Introduction
Endometrial cancer is the most prevalent malignant
gynecological neoplasm in the United States, with ~63,230 new cases
and 11,350 cases of mortality predicted for 2018 (1). It accounts for ~6% of all cancers in
women. Endometrial cancer arises from the endometrium due to the
abnormal growth of cells with the ability to invade or metastasize
(2). It occurs frequently in
postmenopausal women with a mean age of 60 years at the time of
diagnosis (3). Vaginal bleeding or
discharge in menopausal women is associated with ~90% of
endometrial cancer (4). Patients
with endometrial cancer are often diagnosed at an early stage and
have a good outcome with ~82% 5-year relative survival (5). The standard treatment for endometrial
cancer includes radical hysterectomy, bilateral
salpingo-oophorectomy, abdominopelvic washing and lymph node
dissection followed by chemotherapy with or without radiotherapy
according to the grade and stage of the disease (6). However, patients with advanced or
recurrent endometrial cancer do not typically respond well to the
standard treatment (7). In addition,
resistance to chemotherapy remains a challenge and limits the
success of anticancer treatment. Various mechanisms contribute to
chemotherapy resistance including the phosphoinositide
3-kinase/protein kinase B (AKT) pathway (8), apoptotic pathways (9) and hormone receptor signaling pathways
(10). Therefore, it is important to
find alternative strategies to overcome chemotherapy
resistance.
microRNAs (miRNAs or miRs) are ~21–23 nucleotides in
length, and are highly conserved non-coding small RNA molecules
with the biological ability to induce gene silencing (11). Previous studies have demonstrated
that miRNA expression is associated with various biological
activities including embryo development, proliferation,
differentiation, apoptosis, metabolism and tumorigenesis (12,13).
Altered miRNA expression pattern has been demonstrated in
endometrial cancer. Hiroki et al (14) demonstrated that miR-34b expression is
associated with proliferation and invasion of endometrial cancer
cells. Wang et al (15)
reported that miR-34a expression was significantly reduced in
endometrial cancer tissues and miR-34a suppressed the
proliferation, migration and invasion by targeting Notch1 in
endometrial cancer cells. Tores et al (16) demonstrated that miR-99a, miR-100 and
miR-199b levels were increased in serum of patients with
endometrioid cancer. These findings indicate that the miRNAs may be
used as diagnostic markers in endometrioid cancer.
In the present study the effect of miR-423 in
proliferation, invasion, migration and chemoresistance of
endometrial cancer cell lines was examined.
Materials and methods
Cell lines
HEC-1B and Ishikawa cells, human endometrial
epithelial cancer cell lines, were obtained from Shanghai Cell
Bank, Chinese Academy of Sciences (Shanghai, China). These cells
were grown in Dulbecco's modified Eagle's medium (DMEM; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin (Invitrogen;
Thermo Fisher Scientific, Inc.), and cultured at 37°C in a
humidified atmosphere containing 5% CO2. When cells
reached 70–80% confluence, both cells were harvested for use in
further experiments.
Cell transfection
Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to transfect miR-423 mimic (100 ng;
5′-GCCTGAGGGGCAGAGAGC-3′), miR-423 inhibitor (100 ng;
5′-ATCTTTGGTGGCCGTAGACCT-3′) and scrambled negative control RNAs
(100 ng; 5′-GCCTAACTGTGTCAGAAGGAA-3′; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) into endometrial cancer cells. The cells were
harvested 48 h following transfection and the expression of miR-423
was detected via reverse transcription-quantitative polymerase
chain reaction (RT-qPCR).
RT-qPCR
Total RNA was extracted from the transfected
endometrial cancer cells using TRIzol reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
miRNA analysis was performed using Taqman MicroRNA Reverse
Transcription kit (Thermo Fisher Scientific, Inc.). qPCR was
performed as follows: 95°C for 10 min, followed by 40 cycles of
95°C for 15 sec and 60°C for 60 sec. The primer sequences used were
as follows: miR-423, forward 5′-GCCTGAGGGGCAGAGAGC-3′ and reverse
5′-CCACGTGTCGTGGAGTC-3′; and U6, forward 5′-GACTATCATATGCTTACCGT-3′
and reverse 5′-GGGCAGGAAGAGGGCCTAT-3′. U6 was used as an endogenous
control to calculate expression of miR-423 in endometrial cells.
miRNA expression levels were measured based on the threshold cycle
(Cq) and relative expression levels were calculated using the
2−ΔΔCq method (17).
WST-1 assay
To assess the effect of miR-423 on the proliferation
and chemotherapy of endometrial cancer cells, the WST-1 assay
(Roche Applied Science, Penzberg, Germany) was performed as
described previously (18). Briefly,
HEC-1B and Ishikawa cells transfected with miR-423 mimics, miR-423
inhibitor and scrambled negative control RNAs were placed in
96-well plates at a density of 1×104 cells/well. The
endometrial cells were cultured overnight at 37°C and the medium
was replaced with DMEM containing different concentration of
cisplatin (0, 1, 2 and 4 µg/ml; Sigma-Aldrich; Merck KGaA).
Endometrial cancer cells were subsequently cultured at 37°C in a
humidified incubator for 7 days. On alternate days, the medium was
removed and 200 µl DMEM containing WST-1 (20 µl/well) as added to
each well and incubated for at least 60 min at 37°C. The absorbance
was determined at 490 nm on a microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA). All experiments were
performed three times in at least triplicate.
Apoptosis analysis
To examine the effect of miR-423 on
cisplatin-induced apoptosis of endometrial cancer cells, the
caspase 3/7 activity was performed according to the manufacturer's
protocol (Caspase-Glo 3/7 assay systems; Promega Corporation,
Madison, WI, USA). The endometrial cancer cells transfected with
either miR-423 mimic or scrambled negative control RNAs were seeded
in 12-well plates as a density of 3×105/well. The cells
were cultured in DMEM overnight at 37°C in a humidified incubator,
and subsequently, the culture medium was replaced with culture
medium containing different concentration of cisplatin (0, 1, 2 and
4 µg/ml). Cells were cultured for a further 48 h. Caspase-Glo
reagent (Promega Corporation) was added to each well and incubated
for 8 h at room temperature with gentle agitation. The caspase 3/7
activity was measured using 1 min lag time and 0.5 sec/well read
time with a luminometer (Thermo Labsystems, Santa Rosa, CA, USA).
All experiments were performed three times in triplicate.
Migration and invasion assays
To determine the impact of miR-423 on migration and
invasion of endometrial cells, Transwell migration and invasion
assays were performed. For invasion assays, the upper chambers of
Transwell plates were coated with Matrigel (BD Biosciences,
Franklin Lakes, NJ, USA). HEC-1B cells and Ishikawa cells
transfected with either miR-423 mimic or miR-423 inhibitor, were
suspended in culture medium without FBS and placed in the upper
chamber at a density of 3×104/well. Culture medium
containing 7% FBS was added to the lower chamber. The cells were
incubated overnight at 37°C in a humidified atmosphere containing
5% CO2. Remaining cells in the upper chamber were
removed with a cotton swab and cells in the lower chamber were
stained at room temperature for 2 min with Diff Quik solution. The
invaded cells were counted under light microscopy in 5 random
visual fields (magnification, ×10). The percentage of invasion was
expressed as the ratio of invading cells/cell number normalized on
day 2 of the growth curve, based on the proliferation assay.
Endometrial cancer cells transfected with scrambled negative
control RNAs were used as the negative control. All experiments
were performed three times in triplicate.
