc. 5:e0028632016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Volkman R and Offen D: Concise review:
Mesenchymal stem cells in neurodegenerative diseases. Stem Cells.
35:1867–1880. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma T, Sun J, Zhao Z, Lei W, Chen Y, Wang
X, Yang J and Shen Z: A brief review: Adipose-derived stem cells
and their therapeutic potential in cardiovascular diseases. Stem
Cell Res. 8:1242017. View Article : Google Scholar
|
|
12
|
Kane C and Terracciano CMN: Concise
review: Criteria for chamber-specific categorization of human
cardiac myocytes derived from pluripotent stem cells. Stem Cells.
35:1881–1897. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu X, Xia R, Zhang B and Gao F: MRI
tracking stem cells transplantation for coronary heart disease. Pak
J Med Sci. 30:899–903. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu YH, Wu HQ and Qi X: Influence of shenfu
injection on heart function and bone marrow stem cell mobilization
in patients with chronic heart failure of coronary heart disease.
Zhongguo Zhong Xi Yi Jie He Za Zhi. 29:309–312. 2009.(In Chinese).
PubMed/NCBI
|
|
15
|
Ghem C, Dias LD, Sant'Anna RT, Kalil RAK,
Markoski M and Nardi NB: Combined analysis of endothelial,
hematopoietic, and mesenchymal stem cell compartments shows
simultaneous but independent effects of age and heart disease. Stem
Cells Int. 2017:52376342017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dirani M, Nasreddine W, Abdulla F and
Beydoun A: Seizure control and improvement of neurological
dysfunction in Lafora disease with perampanel. Epilepsy Behav Case
Rep. 2:164–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li D, Wei W, Ran X, Yu J, Li H, Zhao L,
Zeng H, Cao Y, Zeng Z and Wan Z: Lipoprotein-associated
phospholipase A2 and risks of coronary heart disease and ischemic
stroke in the general population: A systematic review and
meta-analysis. Clin Chim Acta. 471:38–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng B, Chen S, Gordon AD and Chakrabarti
S: miR-146a mediates inflammatory changes and fibrosis in the heart
in diabetes. J Mol Cell Cardiol. 105:70–76. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tzatzalos E, Abilez OJ, Shukla P and Wu
JC: Engineered heart tissues and induced pluripotent stem cells:
Macro- and microstructures for disease modeling, drug screening,
and translational studies. Adv Drug Deliv Rev. 96:234–244. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gidron Y, Kupper N, Kwaijtaal M, Winter J
and Denollet J: Vagus-brain communication in
atherosclerosis-related inflammation: A neuroimmunomodulation
perspective of CAD. Atherosclerosis. 195:e1–e9. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bujak M and Frangogiannis NG: The role of
IL-1 in the pathogenesis of heart disease. Arch Immunol Ther Exp.
57:165–176. 2009. View Article : Google Scholar
|
|
23
|
Li Z and Wu JC, Sheikh AY, Kraft D, Cao F,
Xie X, Patel M, Gambhir SS, Robbins RC, Cooke JP and Wu JC:
Differentiation, survival, and function of embryonic stem cell
derived endothelial cells for ischemic heart disease. Circulation.
116:I46–54. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu ZW, Liu YF, Wang S and Li B: miRNA-146a
induces vascular smooth muscle cell apoptosis in a rat model of
coronary heart disease via NF-kappaB pathway. Genet Mole Res.
14:18703–18712. 2015. View Article : Google Scholar
|