Introduction
Dental fluorosis is an enamel defect caused by the
excessive intake of fluoride during enamel formation. In deciduous
teeth, it occurs during the embryonic phase, while in permanent
teeth, it occurs primarily in children aged 2–8 years old (1–4). The
primary sources of fluoride intake are from food and water, as well
as from toothpaste, which contains added fluoride. Fluoride is also
being added to different materials, including fluoride varnish,
fluoride foam and dental resin to prevent the occurrence of dental
caries. All these methods increase the morbidity of dental
fluorosis (1). The incidence of
dental fluorosis is currently a problem worldwide, although the
prevalence of dental fluorosis varies in different countries. In
the USA, ~25% of the population have dental fluorosis and its
incidence is also high in China, ~11.7% of adolescents of 12 years
old suffer dental fluorosis (2,4).
According to the Dean's index, dental fluorosis may be classified
into five types: Questionable, very mild, mild, moderate and severe
(5). In clinical practice, dental
fluorosis may be classified into three types: Chalk, discoloration
and defective (6).
Enamel formation by ameloblasts is a complex
process. The primary elements of enamel hydroxyapatite are present
in the crystalline form (7,8). It has been demonstrated that 8–14
H+ are released in the extracellular environment as the
minimum repeating structure of hydroxyapatite crystals are formed,
thus lowering the pH (7–9). To maintain the pH balance, ameloblasts
must buffer the protons. During the secretory stage, amelogenins
may serve an important role in buffering the pH (9,10);
however, during the maturation stage, ameloblasts secrete
bicarbonate into the enamel matrix to neutralize the
microenvironment (7,9–11). The
process of enamel formation requires strict control of
extracellular pH (7–9,11);
hydroxyapatite crystal growth and proteinase activity in the
extracellular space are pH-dependent phenomena (7,8,12). pH therefore serves an important role
during amelogenesis, which is the basis of enamel formation.
The current review discusses the regulation of pH
during amelogenesis in dental fluorosis and also explores the
effects of changes in ion transporters on dental fluorosis. This
may identify future directions of research to identify potential
novel treatments of dental fluorosis.
Mechanism of dental fluorosis
There are multiple potential causes of dental
fluorosis; however, the precise mechanism by which dental fluorosis
occurs remain controversial (13–15).
Current investigations into these mechanisms primarily focus on the
direct effects on the ameloblasts and the indirect effects on the
forming matrix (16). Fluoride has
three distinct effects on ameloblasts. Firstly, it has two
contrasting effects on cell proliferation. Micromolar F−
promotes the proliferation of ameloblasts, while millimolar
F− inhibits their proliferation, suggesting that higher
levels of fluoride may inhibit the proliferation of ameloblasts
(17,18). Secondly, the differentiation of
ameloblasts may be regulated by F− via the
mitogen-activated protein kinase pathway (19). Thirdly, the apoptosis of ameloblasts
increases during oxidative stress if F− levels are high
(20). Yang et al (21) demonstrated that high levels of
fluoride induce the apoptosis of ameloblasts by downregulating
Bcl-2. In terms of indirect fluoride-associated effects, fluoride
may interfere with the synthesis, secretion and intracellular
transportation of enamel matrix proteins in ameloblasts (18,22). The
retention of matrix proteins is not only the result of the
decreased activity of proteases, including matrix
metalloproteinase-20 (MMP-20) (23)
and Kallikrein 4 (KLK4) (24), but
is also the result of more effective binding to fluoridated apatite
(1,13). Furthermore, high levels of fluoride
may induce changes the structure and function of amelogenin, which
serves an important role in buffering pH, thereby contributing to
the disruption of pH regulation (25). pH is important in the mechanisms
mentioned above and pH may directly or indirectly regulatet these
mechanisms. Duan et al (26)
identified that residual protein (emdogain) exists in the enamel of
patients with cystic fibrosis and dental fluorosis. This was
determined to be a consequence of disordered pH levels, which lead
to abnormal proteolytic activity and defective endocytosis. Thus,
pH serves an important role during the entire process of
amelogenesis. It is therefore important to determine how pH is
regulated during amelogenesis when intake of fluoride remains high
for a prolonged period of time.
