Introduction
Acute pancreatitis (AP) is a frequently occurring
acute abdominal condition with characteristics of acute onset and
rapid progression; however, its severity differs considerably among
affected patients. In the majority of cases, AP is classified as
mild, which is a self-limited disease. Only 10–20% of patients with
AP progress to severe AP (SAP) (1),
which is an acute, life-threatening condition with a case fatality
rate of ~20%. SAP may result in persistent organ failure (OF) with
local and/or systemic complications (2). AP may be classified into two types:
Acute interstitial edematous pancreatitis and acute necrotizing
pancreatitis (NP) (3). Furthermore,
the necrosis may be classified as aseptic or infective. For
instance, secondary infection of pancreatic or peri-pancreatic
tissue in advanced NP may induce infectious pancreatic necrosis
(IPN). The major causes of secondary infection of NP include
bacterial translocation, biliary-system source and hematogenous
dissemination. The infection is closely correlated with the degree
of PN (4).
AP has numerous causes, with the major ones being
excessive alcohol consumption and intrabiliary calculi. Certain
studies suggested that hyperlipidemia is another major cause of AP,
and that the prevalence of hyperlipidemic AP (HLAP) has increased
(5). Furthermore, HLAP is generally
considered to have no correlation with elevated blood cholesterol
levels, but to be closely associated with elevated blood
triglycerides (TG). Compared with biliary pancreatitis and
alcoholic pancreatitis, HLAP is more dangerous, with a larger
amount of associated complications and a higher mortality rate
(6); in addition, HLAP cases more
easily and rapidly progress to NP and OF (7).
Numerous studies have investigated the differences
in clinical characteristics between HLAP and non-HLAP. However, to
date, only few studies have assessed the early risk factors of
HLAP. In the present study, data from patients with HLAP obtained
within 48 h of admission were analyzed in order to characterize the
early risk factors of HLAP and provide novel approaches for its
prevention and treatment.
Materials and methods
Case information
The complete case data for a total of 111 patients
with HLAP, who were admitted to Ordos Central Hospital (Ordos,
China) between January 2015 and October 2017, were retrospectively
analyzed. The present study was approved by the Ethics Committee of
Ordos Central Hospital (Ordos, China) and all patients provided
written informed consent.
The inclusion criteria were as follows: i) Patients
who meet the diagnostic criteria for AP. If the patient satisfied
two of the following three criteria, they were considered to have
AP: Abdominal pain; serum amylase and (or) lipase concentration ≥3
times higher than the normal value; and abdominal imaging
examination in line with imaging changes typical for AP (8). ii) Patients who meet the criteria for