Western blotting
To extract total protein from the transfected
endometrial cancer cells, ice-cold radioimmunoprecipitation assay
lysis and extraction buffer (Invitrogen; Thermo Fisher Scientific,
Inc.) was used to suspend the cells. The protein concentration was
measured by bicinchoninic acid assay. The total protein was mixed
with loading buffer (Abcam, Cambridge, MA, USA) and boiled for 10
min. Then the protein concentration was measured and separated by
10% SDS-PAGE and transferred to polyvinylidene difluoride membranes
(Sigma-Aldrich; Merck KGaA). Following blocking in 5% non-fat dry
milk in Tris-buffered saline Tween-20 buffer (TBST; Abcam) for 1 h
at room temperature, membranes were incubated with primary
antibodies (Table I) overnight at
4°C with gentle agitation. Membranes were washed with TBST at least
3 times with gentle agitation followed by incubation with the
horseradish peroxidase-conjugated secondary antibody (1:1,000;
anti-rabbit IgG; #7074S; Cell Signaling Technology, Inc., Danvers,
MA, USA) for 1 h at room temperature with gentle agitation. The
proteins were visualized using an EasySee Western Blot kit (Beijing
Transgen Biotech Co., Ltd., Beijing, China). GAPDH was used as the
loading control and iBright imaging systems (CL1000; Thermo Fisher