Amelogenesis and the regulation of pH
There are five stages of amelogenesis, which
include: The pre-ameloblast stage, the pre-secretory stage, the
secretory stage, the transition stage and the maturation stage
(25,27). Of these five stages, two are the most
important: The secretory and the maturation stages (4,28).
During the secretory stage, ameloblasts secrete a number of
proteins, including amelogenin, ameloblastin, enamelin and MMP20
(29,30). These proteins organize the nascent
structure of ameloblasts, which are composed of long thin crystal
ribbons (29). During the secretory
stage, ameloblasts construct the full length of the enamel ribbons;
however, this matrix remains only partly mineralized until the
maturation stage (29,31,32).
During this stage, the extracellular pH is ~7.23 (9,29,33).
During the maturation stage, matrix proteins are degraded by a
stage-specific protease and crystals develop into their final
hardened forms (4,7,28). This
stage-specific protease is KLK4, which can degrade matrix proteins
and facilitate their resorption (4,34). In
addition, high numbers of calcium and phosphate ions are secreted
into the enamel matrix (30). This
allows enamel ribbons to widen, leading to increased hydrogen
release (7,29). During the efflux of calcium and
phosphate ions, hydroxyapatite (HA) may be deposited. At the same
time with HA deposition, hydrogen ions are released, lowering the
pH of the enamel matrix (33).
Depending on the phosphate precursor, the precipitation of HA
releases 8–14 moles of hydrogen ions per mole of HA, which
acidifies the enamel matrix (4,10,29,35).
At this stage, extracellular pH may decrease to <6.0 (9,29,33).
However, pH levels may vary during the maturation
stage. Due to cyclic oscillations of ameloblasts between the
smooth-ended (SE) and ruffle-ended (RE) forms, the pH in the enamel
matrix periodically fluctuates between neutral (pH 7.2) and acidic
(pH 5.8) (7,11,30,36).
To ensure that pH levels change as required during
the different stages, an effective pH regulation mechanism is
required. The primary extracellular buffering mechanism used by
ameloblasts is the bicarbonate buffer system (7,9–11), particularly during the maturation
stage (9,37). During amelogenesis, ameloblasts
secrete bicarbonate into the enamel matrix to buffer the protons
produced by the growth of hydroxyapatite crystals. During the
maturation of enamel, carbonic anhydrase type VI, which is a
secreted type of carbonic anhydrase, may catalyze the formation of
CO2 and H2O from carbonic acid, formed by
bicarbonate buffering of the protons in the enamel space (9,38).
Furthermore, Sasaki et al (11) suggested that an acidic pH in RE
ameloblasts may be due to the release of protons, which may
contribute to pH regulation. Due to the transportation of protons
and bicarbonate, the balance between the intra- and extracellular
pH of ameloblasts may be maintained.
Electrolyte transport processes involved in
pH regulation during amelogenesis
The transport of ions in and out of cells occurs in
two ways, active transport and passive diffusion, and this is also
the case in ameloblasts. Ion transporters participate in this
process. Ions associated with amelogenesis predominantly include
calcium, phosphonium, chloridion, protons and bicarbonate, and
primarily rely on ion transporters to enter and leave ameloblasts.
Previous studies have demonstrated that there are many ion
transporters on the membrane of ameloblasts, including
Caγ1, inwardly rectifying potassium channel
(Kir1.1), epithelial sodium channel, anion exchange
protein (AE)1, AE2, electrogenic sodium biocarbonate cotransporter
(NBC)1, NBCe1, sodium-calcium exchanger 1–3, sodium-hydrogen
antiporter 1 (NHE-1), sodium/potassium/calcium exchanger 4,
H+-adenosine triphosphate (ATP)ase, cystic fibrosis
transmembrane conductance regulator (CFTR), H/Cl exchange
transporter (ClC)-3, ClC-5, ClC-7, gap junction α-1 protein (Cx43)
and PAT-1 (16,30,36,39–51).
Among these ion transporters, some are responsible for regulating
pH and include ClC-5, ClC-7, CFTR, NHE-1, NBCe1, AE1, AE2 and
pendrin. As aforementioned, the formation of hydroxyapatite during
the maturation stage of amelogenesis generates a large quantity of
protons; to sustain the growth of crystals, these protons must be
neutralized (7,42,52),
potentially via the secretion of neutralizing ions, such as
bicarbonate (30).