hyperlipidemia: Serum TG levels of ≥1,000 mg/dl or TG levels
between 500 and 1,000 mg/dl, accompanied by lactescent serum in the
absence of other causes of pancreatitis, including gallstone
disease, alcoholism or trauma (9–11). iii)
Patients who underwent abdominal enhanced computed tomography (CT)
imaging within 48 h of admission.
The exclusion criteria were traumatic, biliary,
alcoholic, medical, self-limited, pregnant and tumorous
pancreatitis.
Data collection
The clinical characteristics of the subjects,
including age, sex, body mass index (BMI) and history of diabetes
were recorded. Within 48 h of admission, the following laboratory
parameters were determined: Hematocrit, albumin, glucose, calcium
ions, urea nitrogen, C-reactive protein (CRP), white blood cell
(WBC) count, procalcitonin (PTC), fibrinogen (FIB) and red cell
distribution width (RDW). Enhanced CT was performed to determine
the necrotic tissue extent and the fluid locus. The modified CT
severity index (MCTSI) was also determined within 48 h of onset
(12). Abdominal enhanced CT was
used to diagnose AP and determine the volume ratio between necrotic
and non-necrotic pancreatic tissues, as well as peri-pancreatic
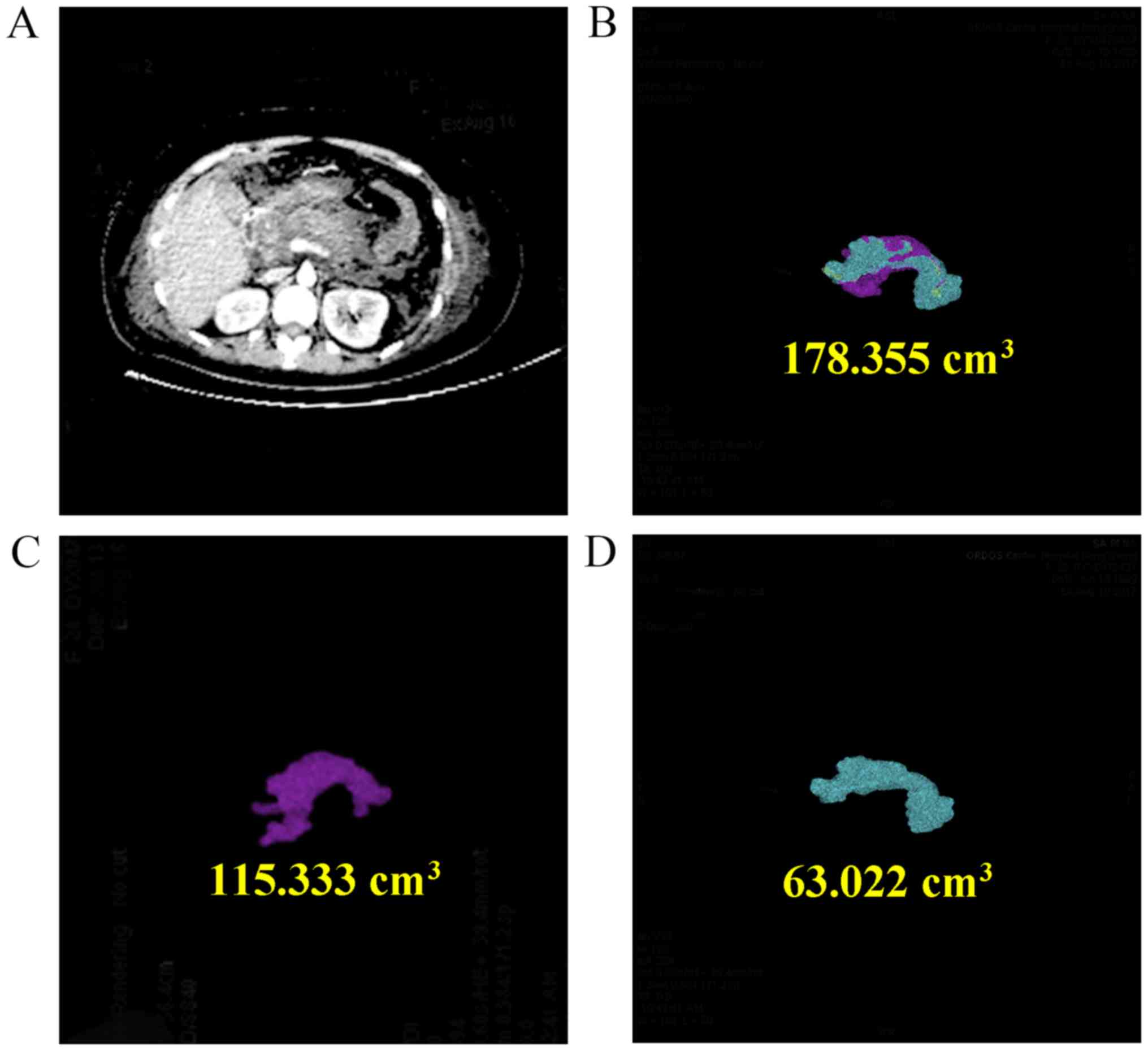
effusion (13). A roentgenologist
and a surgeon co-analyzed 3-dimensional reconstructions of
abdominally-enhanced CT images of necrotic and non-necrotic
pancreatic tissues to determine the volume of necrotic and
non-necrotic pancreatic tissues as exemplified in Fig. 1.
Treatment
Initially, all enrolled patients received targeted
lipidemia-lowering and general therapy, including fasting,
gastrointestinal decompression, fluid resuscitation, nutritional
therapy, organ function maintenance, preventive usage of
antibiotics against gram-negative bacilli and Traditional
Chinese Medicine approaches, including Radix Bupleuri, Radix
Paeoniae Alba and Radix et Rhizoma Rhei, in order to restore
gastrointestinal tract dynamics and treat the pancreatitis.
Study outcomes
The outcome of the study was the progression of HLAP
to IPN or OF at discharge; patients were not involved in a
subsequent follow-up. Pancreatic and peri-PN tissues may remain
uninfected or become infected; most of the studies available
suggest no correlation between the extent of necrosis and the risk
of infection and symptom duration (14,15). The
presence of IPN may be presumed when extraluminal gas is visible in
pancreatic and/or peri-pancreatic tissues on CECT, or when
percutaneous, image-guided, fine-needle aspiration is positive for
bacteria and/or fungi on Gram stain and culture. Three organ
systems should be assessed to determine OF: The respiratory,
cardiovascular and renal systems. OF is defined by a score of ≥2
for one of these three organ systems using the modified Marshall
scoring system, which has the merit of simplicity, universal
applicability and the ability to easily and objectively determine
disease severity (8).
