Introduction
Heart transplantation (HT) is a therapeutic option
for patients with end-stage heart or coronary artery disease when
all other treatments have failed (1,2). The use
of immunosuppressive regimens following HT has demonstrated
improved graft longevity through reduction of early graft loss due
to acute rejection (3,4). However, most immunosuppressive drugs
cause a large spectrum of adverse effects and increase the risk of
malignancy, which contributes to a leading cause of mortality in HT
patients (5,6).
Cyclosporin A (CsA) is an immunosuppressant
medication commonly used for the prophylaxis of graft rejection
with improvement in long-term survival (7,8).
Therapeutic drug monitoring of CsA in transplant patients has
markedly evolved in routine clinical practices incethis drug
requires dosage individualization (9,10).
Although monitoring of the CsA concentration at 2 h after
administration (C2) is recommended for the evaluation of drug
exposure in transplant recipients (11,12), the
optimal time-point for monitoring CsA target levels remains
controversial in HT patients (13).
The aim of the present study was to explore the
optimal time-point for CsA concentration monitoring in the
long-term treatment of HT patients. The results confirm that the C2
approach serves as a clinical tool to monitor CsA target levels and
the occurrence of adverse events in HT recipients.
Materials and methods
Patients and inclusion criteria
A total of 32 patients who received HT surgery
between June 2012 and February 2014 at the Capital Medical
University Affiliated Anzhen Hospital (Beijing, China) were
recruited for the present study. These patients included 25 males
and 7 females aged between 22–53 years (median age, 37.5 years).
The patients' characteristics and the etiologies of heart failure
were evaluated. The left ventricular size and function, evaluated
as the left ventricular end diastolic diameter (LEVDD) and ejection
fraction (EF), are presented in Table
I. The major inclusion criteria for the recipients were as
follows: i) No hypertension or hyperlipidemia diagnosed with in 6
months prior to the HT operation, and no medical history there of;
ii) blood glucose levels within the normal range over 6 months
prior to the operation; iii) normal results in liver and kidney
function tests and iv) no contraindications for steroids,
mycophenolate mofetil (MMF) and CsA. The transplant hearts were
obtained from organ donors who were Chinese citizens with brain
death as defined by The Red Cross Society of China.
 | Table I.Characteristics of patients and
evaluation of left ventricular size and function. |
Table I.
Characteristics of patients and
evaluation of left ventricular size and function.
| Group | No. | Males | Age (years) | Dilated (schemic)
cardiomyopathy | LEVDD (mm) | EF (%) |
|---|
| A | 11 | 7 (36.6) | 42.7±12.1 | 9 (2) | 73.5±15.7 | 19.6±5.7 |
| B | 13 | 8 (61.5) | 45.6±9.7 | 12 (1) | 71.3±8.3 | 22.3±8.7 |
| C | 8 | 6 (75.0) | 41.3±16.7 | 7 (1) | 74.5±6.9 | 20.5±9.6 |
Therapeutic procedures
The subjects were assigned to three groups according
to their plasma CsA levels at C2 after an initial dose of CsA (4
mg/kg/day) was given post-operatively. After one week of treatment,
the recipients in groups A, B and C received oral CsA (Neoral,
Novartis, Switzerland) at the doses of 3.2, 3.5 and 4.4 mg/kg once
a day, respectively, for 6 consecutive months. In the following 6
months, CsA treatment was maintained at the doses of 2.8 mg/kg in
group A, 3.1 mg/kg in group B and 3.9 mg/kg in group C,
respectively. The target plasma concentrations of CsA (ng/ml) at C2
in the patient groups are presented in Table II. All patients were simultaneously
administered oral prednisone (Douglas Pharmaceuticals Ltd.,
Auckland, New Zealand) at 60 mg/day for 15 days and gradually
reduced to 20 mg/day until the end of the study. MMF was purchased
from Hoffmann-LaRoche (Basel, Switzerland) and administered to
patients at 500 mg twice a day during the entire study.
 | Table II.Plasma cyclosporine A concentration
(ng/ml) at C2 in heart transplant recipients. |
Table II.
Plasma cyclosporine A concentration
(ng/ml) at C2 in heart transplant recipients.
| Group | 1st six months | 2nd six months |
|---|
| A | 600–800 | 400–600 |
| B | >800-1,000 | >600–800 |
| C |
>1,000–1,200 | >800-1,000 |
Preparation of samples and analysis of
CsA concentration
Blood samples obtained from patients were prepared
following the manufacturer's protocol and CsA concentrations in
samples were evaluated using a fluorescence polarization
immunoassay (Abbott Pharmaceutical Co., Ltd., Lake Bluff, IL, USA).