Scientific, Inc.), and Image Lab 6.0 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used for densitometry.
 | Table I.Antibodies used in western
blotting. |
Table I.
Antibodies used in western
blotting.
| Antibody | Supplier | Catalogue no. | Dilution |
|---|
| BAX | Cell Signaling
Technology, Inc., Danvers, MA, USA | 2772 | 1:200 |
| Bcl-2 | Santa Cruz
Biotechnology, Inc., Dallas, TX, USA | sc-7382 | 1:500 |
| E-cadherin | Cell Signaling
Technology, Inc. | 3195 | 1:200 |
| N-cadherin | Cell Signaling
Technology, Inc. | 4061 | 1:200 |
| Snail | Cell Signaling
Technology, Inc. | 3879 | 1:200 |
| Vimentin | Cell Signaling
Technology, Inc. | 5741 | 1:100 |
| p-AKT | Abcam, Cambridge,
MA, USA | ab3844 | 1:200 |
| PTEN | Cell Signaling
Technology, Inc. | 9552 | 1:200 |
| AKT | Cell Signaling
Technology, Inc. | 9272 | 1:500 |
| GAPDH | Cell Signaling
Technology, Inc. | 2118 | 1:2,000 |
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analysis was performed using one-way analysis of
variance followed by Tukey's post hoc test for comparisons between
multiple groups using SPSS 12.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-423 promotes proliferation of
endometrial cancer cells and induces cisplatin resistance