Statistical analysis
SPSS v.20.0 software (IBM Corp., Armonk, NY, USA)
was used for statistical analysis. After stratification of the
patients into INP and non-INP, or OF and non-OF groups, inter-group
differences in measurement data were analyzed using a two
independent samples t-test and Mann-Whitney U test, while
differences in enumeration data were analyzed using a chi-square
test. In order to identify early risk factors of HLAP, the data
initially underwent univariate logistic regression analysis to
obtain odds ratios (OR) and 95% confidence intervals (CIs), and
then the parameters that were significantly associated with
progression of HLAP to INP or OF were further subjected to a
multivariate logistic regression analysis. P<0.05 was considered
to indicate a statistically significant difference. The area under
the receiver operating characteristic (ROC) curve (AUC) was
determined to evaluate the performance of the predictive model. The
AUC ranged from 0–1, and a variable with an AUC of >0.7 was
considered useful, while an AUC between 0.8 and 0.9 was considered
to indicate excellent diagnostic accuracy.
Results
Clinicopathological characteristics
associated with the progression of HLAP
Of the 111 patients with HLAP that were enrolled in
the present study, 17 (15.3%) patients progressed to IPN and 14
(12.6%) progressed to OF at the time of discharged. Between the IPN
and non-IPN groups, no significant differences in sex, age, BMI and
diabetes history were present (P>0.05). Furthermore, differences
in calcium ions, CRP, necrotic tissue extent, PTC and MCTSI were
statistically significant (P<0.05). However, differences in
hematocrit, albumin, blood sugar, urea nitrogen, white blood cell
count, fibrinogen, RDW and effusion focus were not statistically
significant (P>0.05; Table
I).
 | Table I.Univariate regression analysis of the
comparison of patients with and without IPN, and of patients with
and without OF. |
Table I.
Univariate regression analysis of the
comparison of patients with and without IPN, and of patients with
and without OF.
| Characteristics | IPN | non-IPN | P-value | OR (95% CI) | OF | non-OF | P-value | OR (95% CI) |
|---|
| Number of
patients | 17 | 94 |
|
| 14 | 97 |
|
|
| Age (years) | 42±11.3 | 40±10.3 | 0.969 | 1.824
(−3.638–7.287) | 43.2±8.6 | 40.1±10.6 | 0.498 | 3.172
(−2.735–9.079) |
| Sex
(male/female) | 11/6 | 79/15 | 0.061 | 0.262