In brief, 2.0 ml venous blood was collected at different
time-points at 2-h intervals over 12 h after oral administration of
CsA. Following lysis and precipitation of blood cells, the
supernatants were collected after the heparin anti-coagulated blood
samples were centrifuged (9,000 × g for 5.0 min at 4°C). For
pharmacokinetic analysis, quantification of the immunosuppressant
CsA in plasma was performed by a TDxFLx-SYSTEManalyzer (Abbott
Laboratories, Irving, TX, USA).
Sample collection was performedby collecting serial
blood samples over a limited period (0–12 h) after administering a
single dose of CsA. The area under the plasma CsA concentration
versus time curve (AUC) was calculated to estimate the extent of
drug absorption after dosing (14).
In the presentstudy, the CsA levels at specific time-points were
compared with the 4-h pharmacokinetic profiles (AUC0–4)
from the recipients, since the latterapproach to monitor CsA
exposure has been used in immunosuppressive regimens (13,15).
Myocardial biopsy
Endomyocardial biopsy (EMB) has been proven to be a
diagnostic tool for the surveillance of cardiac allograft rejection
and identification of myocardial diseases (16). EMB was performed in a supine position
with local anesthesia, and the biopsy was performed through a
catheter threaded into the patient's heart through the jugular vein
under ultrasound guidance. The clinicians used moving images to
guide the catheter to the targeted area. Once in position, a
special device with jaws on the tip was used to remove small pieces
of tissue from the heart muscle (16,17).
The histological grades of organ rejection in EMB
specimens were classified according to the International Society
for Heart and Lung Transplantation (ISHLT) (17).
Clinical observation of the efficacy
and safety of the immunosuppressive therapy
The efficacy of CsA in the management of HT
recipients who were receiving combination therapy with two drugs
(MMF, 500 mg twice a day and prednisone, 20 mg/day) at the same
dosages and time was examined according to the survival rate of HT
patients and the proportion of patients with adverse events. Since
the survival rates vary with the length of time post-HT, the rates
were expressed as a percentage of the population proportion to the
survivors only, at one year after HT.
Adverse events associated with the use of CsA for
the prophylaxis of cardiac allograft rejection were examined. They
mainly included hypertension, diabetes mellitus, hyperlipidemia and
renal in sufficiency diagnosed by a high serum creatinine level
(>133 µmol/l). The incidence of side effects was expressed as
the percentage of affected subjects in each group.
Statistical analysis
Values are expressed as the mean ± standard
deviation and as a percentage/distribution within the study cohort.
Statistical analysis was performed using SPSS version 21.0 (IBM
Corp., Armonk, NY, USA). One-way analysis of variance was
implemented for comparison of independent variables (Tukey's test).
The correlation between the plasma CsA levels at specific sampling
time-points and the AUC0–4 was estimated by calculating
Pearson's correlation coefficient (r2). The
χ2 test was performed to analyze the significance of
population distribution among the grouped recipients. P<0.05 was
considered to indicate a statistic all significant difference.