To assess the effect of miR-423 on cell
proliferation and cisplatin sensitivity, miR-423 mimic or miR-423
inhibitor was transfected to HEC-1B and Ishikawa cells with
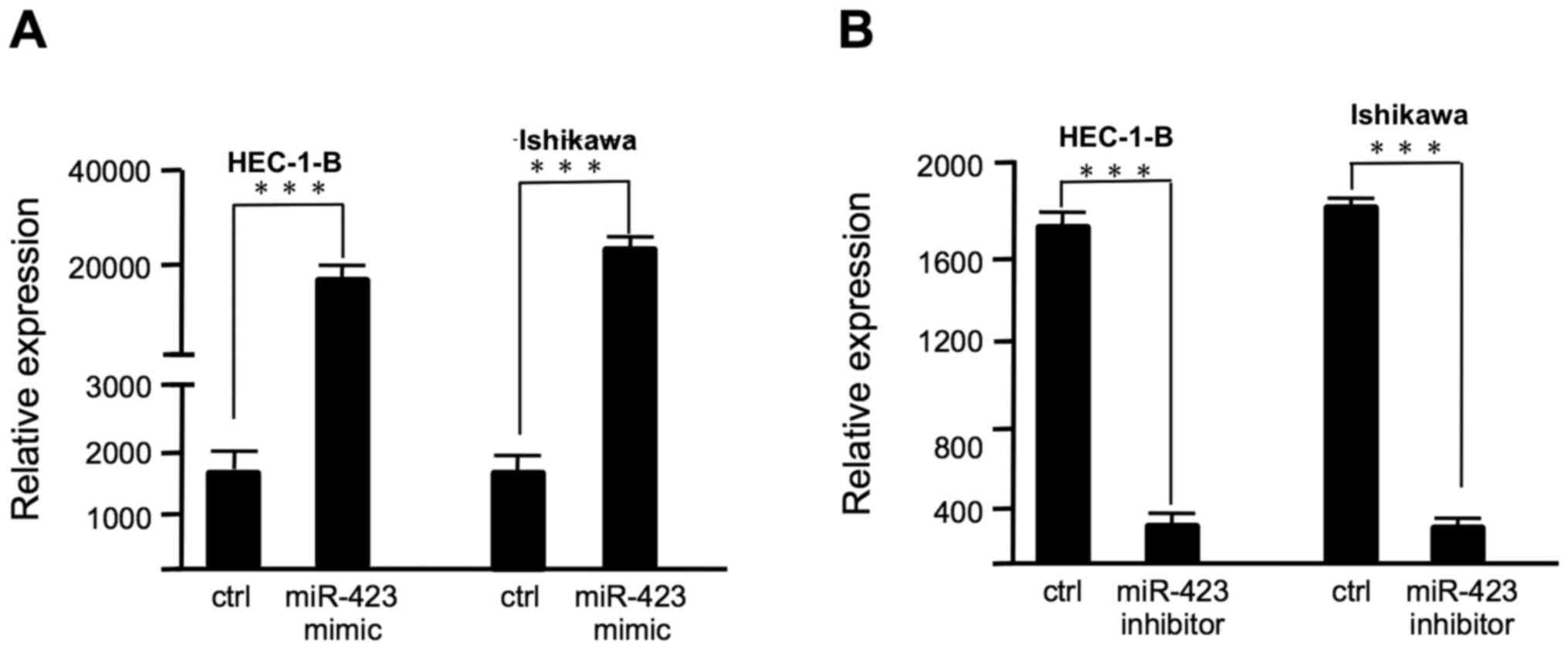
Lipofectamine 2000, as confirmed by RT-qPCR (Fig. 1). The proliferation of HEC-1B and
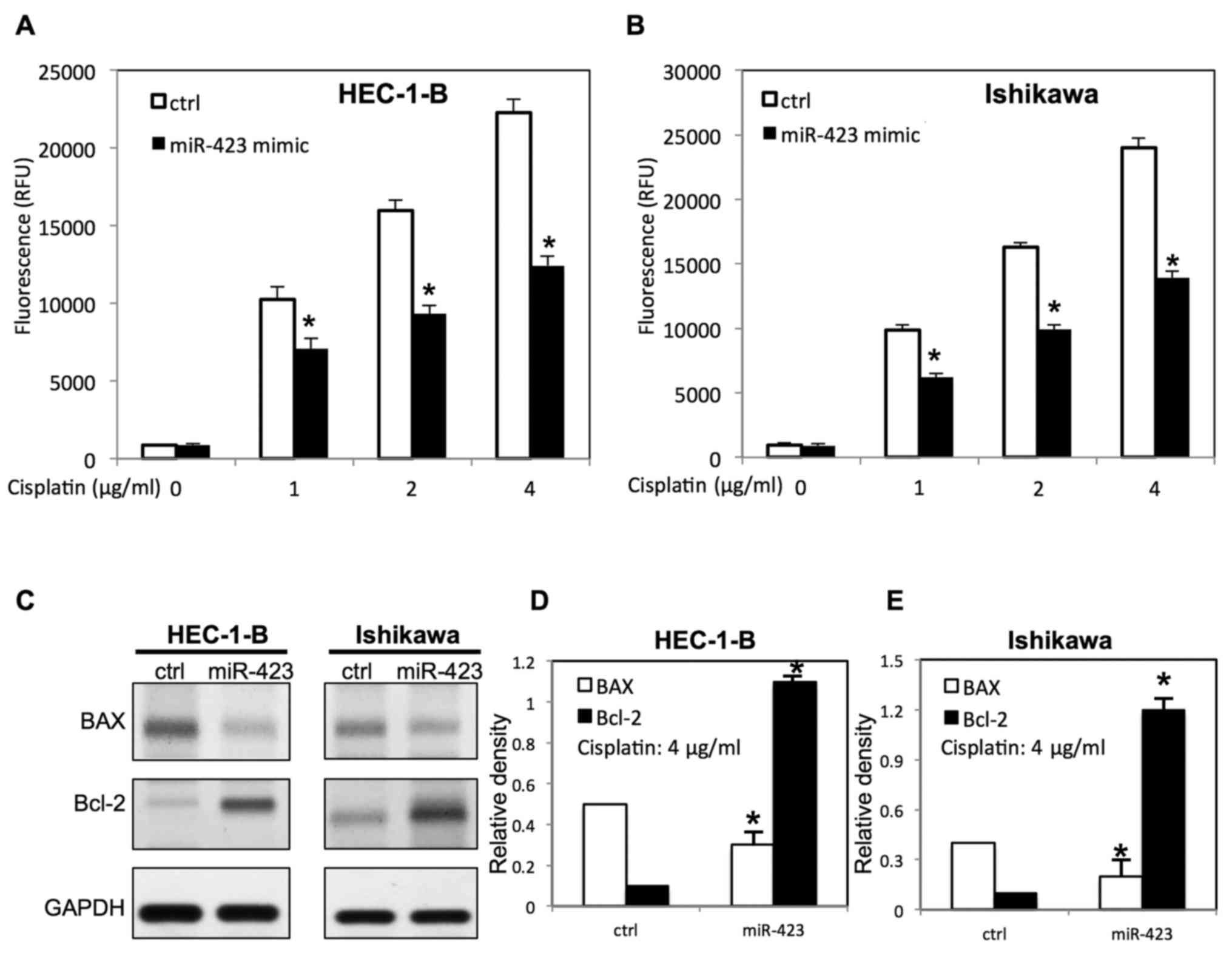
Ishikawa cells was evaluated using WST-1 assay (Figs. 2–4).
The scrambled control RNAs served as negative control. As presented
in Fig. 1A, the miR-423 expression
level was significantly increased in endometrial cancer cells
following transfection with miR-423 mimic. Overexpression of
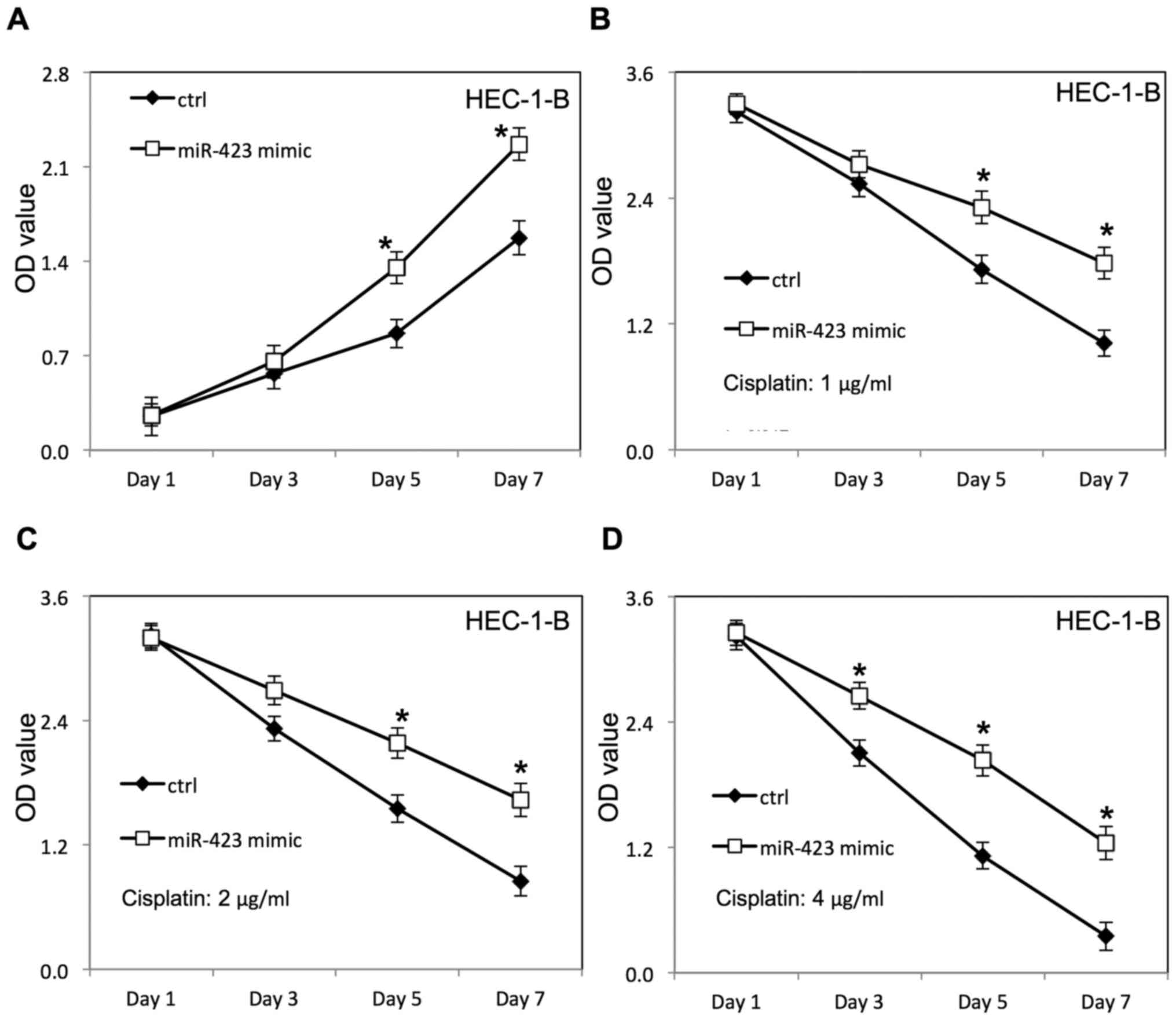
miR-423 significantly improved the proliferation of HEC-1B
(Fig. 2A) and Ishikawa cells
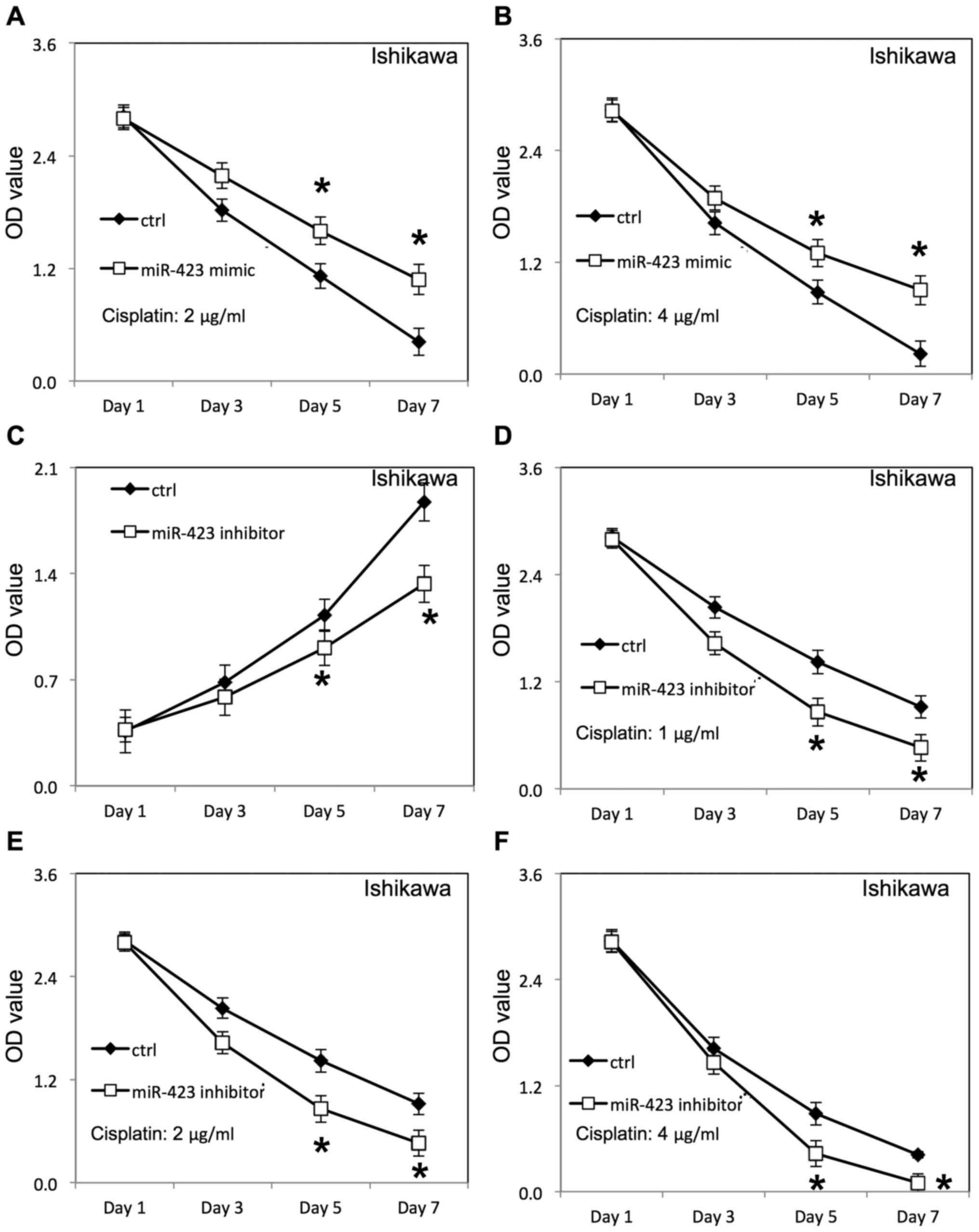
(Fig. 3E), compared with cells
transfected with negative control RNAs. Additionally, the survival
rate of HEC-1B (Fig. 2B-D) and
Ishikawa cells (Figs. 3F, 4A and B) was increased following
overexpression of miR-423 in the presence of cisplatin, compared
with the negative control group. The miR-423 expression level was
decreased in endometrial cancer cells following transfection with
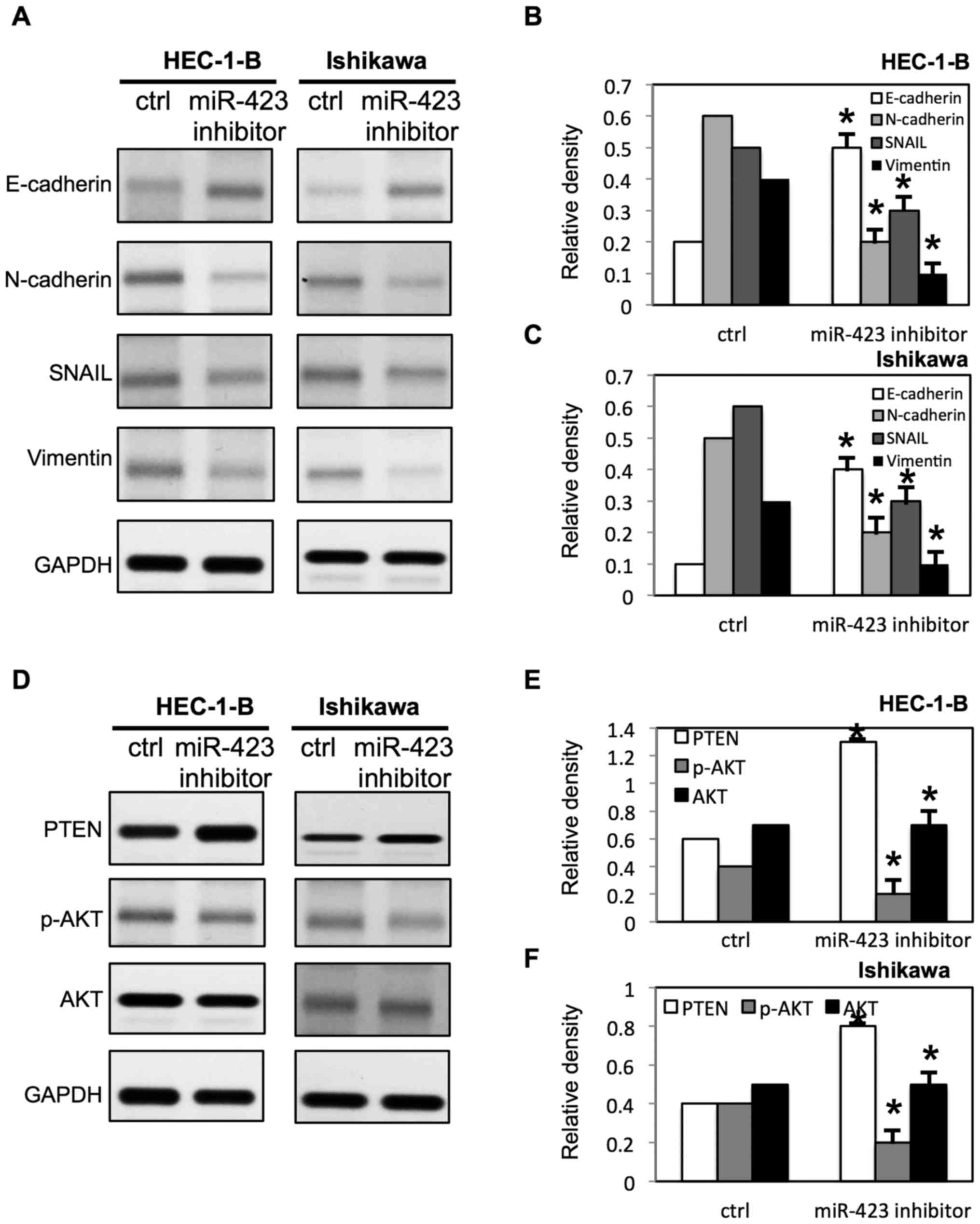
miR-423 inhibitor (Fig. 1B).