(0.062–0.463) | 10/4 | 80/17 | 0.324 | −8.451
(−45.571–28.668) |
| History of
diabetes | 3 (17.6%) | 23 (23.4%) | 0.188 | −0.068
(−0.291–0.154) | 3 (21.4%) | 23 (23.7%) | 0.700 | −0.022
(−0.264–0.219) |
| Body mass index
(kg/m2) | 29±5.4 | 28±4.2 | 0.742 | 1.543
(0.348–9.661) | 29±3.6 | 27±4.9 | 0.823 | 2.672
(−1.622–6.841) |
| Hematocrit (%) | 45.9±3.98 | 36.3±4.39 | 0.876 | −0.445
(−2.713–1.821) | 44.9±4.46 | 46.5±4.28 | 0.637 | −1.537
(−3.981–0.905) |
| Albumin (g/l) | 40.7±6.28 | 43.8±5.72 | 0.477 | −3.105
(−6.144–0.657) | 43.1±6.28 | 43.3±5.87 | 0.742 | −0.217
(−3.575–3.141) |
| Glucose (mmol/l) | 9.6±3.14 | 9.3±2.86 | 0.732 | −2.663
(−13.231–7.904) | 9.01±3.78 | 8.7±2.25 | 0.701 | −1.320
(−3.760–1.119) |
| Calcium ion
(mmol/l) | 1.6±0.42 | 2.2±0.24 | 0.006 | −0.591
(−0.761–0.421) | 1.5±0.23 | 2.0±0.39 | 0.004 | −0.684 (−0.870 -
−0.499) |
| BUN (mmol/l) | 61.8±10.18 | 77.6±10.58 | 0.268 | −15.808
(−49.928–18.310) | 67.8±12.13 | 76.3±29.66 | 0.342 | −8.451
(−45.571–28.668) |
| CRP (mg/l) | 119.9±44.73 | 71.3±21.48 | <0.001 | 38.528
(20.330–56.725) | 99.7±26.38 | 76.5±29.49 | 0.405 | 6.227
(−15.014–27.469) |
| WBC
(109/l) | 15.4±5.87 | 13.7±7.00 | 0.943 | 1.671
(−1.916–5.259) | 16.5±6.24 | 13.5±6.88 | 0.966 | 2.816
(−1.051–6.884) |
| PTC (ng/ml) | 2.1±0.80 | 1.4±0.79 | 0.023 | 0.017
(−0.399–0.434) | 1.5±0.75 | 1.2±0.78 | 0.988 | 0.501
(0.059–0.943) |
| Fibrinogen
(g/l) | 3.6±1.71 | 3.7±1.57 | 0.721 | −0.093
(−0.928–0.741) | 4.0±2.09 | 3.7±1.51 | 0.111 | 0.279
(−0.625–1.183) |
| RDW (%) | 13.3±0.58 | 13.0±0.54 | 0.101 | 0.284
(−1.465–3.426) | 13.6±0.41 | 12.9±0.66 | 0.003 | 0.943
(−0.148–2.465) |
| Extent of
necrosis |
|
<30% | 12 (12.5%) | 84 (87.5%) | 0.037 | 0.286
(0.083–0.980) | 8 (8.5%) | 86 (91.5%) | 0.002 | 0.171
(0.050–0.584) |
| ≥
30% | 5 (33.3%) | 10 (66.7%) |
|
| 6 (35.3) | 11 (64.7%) |
|
|
| Number of fluid
collections |
| 1 | 14 (15.6%) | 76 (84.4%) | 0.884 | 1.105
(0.287–4.258) | 12 (15.4%) | 66 (84.6%) | 0.176 | 2.818
(0.584–13.366) |
| ≥2 | 3 (14.3%) | 18 (85.7%) |
|
| 2 (6.1%) | 31 (93.9%) |
|
|
| MCTSI | 6.5±1.34 | 4.7±1.19 | 0.020 | 1.257
(0.384–2.311) | 6.2±1.67 | 4.8±0.98 | 0.025 | 2.975
(0.521–3.643) |
Between the OF and non-OF groups, no significant
differences in sex, age, BMI and diabetes history were determined
(P>0.05). Furthermore, differences in calcium ions, RDW,
necrotic tissue extent and MCTSI were statistically significant