Results
Pharmacokinetic profile of CsA
absorption
The effect of drugs is frequently associated with
their concentration in the blood; hence it is useful to establish a
concentration-time association after a single administration of
CsA. Changes in the plasma CsA concentration in HT recipients
(n=32) were observed at 2 h intervals over a period of 12 h and CsA
levels were obtained just before drug administration. The
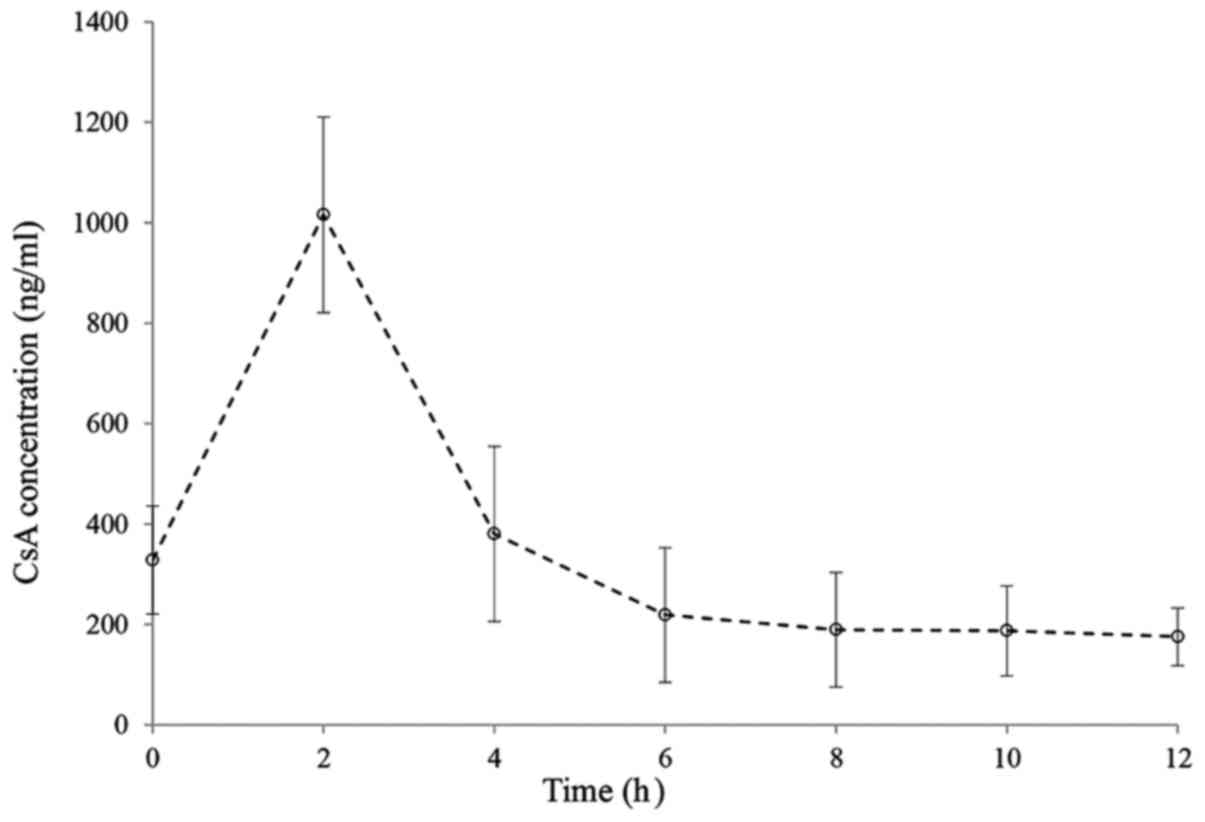
pharmacokinetic profile of CsA is presented in Fig. 1, displaying the mean plasma CsA
concentration in the transplant recipients. Most patients exhibited
similar variation of CsA plasma levels during the 12-h period
following administration. Of note, sampling between 0 and 4 h
appeared to provide a reliable indication of total CsA exposure. In
general, the plasma CsA concentration-time curve of the recipients
exhibited an increasing trend initially and reached a peak level at
2 h following administration, after which the levels dropped again
to reach a stable, lower level from 6–12 h.
Correlation between CsA and AUC
CsA concentrations were measured by collecting blood
samples over the time period of 0–12 h after dosing at 2-h
intervals. The r2 value was calculated for the
correlation between plasma CsA levels taken at specific time-points
and the AUC0–4 h (Fig.
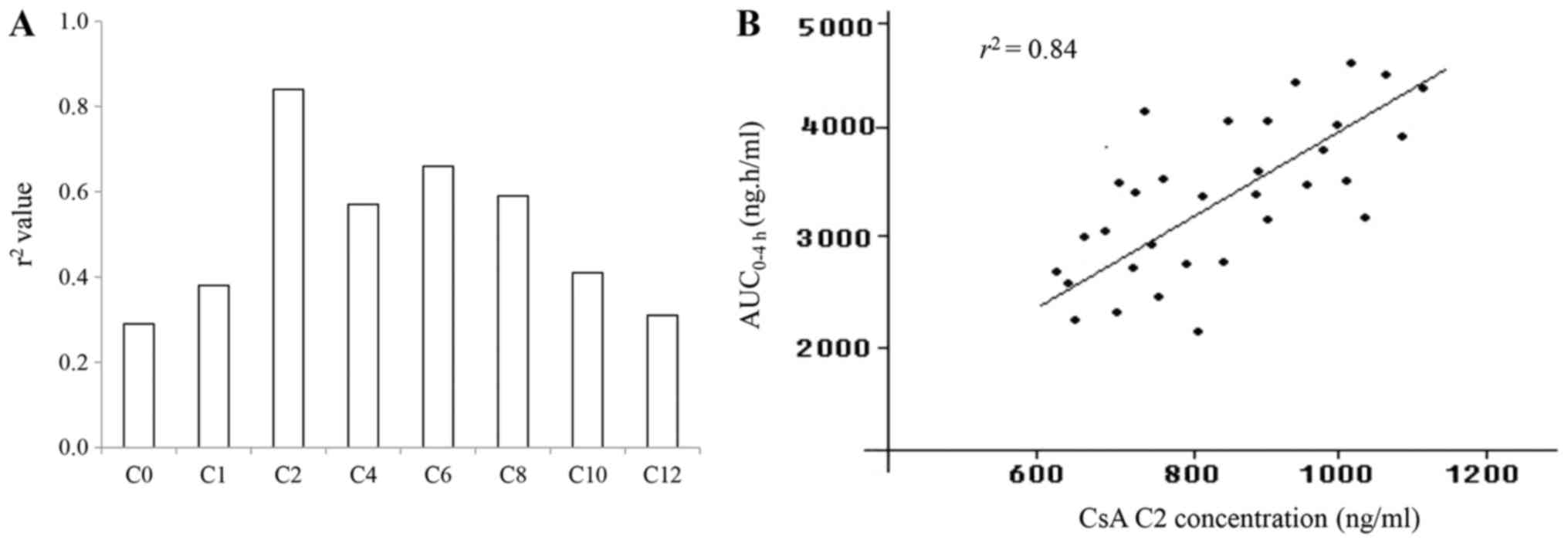
2). According to the regression analysis, the r2
values varied significantly for CsA concentrations detected at
different blood sampling times (Fig.
2A). The r2 values were 0.29 at C0, 0.38 at C1, 0.84
at C2, 0.57 at C4, 0.66 at C6, 0.59 at C8, 0.41 at C10 and 0.31 at
C12. Of note, the highest r2 value was obtained at C2,
thereby indicating the applicability of this time-point for
monitoring the CsA absorption in the transplant recipients.
The correlation of the plasma CsA concentration
examined by measurement of the AUC0–4 h (ng/h/ml) with
that determined at C2 is displayed in (Fig. 2B). The levels of CsA at C2 were