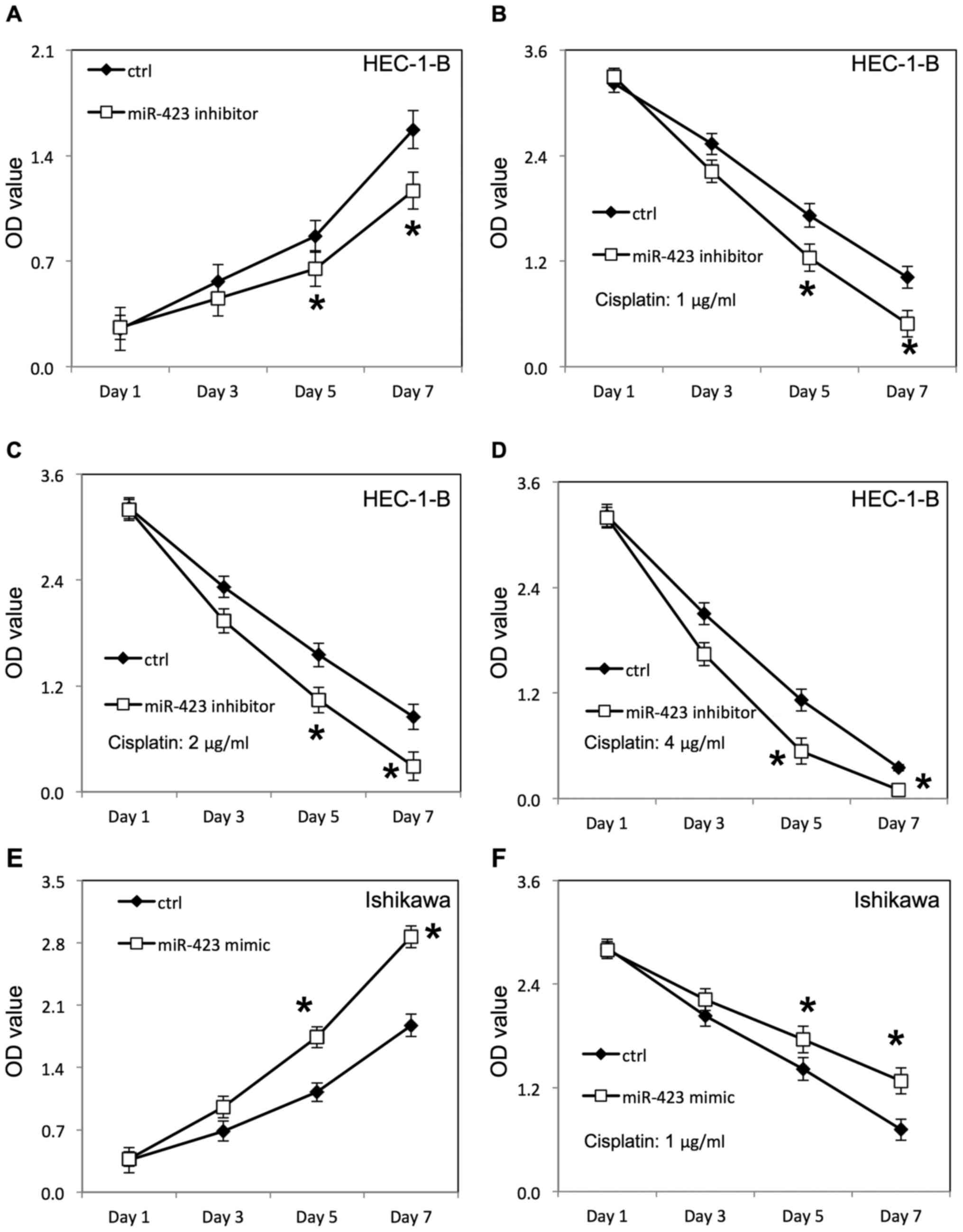
Downregulation of miR-423 inhibited proliferation of HEC-1B
(Fig. 3A) and Ishikawa cells
(Fig. 4C), compared with cells
transfected with negative control RNAs. Additionally, the survival
rate of HEC-1B (Fig. 3B-D) and
Ishikawa cells (Fig. 4D-F) was
decreased following downregulation of miR-423 in the presence of
cisplatin, compared with the negative control group.
miR-423 decreases cisplatin-induced
apoptosis of endometrial cancer cells
To examine the function of miR-423 on
cisplatin-induced apoptosis, transfected endometrial cancer cells
were treated with different doses of cisplatin. The apoptosis
analysis was carried out via evaluating caspase 3/7 activity. It
was demonstrated that upregulation of miR-423 inhibited
cisplatin-induced apoptosis by decreasing caspase3/7 activity
(Fig. 5A and B). Western blotting
was then performed to further study the expression of