(P<0.05). However, differences in hematocrit, albumin, blood
glucose, urea nitrogen, white blood cell count, procalcitonin,
fibrinogen, CRP and effusion focus were not statistically
significant (P>0.05; Table
I).
Multivariate logistic regression analysis indicated
that CRP was significantly and independently associated with IPN
(P=0.014). RDW (P=0.025) and the extent of necrosis (P=0.022) were
significant independent factors associated with the progression to
OF (Table II).
 | Table II.Multivariate regression analysis of
variables independently associated with progression to IPN and
OF. |
Table II.
Multivariate regression analysis of
variables independently associated with progression to IPN and
OF.
|
Event/variables | OR (95% CI) | P-value |
|---|
| IPN |
|
|
|
CRP | 0.961
(0.933–0.991) | 0.014 |
| OF |
|
|
|
RDW | 1.225
(1.051–1.648) | 0.025 |
| Extent
of necrosis (≥30%) | 2.410
(1.210–3.612) | 0.022 |
CRP, RDW and extent of necrosis are
independent prognostic factors for the progression of HLAP
The prognostic value of CRP regarding the
progression to IPN, and that of RDW and the extent of necrosis
regarding the progression to OF, was then evaluated by ROC curve
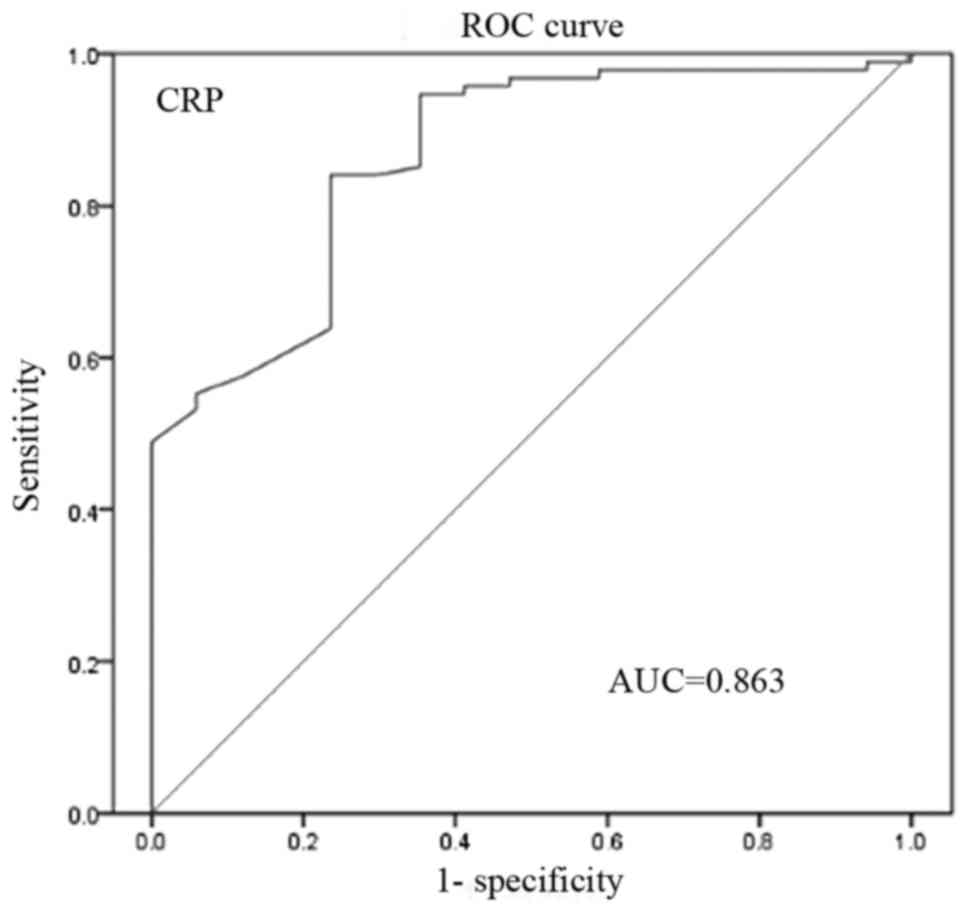
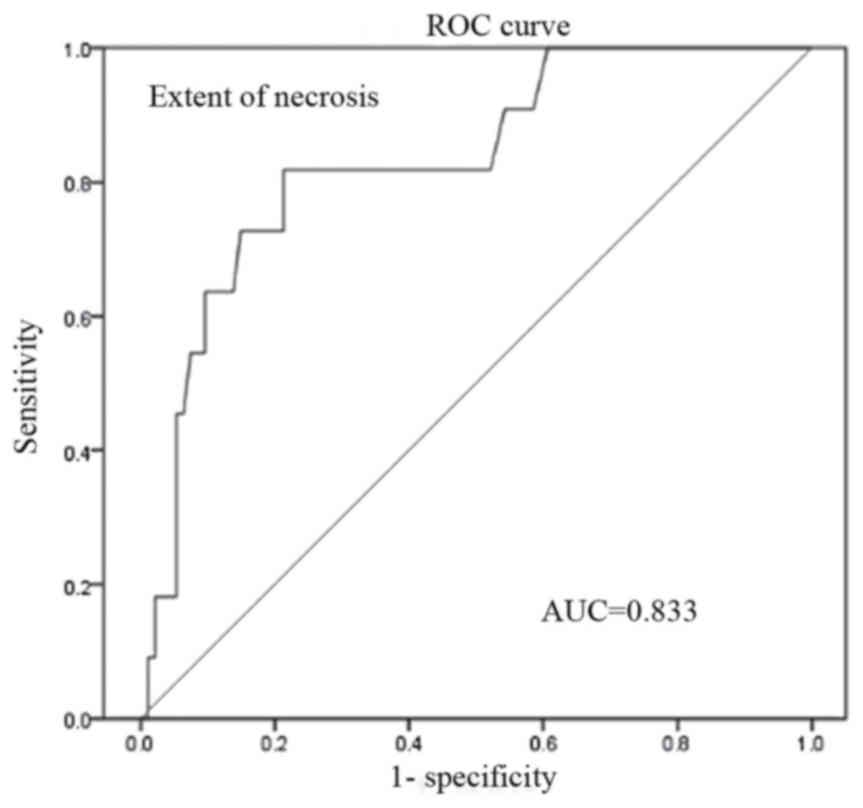
analysis (Figs. 2–4, respectively). The AUC for CRP, RDW and
the extent of necrosis was 0.863 (95% CI, 0.646–0.886), 0.727 (95%
CI, 0.651–0.803) and 0.833 (95% CI, 0.739–0.936), respectively. The
optimal cut-off value of CRP to predict IPN was 77.2 mg/l.
Additionally, the optimal cut-off value of RDW and the extent of
necrosis to predict OF was 13,7 and 18.7%, respectively.
Discussion
HLAP is the third most frequent cause of AP.
Multicenter, retrospective studies have revealed that the
prevalence of HLAP has increased over the past 20 years (16). Other studies have revealed that
increasing TG is a key factor in inducing HLAP, while obesity,
fatty liver, male sex and concomitant diabetes are also important
factors in inducing HLAP (17–19). In
the present study, 90 patients were male, aged <45 years and had
a relatively high BMI. HLAP is more common in younger males; this
may be associated with unhealthy, high-lipid diets, overeating and
excessive alcohol consumption. Similarly, transient increases in TG
levels in the plasma are associated with the competitive
simultaneous oxidation of ethanol and free fatty acids (FFA) in
liver tissues (20).