significantly enhanced with increasing doses of the
immunosuppressive agent and ranged from 600 to 1,200 ng/ml in these
HT recipients (n=32). In parallel, the AUC0–4 h values
(ng/h/ml) displayed a dose-dependent increment in the CsA-treated
recipients. A positive correlation between the CsA levels at C2 and
the AUC0–4 h was determined in the study cohort with an
r2 value of 0.84 (P<0.05). It was therefore indicated
that the C2 monitoring strategy is suitable for assessing the
target levels of CsA in HT recipients at one time-point.
Clinical significance of CsA
therapy
Survival rates and adverse events for recipients
with HT rejection were assessed in line with the strategy of CsA
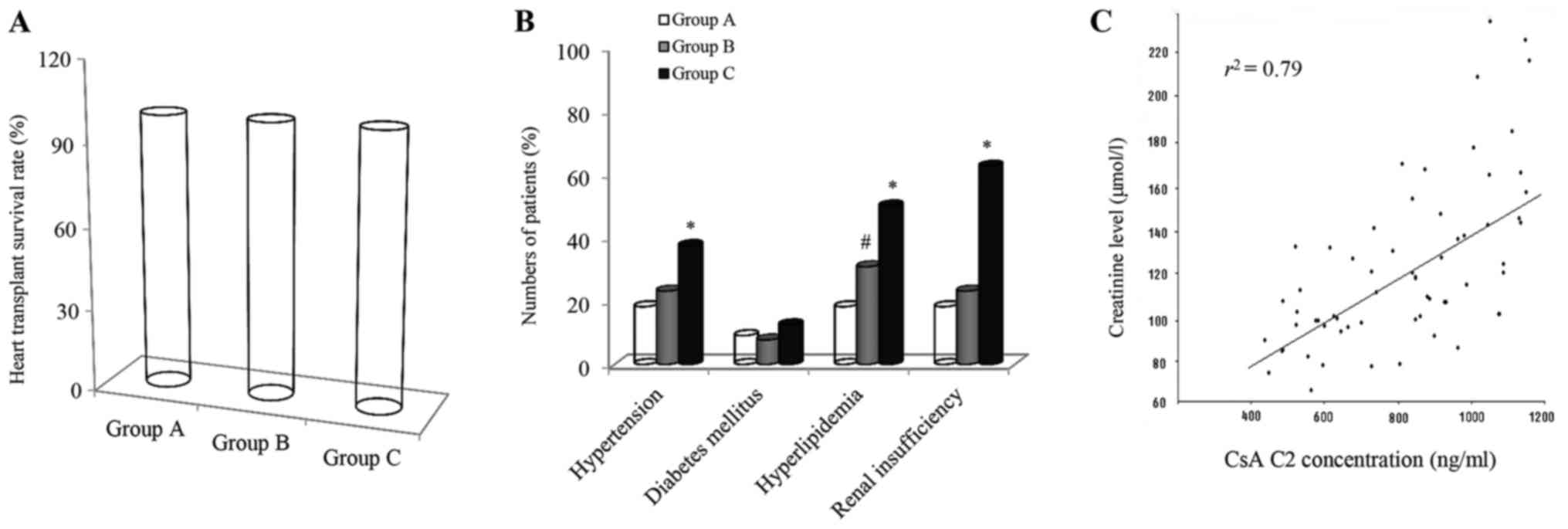
monitoring at C2 and the results are presented in Fig. 3. The efficacy of CsA as an
immunosuppressive therapy was observed to be excellent with a
survival rate of 100% for all patients within the first year after
HT (Fig. 3A). On further analysis by
cardiac pathologic examination, no acute cellular rejection was
detected in the transplanted cohort during the first six months
according to standardized cardiac biopsy grading. In the following
six months, immune responses for certain recipients with ISHLT
grades I–II were notably weakened and gradually recovered to normal
conditions in the CsA-treated recipients after increasing the MMF
dosage to 1.0 g twice a day. The recipients who received CsA
therapy had a survival rate of 100% in the present clinical
trial.
The most common adverse events in the CsA-treated
patients were observed by using the C2 monitoring approach.
Clinical manifestations of the side effects mainly included
hypertension, diabetes mellitus, hyperlipidemia and renal
insufficiency in the recipients (Fig.
3B). The frequencies of adverse events are expressed as a
percentage of affected patients in each recipient group. The
occurrence of hypertension, hyperlipidemia and renal insufficiency
was relatively common in the study cohort, affecting 18.2, 18.2 and