apoptosis-associated proteins of transfected endometrial cells in
the presence of cisplatin. As presented in Fig. 5C-E, overexpression of miR-423
decreased B cell lymphoma-2 (Bcl-2) associated X protein (BAX)
expression, whereas the Bcl-2 expression was increased in both
HEC-1B and Ishikawa cells.
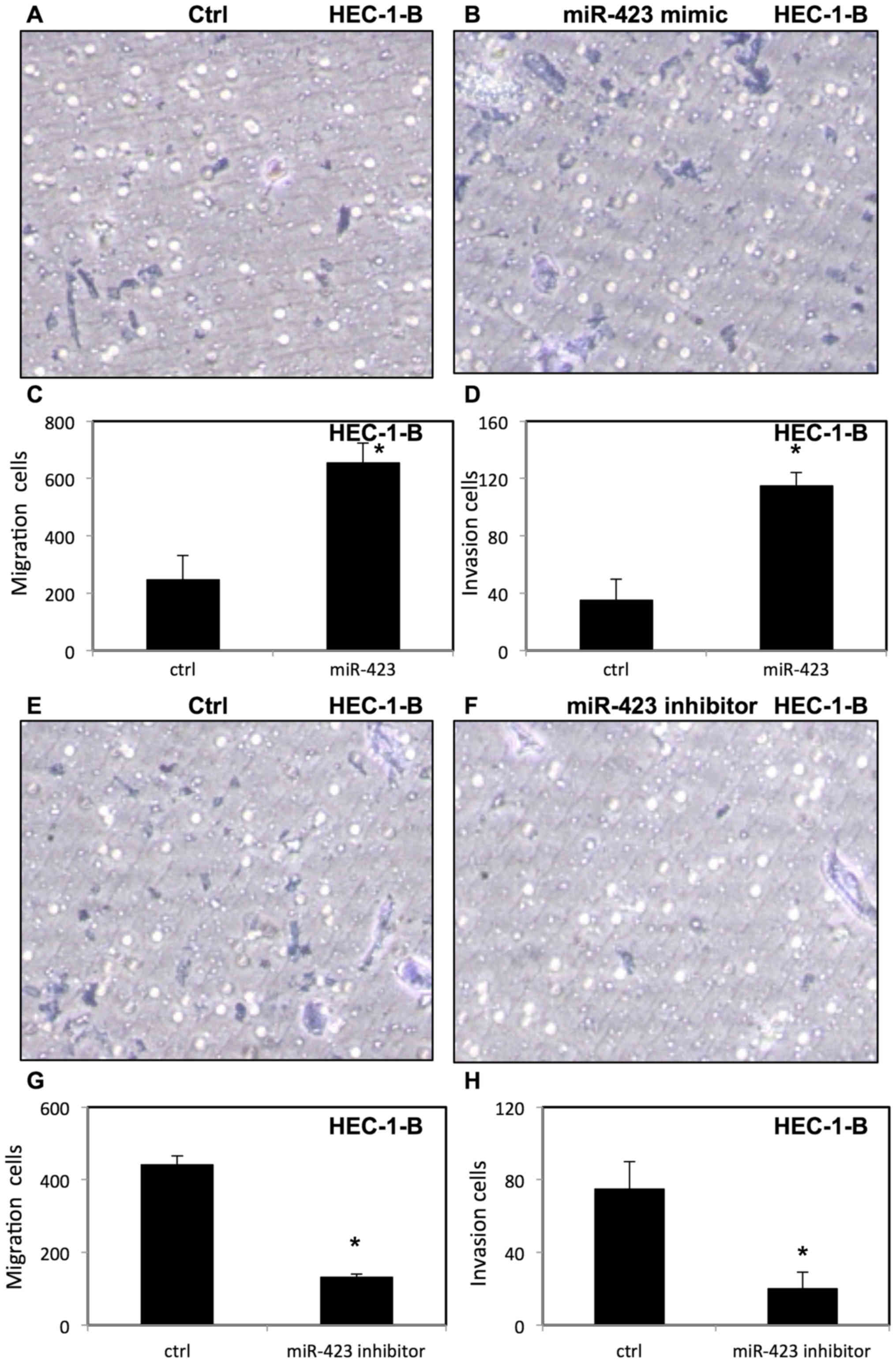
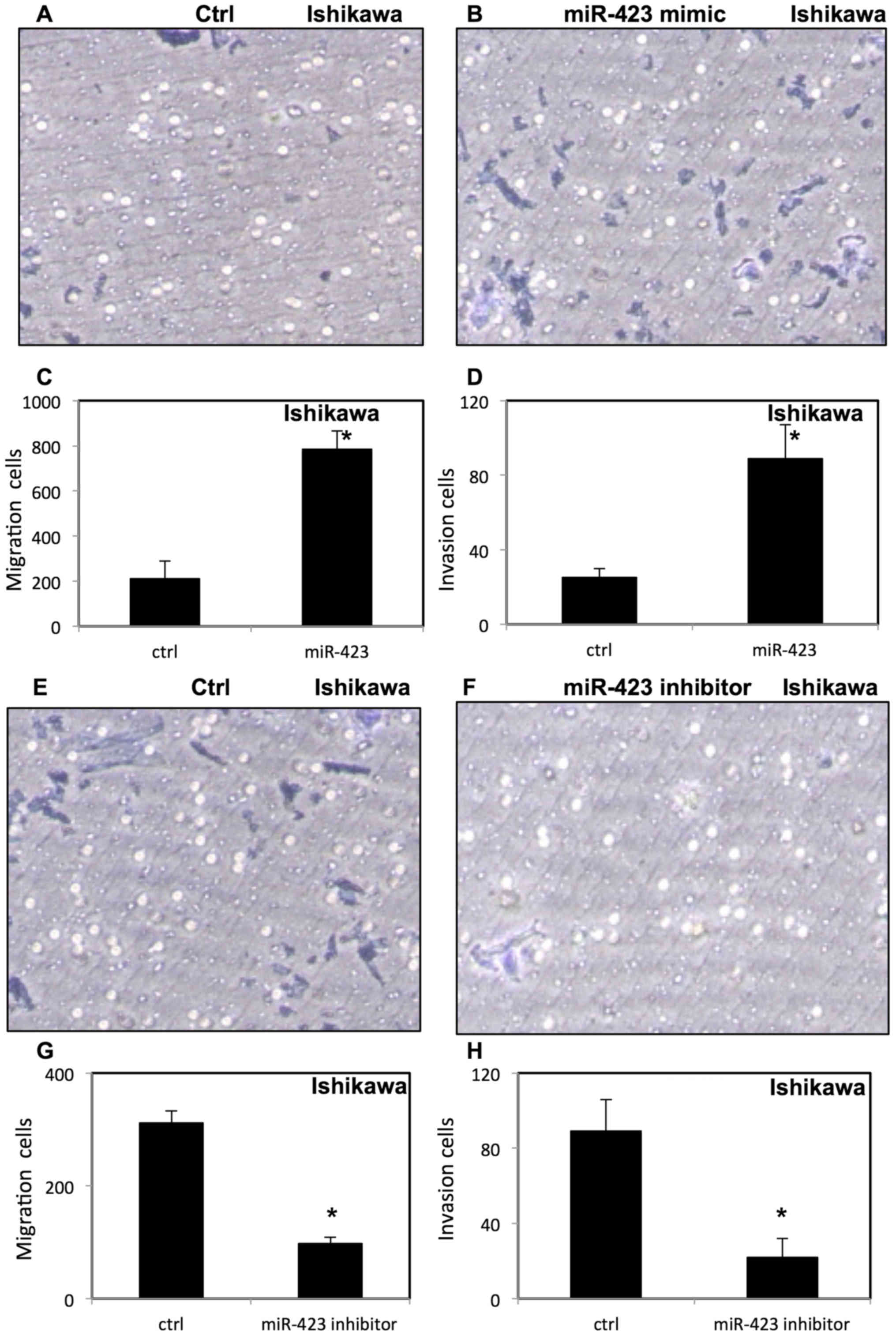
miR-423 increases migration and
invasion of endometrial cancer cells
To evaluate the effect of miR-423 on migration and
invasion of endometrial cancer cells, invasion and migration assays
were performed using transfected HEC-1B (Fig. 6) and Ishikawa cells (Fig. 7) were placed in Transwell chambers
with or without Matrigel. It was demonstrated that upregulation of
miR-423 enhanced migration and invasion of HEC-1B cells (Fig. 6A-D) and Ishikawa cells (Fig. 7A-D). In contrast, downregulation of
miR-423 decreased the migration and invasion of HEC-1B cells
(Fig. 6E-H) and Ishikawa cells
(Fig. 7E-H).