CRP is a non-specific marker for tissue lesions and
inflammation, and is an acute-phase reaction (APR) protein that
activates the complement system, improves phagocytosis and adjusts
immunity. When the body is damaged, an APR occurs and CRP levels
increase. AP is an acute inflammation caused by pancreatitis and
induces the autodigestion of pancreatic tissues. Among patients
with severe AP, particularly in cases of PN complicated with
bacterial infection, CRP may rapidly increase. CRP levels reflect
the extent of inflammation in AP and may predict important indexes
of disease severity, complications and mortality. Detection of CRP
has the advantages of low cost, simplicity, availability, accuracy
and reliability. Furthermore, CRP determined within 48 h of
presentation is more accurate in predicting SAP and PN, with a
higher sensitivity and specificity compared with CRP determined at
other time-points (21). Khanna
et al (22) revealed that CRP
had the highest sensitivity (100%) and specificity (81.4%) for the
prediction of PN, while it had a sensitivity of 86.2% and a
specificity of 100% for the prediction of SAP. The AUC for CRP for
the prediction of PN was higher at 0.90 (95% CI, 0.82–0.77)
compared with the AUC for the multiple organ system score, the
acute physiology and chronic health evaluation II score, systemic
inflammatory response syndrome, the bedside index for severe acute
pancreatitis, interleukin (IL)-6 expression and the CT severity
index. Yin et al (23)
revealed that the for the accurate prediction of severity, the
cutoff value for CRP in HLAP was required to be higher than that in
non-HLAP. Furthermore, the serum CRP concentration in patients with
HLAP, mild AP, moderately severe AP and SAP was notably higher
within four days of disease onset. In the present study, univariate
analysis of early risk factors for HLAP progression indicated that
calcium ions, CRP, extent of necrotic tissue and MCTSI were
significant predictors of IPN. Multivariate analysis then
determined that CRP is an independent predictive factor for
progression of HLAP to IPN (OR, 0.961; 95% CI, 0.933–0.991;
P=0.014).
The diagnostic criteria for OF are in line with the
improved Marshall scoring system, which mainly involves the
evaluation of respiratory, circulation and kidney function of
patients with pancreatitis. In the present study, the clinical
partial oxygen pressure/fraction of inspired oxygen index,
contractive pressure and serum creatinine index were assessed for
this. A score of ≥2 for any organ is regarded as indicative of OF
(8). Studies have revealed that HLAP
more easily progresses to OF through excessive TG levels causing
the release of large quantities of FFA in the process of
pancrelipase hydrolysis (24).
Furthermore, the FFA form an acidic environment in the pancreas,
and a number of cytokines and inflammatory mediators are activated
and released, thereby leading to systemic inflammatory response
syndrome (SIRS), which may in turn lead to multiple organ
dysfunction syndrome.
The clinical presentation of early OF due to AP
includes damage to the respiratory, circulatory and renal systems.
If this is reliably predicted, targeted and appropriate treatments
may be immediately applied and the duration and severity of OF may
be decreased. Searching for markers whose assessment is simple,
economic and non-invasive, and which have good sensitivity and
specificity, is important for predicting OF in AP.
Several parameters, including the concentration of
apolipoprotein (APO)A-I, high-density lipoprotein-cholesterol and
combinations of APOA-I and scoring systems (25), lactate dehydrogenase (26) and calcium (27), have been used for predicting
persistent OF in AP. Peng et al (28) concluded that RDW is independently
associated with persistent OF in AP and may serve as an early
predictor. The predictive value of RDW was superior to that of SIRS
and glucose levels. Another study indicated that RDW is a potential
novel and sensitive predictor of mortality in patients with AP
(29). The AUC for RDW was 0.894
(95% CI, 0.823–0.966) and the optimal cut-off value to predict
mortality was 14.35 (sensitivity, 88.2%; specificity, 91.8%).
RDW, the assessment of which is part of routine
blood tests, is used as a parameter to quantify the extent of
erythrocyte anisocytosis. A meta-analysis has determined that RDW
is an independent prognostic marker for determining the risk of
mortality in numerous pathophysiological conditions, including
cardiovascular diseases and cancer (30). The univariate logistic regression
analysis regarding the early prediction of HLAP progression to OF
performed in the present study revealed that calcium ions, oxygen
partial pressure, extent of necrosis and MCTSI scores are
significant risk factors. Multivariate analysis then indicated that
RDW is an early independent predictor of OF in HLAP (P=0.025).