18.2%, in group A (n=11), 23.1, 30.8 and 23.1% in group B (n=13)
and 37.4, 50.0 and 62.5% in group C (n=8), respectively. Incidence
of diabetes mellitus was had a rate of 9.1% in group A, 7.7% in
group B and 12.5% in group C. Statistically significant differences
in the occurrence of hypertension, hyperlipidemia and renal
insufficiency between group C and either group A or B were
identified (P<0.01). In addition, there was a statistically
significant difference in incidence of hyperlipidemia between
groups A and B (P<0.01).
Blood creatinine is a fairly reliable indicator of
renal health. The dependency between the CsA levels at C2 and the
creatinine content at the same time-point was therefore assessed in
the cohort of HT recipients. The creatinine concentration (µmol/l)
in the blood at C2 was significantly enhanced with the CsA dose
increasing from 400 to 1,200 ng/ml (Fig.
3C). Furthermore, linear regression between the two variables
was clearly exhibited in the cohort (r2=0.79 and
P<0.05).
Discussion
The pharmacokinetic profile of CsA was first
determined in HT recipients after a single administration of the
immunosuppressant based on the fact that CsA has a relatively
narrow therapeutic window (18). The
results of the present study indicated that oral CsA was rapidly
taken up into the recipients' blood with a peak level detected at
~2 h after administration. The pharmacokinetic analysis of CsA
indicated that the plasma CsA concentration rapidly increased in
the transplant recipients, followed by a sharp drop in CsA levels.
The present results were consistent with those of previous studies
reporting that the time window for CsA therapeutic drug monitoring
is narrow (19,20). Given that plasma the CsA
concentration varied cross the serial sampling time-points with a
peak value of the CsA level observed at 2 h after administration,
it is indicated that C2 is an optimal sampling time-point, and is
superior to the other time-points for monitoring the efficacy of
CsA in inhibiting HT rejection.