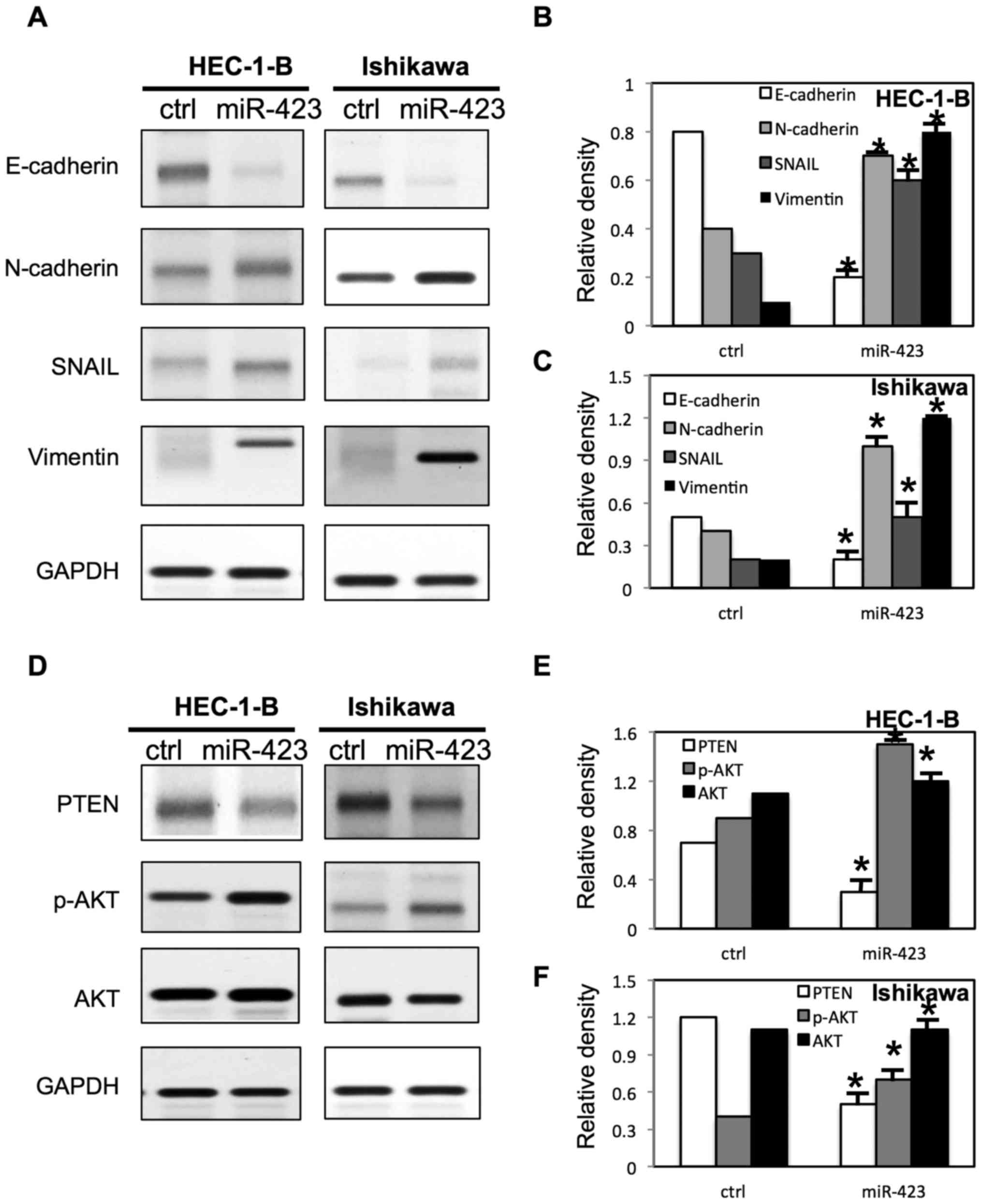
miR-423 changes the expression of EMT
markers and activates AKT pathway in endometrial cancer cells
To further evaluate the molecular mechanisms
underlying miR-423 mediated cell proliferation, migration and
invasion in endometrial cancer cells, multiple signaling pathways
were examined via western blotting (Figs. 8 and 9). It was demonstrated that the E-cadherin
expression was significantly decreased and the expression of
N-cadherin, snail and Vimentin were increased in both HEC-1B cells
and Ishikawa cells following overexpression of miR-423 (Fig. 8A-C). However, the downregulation of
miR-423 increased the expression of E-cadherin, and decreased the
expression of N-cadherin, snail and Vimentin in both HEC-1B cells
and Ishikawa cells (Fig. 9A-C).
Notably, It was demonstrated that miR-423 decreased the phosphatase
and tensin homolog (PTEN) expression level, and increased
phosphorylated (p)-AKT expression level in HEC-1B and Ishikawa
cells (Fig. 8D-F). Meanwhile, the
downregulation of miR-423 increased the expression of PTEN, and
decreased the expression of p-AKT in HEC-1B and Ishikawa cells
(Fig. 9D-F).
Discussion
Growing evidence has demonstrated that miRNA
expression serves essential roles in tumorigenesis and progression.
miR-423 expression level is upregulated in endometrial cancer cells
and involves in carcinogenesis (19). Lin et al (20) reported that miR-423 expression was
upregulated in hepatocellular carcinoma and miR-423 promoted cell
proliferation by inhibiting tumor suppressor p21Cip1/Waf1
expression. Zhao et al (21)
demonstrated that miR-423 enhanced cell proliferation in breast
cancer cell lines and acted as a potentially oncogenic role in
breast tumorigenesis. Another previous study demonstrated that
miR-423 may be an independent marker to predict outcome in patients
with breast cancer (22). The
present study demonstrated that overexpression of miR-423 enhanced
proliferation of endometrial cancer cells, and downregulation of
miR-423 inhibited proliferation of endometrial cancer cells. These
findings indicated that miR-423 serves a role in the proliferation
of endometrial cancer cells.
Cisplatin is a well-known effective anticancer drug
and has been used to treat numerous types of malignant tumors
including endometrial cancer (23,24).
Chemotherapy resistance remains a major challenge, although many
efforts have been made to develop novel chemotherapeutic agents
(25). Previous studies have
demonstrated that chemoresistance may be present prior to therapy
or may develop following treatment of recurrent cancer (26). Therefore, it is urgent to identify an
alternative strategy to increase chemotherapy sensitivity and
reverse resistance. miRNAs have been demonstrated to serve
essential roles in chemotherapy sensitivity (27). Yang et al (28) reported that miR-214 promoted cell
survival and induced cisplatin resistance by targeting PTEN in
ovarian cancer. Kong et al (29) demonstrated that overexpression of
miR-155 promoted BT-474 breast cells resistant to paclitaxel, VP16
and doxorubicin, and downregulation of miR-155 sensitized HS578T
cells to these drugs. Yu et al (30) reported that overexpression of
miR-17/20 increased doxorubicin-induced apoptosis in MCF-7 breast
cancer cells by targeting AKT1. The present study demonstrated that
overexpression of miR-423 increased the survival of endometrial
cancer cells following cisplatin treatment. These results suggested
that miR-423 induces drug resistance of endometrial cancer cells.
By apoptosis analysis, it was demonstrated that the upregulation of
miR-423 decreases the cisplatin-induced apoptosis of endometrial
cancer cells. In addition, overexpression of miR-423 increased
Bcl-2 and decreased BAX expression levels in endometrial cancer
cells. Notably, the survival rate of HEC-1B and Ishikawa cells was
decreased following downregulation of miR-423 in the presence of
cisplatin, compared with the negative control group. These findings
indicated that miR-423 increased the survival rate of HEC-1B and
Ishikawa cells following treatment with cisplatin via inhibiting
apoptosis. These results indicated that miR-423 may serve an
important role in conferring chemosensitivity to endometrial cancer
cells.
Epithelial-mesenchymal transition (EMT) is a
biological process that allows epithelial cells to lose their
epithelial characteristics and acquire a mesenchymal phenotype. EMT
serves critical roles in motility, invasiveness and resistance to
apoptosis of cancer cell (31).
Recent studies demonstrated that miR-200 family members inhibited
EMT by directly targeting zinc finger E-box binding homeobox (ZEB)1
and ZEB2 (32). It was demonstrated
in the present study that overexpression of miR-423 increased
migration and invasion in endometrial cancer cells via Transwell
migration and invasion assays. In contrast, knockdown of miR-423
decreased the migration and invasion of endometrial cancer cells.
As detailed above, miR-423 promoted proliferation and inhibited
apoptosis of endometrial cancer cells. Although the ratio of
invading cells to the total cell number was normalized on day 2 of
the growth curve, there is a possibility that the invasion of
endometrial cancer cells, at least to some extent, was affected by
miR-423 associated proliferation or apoptosis. Therefore, further
experiments were performed to explore the effect of miR-423 on
expression of EMT-associated proteins in endometrial cancer cells.
It was demonstrated that miR-423 inhibited E-cadherin expression
and promoted the expression of N-cadherin, snail and Vimentin in
endometrial cancer cells. The present study indicated that the
expression level of miR-423 is associated with endometrial cancer
progression.
PTEN is a well-known tumor suppressor gene in human
cancer and regulates multiple biological processes including
apoptosis, cell proliferation, invasion, adhesion and metabolism
(33). PTEN negatively regulates AKT
activity through the dephosphorylation of
phosphatidylinositol-trisphosphate (PIP3) to PIP2 (34). PTEN mutations have been reported in
55% of precancerous lesions, up to 80% of endometrioid endometrial
cancer and up to 90% of high-grade tumors (35). Loss of PTEN and activation of AKT are
associated with resistance to small molecule compound treatment in
endometrial cancer. It was demonstrated that miR-423 decreased the
PTEN expression level, and increased the p-AKT expression level in
endometrial cancer cells. These data suggested that miR-423
regulated proliferation of endometrial cancer cells by targeting
the AKT signaling pathway. Further studies are required to
demonstrate the molecular mechanism.