Meyrignac et al (31) investigated the aptness of the
extra-PN volume for early prediction of AP severity. The AUC for
extra-PN in the prediction of OF was 0.94 (95% CI, 0.90–0.97),
which was significantly higher than that of the Balthazar score
(AUC, 0.83; 95% CI, 0.76–0.88), the CTSI (AUC, 0.84; 95% CI,
0.78–0.89) and CRP levels (AUC, 0.78; 95% CI, 0.72–0.84). With a
cutoff value of 100 ml, extra-PN had a sensitivity of 95% (19/20)
and a specificity of 83% (142/172) in the prediction of OF. Mentula
et al (32) demonstrated that
IL-10, high blood sugar and serum calcium are independent
predictive factors for the progression of AP to OF. Furthermore,
calcium levels were identified to be associated with the clinical
onset of OF. The combined predictive value of IL-10 (>50 µg/ml)
and calcium (<1.65 mmol/l) was greater than that of any single
factor or of the APACHE II score, with a sensitivity of 88%, a
specificity of 93% and an adjusted OR of 94. In conclusion, the
extent of necrosis is another independent prognostic/predictive
factor of the progression of HLAP to OF, while serum calcium is
also closely associated with OF in HLAP. However, calcium ions are
not an independent predictive factor, as indicated by multivariate
logistic regression analysis.
The aim of the present study was to investigate the
early risk factors of HLAP progression in order to facilitate
intervention/prevention, as well as to open up novel avenues for
its clinical treatment. However, due to its retrospective nature,
the present study only included a limited number of subjects with
correlative factors. Further studies with larger sample sizes and
multicenter studies are therefore required to verify the present
results.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC and ZS designed the study. HW, XY, HD, EC, SW and
YD collected the data. XC analyzed the data and drafted the
manuscript. All authors have read and approved the final
manuscript.
Ethical approval and consent to
participate
The present study was approved by the ethical review
committee of Ordos Central Hospital (Ordos, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Al Mofleh IA: Severe acute pancreatitis:
Pathogenetic aspects and prognostic factors. World J Gastroenteml.
14:675–684. 2008. View Article : Google Scholar
|
|
2
|
Zerem E, Imamovic G, Omerović S and
Imširović B: Randomized controlled trial on sterile fluid
collections management in acute pancreatitis: Should they be
removed? Surg Erldosc. 23:2770–2777. 2009. View Article : Google Scholar
|
|
3
|
Bollen TL: Imaging of acute pancreatitis:
Update of the revised Atlanta classification. Radiol Clin North Am.
50:429–445. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beger HG and Rau BM: Severe acute
pancreatitis: Clinical course and management. World J
Gastroenterol. 13:5043–5051. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yadav D and Lowenfels AB: The epidemiology
of pancreatitis and pancreatic cancer. Gastroenterology.
144:1252–1261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rashid N, Sharma PP, Scott RD, Lin KJ and
Toth PP: All-cause and acute pancreatitis health care costs in
patients with severe hypertriglyceridemia. Pancreas. 46:57–63.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qiu L, Sun RQ, Jia RR, Ma XY, Cheng L,
Tang MC and Zhao Y: Comparison of existing clinical scoring systems
in predicting severity and prognoses of hyperlipidemic acute
pancreatitis in Chinese patients: A retrospective study. Medicine
(Baltimore). 94:e9572015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Banks PA, Bollen TL, Dervenis C, Gooszen
HG, Johnson CD, Sarr MG, Tsiotos GG and Vege SS: AcutePancreatitis
Classification Working Group: Classification of acute
pancreatitis-2012: Revision of the Atlanta classification and
definitions by international consensus. Gut. 62:102–111. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cameron JL, Crisler C, Margolis S,
DeMeester TR and Zuidema GD: Acute pancreatitis with hyperlipemia.
Surgery. 70:53–61. 1971.PubMed/NCBI
|
|
10
|
Fortson MR, Freedman SN and Webster PD
III: Clinical assessment of hyperlipidemic pancreatitis. Am J
Gastroenterol. 90:2134–2139. 1995.PubMed/NCBI
|
|
11
|
Yadav D and Pitchumoni CS: Issues in
hyperlipidemic pancreatitis. J Clin Gastroenterol. 36:54–62. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rehan A, Shabbir Z, Shaukat A and Riaz O:
Diagnostic accuracy of modified CT severity index in assessing
severity of acute pancreatitis. J Coll Physicians Surg Pak.
26:967–970. 2016.PubMed/NCBI
|
|
13
|
Cao X, Cao F, Li A, Gao X, Wang XH, Liu
DG, Fang Y, Guo DH and Li F: Predictive factors of pancreatic
necrosectomy following percutaneous catheter drainage as a primary
treatment of patients with infected necrotizing pancreatitis. Exp
Ther Med. 14:4397–4404. 2017.PubMed/NCBI
|
|
14
|
Besselink M, Van-Santvoort HM, Boermeester
MA, Nieuwenhuijs VB, van Goor H, Dejong CH, Schaapherder AF and
Gooszen HG: Dutch Acute Pancreatitis Study Group: Timing and impact
of infections in acute pancreatitis. Br J Surg. 96:267–273. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van Santvoort HC, Bakker OJ, Bollen TL,
Besselink MG, Ali Ahmed U, Schrijver AM, Boermeester MA, van Goor
H, Dejong CH, van Eijck CH, et al: A conservative and minimally
invasive approach to necrotizing pancreatitis improves outcome.