To explore if a blood sample taken at 2 h after oral
administration of CsA is a better estimate of the AUC0–4
h than other time-points in HT recipients, the r2
value for the correlation of the AUC0–4 h with the
plasma CsA concentration at each sampling time-point from 0 to 12 h
was determined. The values of the Pearson's Correlation indicated
that at C2, the r2 value was markedly higher than those
at the other time-points, and this high correlation suggested that
the CsA target levels at this single time-point reflected the drug
exposure, therefore rendering it suitable for therapeutic
monitoring of the immunosuppressant levels. The utility of the
AUC0–12 h has been studied in transplant recipients
receiving CsA therapy (21,22); however, this monitoring approach is
not clinically feasible due to the added time, expense and
inconvenience for transplant recipients. The area under the curve
over 4 h after administration has been conceived for monitoring of
the plasma CsA concentration in patients receiving
immunosuppressive therapy (15,23). The
results of the present clinical trial demonstrated that the C2
levels exhibited a good correlation with the measured AUC0–4
h value, confirming that CsA monitoring at C2 not only
accurately reflected the drug absorption but the implementation of
this strategy also reduces cost and assessment time in the
long-term management of HT recipients.
While monitoring at C2 has been applied for HT
recipients in clinical practice (12,24),
efficacy of this type of monitoring remains controversial (25–27). To
verify the feasibility of the C2 strategy in therapeutic drug
monitoring, a regression analysis was employed to evaluate the
correlation between the CsA level at C2 and the AUC0–4
h, which was during the absorption phase of CsA in the
recipients. The present results indicated that the CsA levels were
elevated in parallel with the AUC0–4 h with increasing
doses of CsA administered, and at C2, the highest correlation was
obtained with an r2 value of 0.84. These results
indicated that monitoring at C2 may provide a suitable means of
determining subsequent dosing in clinical trials. Due to the high
correlation between the drug level at C2 and AUC0–4 h in
CsA-based immunosuppression, it led us to speculate that C2 may
serve as a useful tool for monitoring drug absorption in HT
recipients as well as providing information for individualized drug
dosing for each patient.
HT recipients who received CsA were grouped
according to their plasma CsA levels. The utility of CsA
measurement at C2 in achieving optimal dosing for the prophylaxis
of cardiac allograft rejection was examined in the grouped
recipients within one year of oral administration of CsA. The
results indicated that in the cohort of recipients, a graft
survival rate of 100% was achieved at the indicated time period (1
year) following HT, suggesting that C2 may be considered as a
suitable time-point to assess the efficacy of the immunosuppressant
in a broad concentration range for the inhibition of organ
rejection. Previous studies have reported that a high survival rate
of HT of >90% was achieved in the first year after surgery
(28–30), which is similar to the present
results. However, it is worth noting that this survival rate is
only for one year after HT, and that this time is critical to the
survival of the recipients. It has been previously reported that a
CsA blood concentration of 1,000–1,400 ng/ml achieved satisfactory
efficacy and safety over six months after HT (31). The present results indicated that
intervention with CsA at low plasma concentrations (600–800 ng/ml)
had a similar therapeutic effect to that achieved with higher doses
(800–1,200 ng/ml) in the patients monitored at C2, providing
evidence that managing the target drug concentration at a
relatively low level was necessary in the monitored recipients.
Additionally, the lower dosing of the immunosuppressant and less
frequent sampling reduced the overall cost during patient
treatment.
The incidence of adverse events, which mainly
included hypertension, diabetes, hyperlipidemia and renal
insufficiency, was examined in the grouped recipients since the
immunosuppressant has a narrow therapeutic range and only a small
difference between toxic and therapeutic concentrations in clinical
practice (19). With increasing CsA
levels, the occurrence rate of hypertension, hyperlipidemia and
renal impairment was significantly higher in the groups B and C
than that in group A, indicating a high incidence of adverse events
at higher doses of CsA. Consequently, the monitoring of CsA
concentration at C2 in clinical practice may serve as a sampling
strategy for re-adjusting the dosage of the immunosuppressant to
reach minimal toxicity in the recipients.
The principal adverse reaction to CsA therapy in
transplant recipients is renal dysfunction, so that kidney damage
caused by oral CsA requires medical attention (32,33).
Treatment with an increasing doses of CsA resulted in elevated
creatinine levels, which were correlated with the CsA level at C2
(r2=0.79). Since the peak level of CsA is associated
with nephrotoxicity, it may be concluded that monitoring at C2 is a
reliable approach for adequately observing the occurrence of
adverse events, and measurement of CsA at C2 may offer a strategy
for reducing the incidence of side effects through providing
information for adjusting CsA dosing.
Although previous studies have also reported similar
benefits of CsA monitoring at C2 (9,12,24), the
present study is of particular importance with regard to the
adjustment of the drug treatment regimen for HT recipients,
emphasizing that low plasma levels of CsA (400–600 ng/ml) combined
with prednisone and MMF achieved a highly satisfactory efficacy and
reduced the risk of adverse events during the maintenance treatment
of recipients with monitoring at C2. However, the small number of
recruited patients is a limitation of the present study.