In conclusion, miR-423 serves important roles in
tumorigenesis and malignant progression of endometrial cancer. The
present study indicates that miR-423 may be used as a potential
biomarker to predict the chemotherapy response and prognosis in
endometrial cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL performed the experiments. HS and TL performed
data analysis. JK designed the project. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu YY, Wu HJ, Ma HD, Xu LP, Huo Y and Yin
LR: MicroRNA-503 suppresses proliferation and cell-cycle
progression of endometrioid endometrial cancer by negatively
regulating cyclin D1. FEBS J. 280:3768–3779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prat J: Pathology of cancers of the female
genital tract. Int J Gynaecol Obstet. 131 Suppl 2:S132–S145. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jin J: Vaginal and urinary symptoms of
menopause. JAMA. 317:13882017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dinkelspiel HE, Wright JD, Lewin SN and
Herzog TJ: Contemporary clinical management of endometrial cancer.
Obstet Gynecol Int. 2013:5838912013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Colombo N, Preti E, Landoni F, Carinelli
S, Colombo A, Marini C and Sessa C: ESMO Guidelines Working Group:
Endometrial cancer: ESMO clinical practice guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 24 Suppl
6:vi33–vi38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davidson BA, Foote J, Clark LH, Broadwater
G, Ehrisman J, Gehrig P, Graybill W, Secord Alvarez A and
Havrilesky LJ: Tumor grade and chemotherapy response in
endometrioid endometrial cancer. Gynecol Oncol Rep. 17:3–6. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
West KA, Castillo SS and Dennis PA:
Activation of the PI3K/Akt pathway and chemotherapeutic resistance.
Drug Resist Updat. 5:234–248. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mohammad RM, Muqbil I, Lowe L, Yedjou C,
Hsu HY, Lin LT, Siegelin MD, Fimognari C, Kumar NB, Dou QP, et al:
Broad targeting of resistance to apoptosis in cancer. Semin Cancer
Biol. 35 Suppl:S78–S103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johnston SR: Enhancing endocrine therapy
for hormone receptor-positive advanced breast cancer: Cotargeting
signaling pathways. J Natl Cancer Inst. 107:pii: djv212. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Catalanotto C, Cogoni C and Zardo G:
MicroRNA in control of gene expression: An overview of nuclear
functions. Int J Mol Sci. 17:pii: E1712. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Macfarlane LA and Murphy PR: MicroRNA:
Biogenesis, function and role in cancer. Curr Genomics. 11:537–561.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pillai RS: MicroRNA function: Multiple
mechanisms for a tiny RNA? RNA. 11:1753–1761. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hiroki E, Suzuki F, Akahira J, Nagase S,
Ito K, Sugawara J, Miki Y, Suzuki T, Sasano H and Yaegashi N:
MicroRNA-34b functions as a potential tumor suppressor in
endometrial serous adenocarcinoma. Int J Cancer. 131:E395–E404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Z, Wang W, Huang K, Wang Y, Li J and
Yang X: MicroRNA-34a inhibits cells proliferation and invasion by
downregulating Notch1 in endometrial cancer. Oncotarget.
8:111258–111270. 2017.PubMed/NCBI
|
|
16
|
Torres A, Torres K, Pesci A, Ceccaroni M,
Paszkowski T, Cassandrini P, Zamboni G and Maciejewski R:
Deregulation of miR-100, miR-99a and miR-199b in tissues and plasma
coexists with increased expression of mTOR kinase in endometrioid
endometrial carcinoma. BMC Cancer. 12:3692012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Riss TL, Moravec RA, Niles AL, et al: Cell
viability assaysSittampalam GS, Coussens NP, Brimacombe K, et al:
Assay Guidance Manual. Bethesda (MD): 2004, View Article : Google Scholar
|
|
19
|
Yanokura M, Banno K, Iida M, Irie H, Umene
K, Masuda K, Kobayashi Y, Tominaga E and Aoki D: MicroRNAS in
endometrial cancer: Recent advances and potential clinical
applications. EXCLI J. 14:190–198. 2015.PubMed/NCBI
|
|
20
|
Lin J, Huang S, Wu S, Ding J, Zhao Y,
Liang L, Tian Q, Zha R, Zhan R and He X: MicroRNA-423 promotes cell
growth and regulates G(1)/S transition by targeting p21Cip1/Waf1 in
hepatocellular carcinoma. Carcinogenesis. 32:1641–1647. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao H, Gao A, Zhang Z, Tian R, Luo A, Li
M, Zhao D, Fu L, Fu L, Dong JT and Zhu Z: Genetic analysis and
preliminary function study of miR-423 in breast cancer. Tumour
Biol. 36:4763–4771. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Volinia S, Galasso M, Sana ME, Wise TF,
Palatini J, Huebner K and Croce CM: Breast cancer signatures for
invasiveness and prognosis defined by deep sequencing of microRNA.
Proc Natl Acad Sci USA. 109:3024–3029. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moxley KM and McMeekin DS: Endometrial
carcinoma: A review of chemotherapy, drug resistance, and the
search for new agents. Oncologist. 15:1026–1033. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abdullah LN and Chow EK: Mechanisms of
chemoresistance in cancer stem cells. Clin Transl Med. 2:32013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng HC: The molecular mechanisms of
chemoresistance in cancers. Oncotarget. 8:59950–59964.
2017.PubMed/NCBI
|
|
27
|
Meng F, Henson R, Lang M, Wehbe H,
Maheshwari S, Mendell JT, Jiang J, Schmittgen TD and Patel T:
Involvement of human micro-RNA in growth and response to
chemotherapy in human cholangiocarcinoma cell lines.
Gastroenterology. 130:2113–2129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang H, Kong W, He L, Zhao JJ, O'Donnell
JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV and Cheng JQ:
MicroRNA expression profiling in human ovarian cancer: miR-214
induces cell survival and cisplatin resistance by targeting PTEN.
Cancer Res. 68:425–433. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kong W, He L, Coppola M, Esposito NN,
Coppola D and Cheng JQ: MicroRNA-155 regulates cell survival,
growth, and chemosensitivity by targeting FOXO3a in breast cancer.
J Biol Chem. 291:228552016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu Z, Xu Z, Disante G, Wright J, Wang M,
Li Y, Zhao Q, Ren T, Ju X, Gutman E, et al: miR-17/20 sensitization
of breast cancer cells to chemotherapy-induced apoptosis requires
Akt1. Oncotarget. 5:1083–1090. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li L and Li W: Epithelial-mesenchymal
transition in human cancer: Comprehensive reprogramming of
metabolism, epigenetics, and differentiation. Pharmacol Ther.
150:33–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie Y, Naizabekov S, Chen Z and Tokay T:
Power of PTEN/AKT: Molecular switch between tumor suppressors and
oncogenes. Oncol Lett. 12:375–378. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu W, Yang Z, Zhou SF and Lu N:
Posttranslational regulation of phosphatase and tensin homolog
(PTEN) and its functional impact on cancer behaviors. Drug Des
Devel Ther. 8:1745–1751. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Djordjevic B, Hennessy BT, Li J, Barkoh
BA, Luthra R, Mills GB and Broaddus RR: Clinical assessment of PTEN
loss in endometrial carcinoma: Immunohistochemistry outperforms
gene sequencing. Mod Pathol. 25:699–708. 2012. View Article : Google Scholar : PubMed/NCBI
|