Gastroenterology. 141:1254–1263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang YX, Jia L, Jiang SM, Wang SB, Li MX
and Yang BH: Incidence and clinical features of hyperlipidemic
acute pancreatitis from Guangdong, China: A retrospective
multicenter study. Pancreas. 43:548–552. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang YT, Chang MC, Tung CC, Wei SC and
Wong JM: Distinctive roles of unsaturated and saturated fatty acids
in hyperlipidemic pancreatitis. World J Gastroenterol.
21:9534–9543. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nair S, Yadav D and Pitchumoni CS:
Association of diabetic ketoacidosis and acute pancreatitis:
Observations in 100 consecutive episodes of DKA. Am J
Gastroenterol. 95:2795–2800. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morita Y, Yoshikawa T, Takeda S, Matsuyama
K, Takahashi S, Yoshida N, Clemens MG and Kondo M: Involvement of
lipid peroxidation in free fatty acid-induced isolated rat
pancreatic acinar cell injury. Pancreas. 17:383–389. 1999.
View Article : Google Scholar
|
|
20
|
Saharia P, Margolis S, Zuidema GD and
Cameron JL: Acute pancreatitis with hyperlipemia: Studies with an
isolated perfused canine pancreas. Surgery. 82:60–67.
1977.PubMed/NCBI
|
|
21
|
Cardoso FS, Ricardo LB, Oliveira AM,
Canena JM, Horta DV, Papoila AL and Deus JR: C-reactive protein
prognostic accuracy in acute pancreatitis: Timing of measurement
and cutoff points. Eur J Gastroenterol Hepatol. 25:784–789. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khanna AK, Meher S, Prakash S, Tiwary SK,
Singh U, Srivastava A and Dixit VK: Comparison of Ranson, Glasgow,
MOSS, SIRS, BISAP, APACHE-II, CTSI scores, IL-6, CRP, and
procalcitonin in predicting severity, organ failure, pancreatic
necrosis, and mortality in acute pancreatitis. HPB Surg.
2013:3675812013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin G, Hu G, Cang X, Yu G, Hu Y, Xing M,
Chen C, Huang Y, Tang M, Zhao Y, et al: C-reactive protein:
Rethinking its role in evaluating the severity of hyperlipidemic
acute pancreatitis. Pancreas. 43:1323–1328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsuang W, Navaneethan U, Ruiz L, Palascak
JB and Gelrud A: Hypertriglyceridemic pancreatitis: Presentation
and management. Am J Gastroenterol. 104:984–991. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou CL, Zhang CH, Zhao XY, Chen SH, Liang
HJ, Hu CL and Chen NW: Early prediction of persistent organ failure
by serum apolipoprotein A-I and high-density lipoprotein
cholesterol in patients with acute pancreatitis. Clin Chim Acta.
476:139–145. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cui J, Xiong J, Zhang Y, Peng T, Huang M,
Lin Y, Guo Y, Wu H and Wang C: Serum lactate dehydrogenase is
predictive of persistent organ failure in acute pancreatitis. J
Crit Care. 41:161–165. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peng T, Peng X, Huang M, Cui J, Zhang Y,
Wu H and Wang C: Serum calcium as an indicator of persistent organ
failure in acute pancreatitis. Am J Emerg Med. 35:978–982. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peng T, Zhang Y, Wu H and Wang C:
Assessment of red blood cell distribution width as an early
predictor of persistent organ failure in patients with acute
pancreatitis. Pancreas. 18:393–398. 2017.
|
|
29
|
Wang D, Yang J, Zhang J, Zhang S, Wang B,
Wang R and Liu M: Red cell distribution width predicts deaths in
patients with acute pancreatitis. J Res Med Sci. 20:424–428. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Patel KV, Semba RD, Ferrucci L, Newman AB,
Fried LP, Wallace RB, Bandinelli S, Phillips CS, Yu B, Connelly S,
et al: Red cell distribution width and mortality in older adults: A
meta-analysis. J Gerontol A Biol Sci Med Sci. 65:258–265. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Meyrignac O, Lagarde S, Bournet B, Mokrane
FZ, Buscail L, Rousseau H and Otal P: Acute pancreatitis:
Extrapancreatic necrosis volume as early predictor of severity.
Radiology. 276:119–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mentula P, Kylänpää ML, Kemppainen E,
Jansson SE, Sarna S, Puolakkainen P, Haapiainen R and Repo H: Early
prediction of organ failure by combined markers in patients with
acute pancreatitis. Br J Surg. 92:68–75. 2010. View Article : Google Scholar
|