Consequently, a study with a larger cohort is necessary in the
future.
In conclusion, C2 is the optimal single time-point
to measure CsA in HC recipients and is representative of the
AUC0–4 h. C2 monitoring may serve as a reliable
technique to optimize the efficacy and safety of CsA therapy by
allowing for adjustment of the individualized drug dosage for each
recipient in the long-term treatment with the immunosuppressant
CsA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All relevant data generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
YJ designed the experiments and drafted the
manuscript. XM, YL, CX, WZ and YJ produced the figures, tables and
references. WH provided technical assistance and advice. All
authors read and approved the final manuscript and each author
believes that the manuscript represents honest work.
Ethical approval and consent to
participate
The study was approved by the Ethics Committee of
Capital Medical University Affiliated Anzhen Hospital (Beijing,
China). The Ethics Committee also approved the treatments and
tissue collection from patients in accordance with the experimental
design and long-term clinical outcome assessment of CsA therapy.
The patients and their family members provided written informed
consent for inclusion in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CsA
|
cyclosporin A
|
|
r2
|
correlation coefficient
|
|
HT
|
heart transplantation
|
|
MMF
|
mycophenolate mofetil
|
|
EMB
|
endomyocardial biopsy
|
References
|
1
|
Burchill LJ: Heart transplantation in
adult congenital heart disease. Heart. 102:1871–1877. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Korewicki J: Cardiac transplantation is
still the method of choice in the treatment of patients with severe
heart failure. Cardiol J. 16:493–499. 2009.PubMed/NCBI
|
|
3
|
Patel JK, Kittleson M and Kobashigawa JA:
Cardiac allograft rejection. Surgeon. 9:160–167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patel JK and Kobashigawa JA: Improving
survival during heart transplantation: Diagnosis of
antibody-mediated rejection and techniques for the prevention of
graft injury. Future Cardiol. 8:623–635. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ippoliti G, Rinaldi M, Pellegrini C and
Viganò M: Incidence of cancer after immunosuppressive treatment for
heart transplantation. Crit Rev Oncol Hematol. 56:101–113. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Söderlund C and Rådegran G:
Immunosuppressive therapies after heart transplantation-the balance
between under- and over-immunosuppression. Transplant Rev
(Orlando). 29:181–189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Banner NR and Yacoub MH: Cyclosporine in
thoracic organ transplantation. Transplant Proc. 36 2
Suppl:302S–308S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haverich A and Gorler H: Experience with
cyclosporine: From revolution to evolution of immunosuppressive
protocols in thoracic organ transplantation. Transplant Proc. 36 2
Suppl:314S–317S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cantarovich M, Barkun J, Giannetti N,
Cecere R, Besner JG and Tchervenkov J: History of C2 monitoring in
heart and liver transplant patients treated with cyclosporine
microemulsion. Transplant Proc. 36 2 Suppl:442S–447S. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oellerich M and Armstrong VW: Two-hour
cyclosporine concentration determination: An appropriate tool to
monitor neoral therapy? Ther Drug Monit. 24:40–46. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Levy GA: C2 monitoring strategy for
optimising cyclosporin immunosuppression from the Neoral
formulation. BioDrugs. 15:279–290. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Delgado DH, Rao V, Hamel J, Miriuka S,
Cusimano RJ and Ross HJ: Monitoring of cyclosporine 2-hour
post-dose levels in heart transplantation: Improvement in clinical
outcomes. J Heart Lung Transplant. 24:1343–1346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Balram C, Sivathasan C, Cheung YB, Tan SB
and Tan YS: A limited sampling strategy for the estimation of
12-hour Neoral systemic drug exposure in heart transplant
recipients. J Heart Lung Transplant. 21:1016–1021. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scheff JD, Almon RR, Dubois DC, Jusko WJ
and Androulakis IP: Assessment of pharmacologic area under the
curve when baselines are variable. Pharm Res. 28:1081–1089. 2001.
View Article : Google Scholar
|
|
15
|
Banner NR, David OJ, Leaver N, Davis J,
Breen J, Johnston A and Yacoub MH: Pharmacokinetics of oral
cyclosporine (Neoral) in heart transplant recipients during the
immediate period after surgery. Transpl Int. 15:649–654. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schulz E, Jabs A, Gori T, Hink U, Sotiriou
E, Tschöpe C, Schultheiss HP, Münzel T and Wenzel P: Feasibility
and safety of left ventricular endomyocardial biopsy via
transradial access: Technique and initial experience. Catheter
Cardiovasc Interv. 86:761–765. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stewart S, Winters GL, Fishbein MC,
Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A,
Berry GJ, Burke MM, et al: Revision of the 1990 working formulation
for the standardization of nomenclature in the diagnosis of heart
rejection. J Heart Lung Transplant. 24:1710–1720. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morris RG: Cyclosporin therapeutic drug
monitoring-an established service revisited. Clin Biochem Rev.
24:33–46. 2003.PubMed/NCBI
|
|
19
|
Jorga A, Holt DW and Johnston A:
Therapeutic drug monitoring of cyclosporine. Transplant Proc. 36 2
Suppl:396S–403S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Midtvedt K: Therapeutic drug monitoring of
cyclosporine. Transplant Proc. 36 2 Suppl:430S–433S. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Frassetto LA, Tan-Tam CC, Barin B, Browne
M, Wolfe AR, Stock PG, Roland M and Benet LZ: Best single time
point correlations with AUC for cyclosporine and tacrolimus in
HIV-infected kidney and liver transplant recipients.
Transplantation. 97:702–707. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mardigyan V, Giannetti N, Cecere R, Besner
JG and Cantarovich M: Best single time points to predict the
area-under-the-curve in long-term heart transplant patients taking
mycophenolate mofetil in combination with cyclosporine or
tacrolimus. J Heart Lung Transplant. 24:1614–1618. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Monchaud C and Marquet P: Pharmacokinetic
optimization of immunosuppressive therapy in thoracic
transplantation: Part I. Clin Pharmacokinet. 48:419–462. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mathias HC, Ozalp F, Will MB, Borland W,
Payne C, Kerr M, Lockhart J and Murday A: A randomized, controlled
trial of C0- Vs C2-guided therapeutic drug monitoring of
cyclosporine in stable heart transplant patients. J Heart Lung
Transplant. 24:2137–2143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Knight SR and Morris PJ: The clinical
benefits of cyclosporine C2-level monitoring: A systematic review.
Transplantation. 83:1525–1535. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Narula AS, Murthy M, Patrulu K and Saxena
VK: Routine Cyclosporine concentration-C2 level monitoring. Is it
helpful during the early post transplant period? Med J Armed Forces
India. 60:326–328. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zakliczynski M, Krynicka A, Szewczyk M,
Wojarski J and Zembala M: Limited utility of cyclosporine C2
monitoring in heart transplant recipients receiving ketoconazole.
Transplant Proc. 35:2333–2334. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Blanche C, Blanche DA, Kearney B, Sandhu
M, Czer LS, Kamlot A, Hickey A and Trento A: Heart transplantation
in patients seventy years of age and older: A comparative analysis
of outcome. J Thorac Cardiovasc Surg. 121:532–541. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stehlik J, Edwards LB, Kucheryavaya AY,
Benden C, Christie JD, Dipchand AI, Dobbels F, Kirk R, Rahmel AO
and Hertz MI: International Society of Heart and Lung
Transplantation: The registry of the international society for
heart and lung transplantation: 29th official adult heart
transplant report-2012. J Heart Lung Transplant. 31:1052–1064.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Colvin-Adams M, Smith JM, Heubner BM,
Skeans MA, Edwards LB, Waller C, Snyder JJ, Israni AK and Kasiske
BL: OPTN/SRTR 2011 annual data report: Heart. Am J Transplant. 13
Suppl 1:S119–S148. 2013. View Article : Google Scholar
|
|
31
|
Wang SS, Chou NK, Chi NH, Huang SC, Wu IH,
Wang CH, Yu HY, Chen YS, Tsao CI, Ko WJ and Shun CT: Can
cyclosporine blood level be reduced to half after heart
transplantation? Transplant Proc. 42:930–933. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boffini M, Sansone F, Patanè F, Bonato R,
Ribezzo M, Iacovino C, Comoglio C and Rinaldi M: Does everolimus
associated with a low dose of cyclosporine in long-term cardiac
transplant recipients improve renal function? Initial experience.
Transplant Proc. 41:1349–1352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Damiano S, Ciarcia R, Montagnaro S,
Pagnini U, Garofano T, Capasso G, Florio S and Giordano A:
Prevention of nephrotoxicity induced by cyclosporine-A: Role of
antioxidants. J Cell Biochem. 116:364–369. 2015. View Article : Google Scholar : PubMed/NCBI
|