CFTR serves an important role in transporting
bicarbonate into the enamel space to buffer protons in ameloblasts
and locates on the apical plasma membrane during the maturation
stage of ameloblasts (52). Paine
et al (8) demonstrated that
bicarbonate was transported by apical AE2a, basolateral NBCe1 and
apical CFTR. Furthermore, previous studies have identified that
CFTR is a critical factor in the regulation of pH during the
maturation of ameloblasts and is essential for enamel
mineralization (52–54). Duan et al (26) demonstrated that CFTR inhibition and
treatment with CFTR siRNA may increase intracellular pH. Sui et
al (53) placed incisors taken
from mice with the cystic fibrosis gene knocked out in pH indicator
solution and indicated that they were acidic. CFTR is a classical
Cl− channel and transports bicarbonate in two main ways,
accompanied by the transportation of chloride. CFTR stimulates the
transport activity of Slc26a members, leading to bicarbonate efflux
(54–56). Additionally, CFTR is permeable to
bicarbonate (54,57,58).
Although the results of previous studies have indicated that CFTR
is more permeable to Cl− than to bicarbonate, studies
have revealed that CFTR may be responsible for >50% of the total
bicarbonate efflux in pancreatic duct cells (54,57).
Solute carrier (SLC) 4 bicarbonate transporters
serve an important role in the transport of bicarbonate and the
regulation of pH in different types of cells (8,59).
SLC4A2 codes for AE2, an anion-exchanger; whereas SLC4A4 codes for
NBCe1, a bidirectionally electrogenic transmembrane ion-transporter
(60). Depending on different cell
types, NBCe1 is able to co-transport two or three bicarbonate ions
per Na+ ion (60).
However, the location of AE2 and NBCe1 remains controversial. Paine
et al (8) and Lacruz et
al (43) indicated that NBCe1 is
located on the basolateral membrane and AE2 is located on the
apical plasma membrane. However, Bronckers et al (61) demonstrated that AE2 is located on the
basolateral membrane and that NBCe1 is located in the papillary
layer cells of the enamel organ. The difference between these
studies may be due to the different methods employed and the
different age groups of the animals in each of the studies
(49). Overall, it is considered
that, following basolateral bicarbonate uptake by NBCe1 and AE2,
apical bicarbonate secretion is mediated by CFTR (62). Gawenis et al (63) demonstrated that mice lacking AE2 were
edentulous (63). Additionally,
patients harboring NBCe1 mutations exhibit different levels of
enamel abnormalities (64,65). These results all confirm the
important role of AE2 and NBCe1 in the transport of bicarbonate in
ameloblasts and in regulating pH levels (8).
Pendrin is another member of SLC family, which is
encoded by SLC26A4 located on the apical membrane of ameloblasts
(45). It is able to transport
chloride, bicarbonate, iodine and formate (45). A number of studies have demonstrated
that it is able to regulate luminal pH in the kidney, inner ear and
thyroid (66–68). However, the transport of bicarbonate
by pendrin is not critical for enamel formation. Bronckers et
al (45) identified that
ameloblasts may achieve the normal mineralization of enamel in
pendrin knockout rodents.
ClC-5 and ClC-7 are voltage-gated chloride channels,
which are responsible for transporting Cl− and
H+. Previous studies have generally focused on the
regulation of dentin development by ClC-5 (41,69).
Duan et al (70) demonstrated
that ClC-5 is also expressed by ameloblasts of tooth germ. The
enamel of ClC-5 knockout mice is easily detached from dentin and
this may affect enamel formation (41,70).
ClC-7 is a Cl−/ H+ antiporter (71,72). It
has been proven that, during the maturation stage of ameloblasts,
the highest levels of ClC-7 are immunolocated in ameloblast
vesicles (48,71,72).
Osteopetrosis-associated transmembrane protein 1, which centralizes
to the lysosomes of all cells and lies on the ruffled border
membrane of osteoclasts, is essential for the transport activity of
ClC-7 (71–74). In osteoclasts, Kornak et al
(74) demonstrated that ClC-7 was
important for the acidification of the resorption lacuna; however,
this was not the case in lysosomes. The lysosomal pH and degree of
enamel mineralization did not change following ClC-7 knockout
(71,75,76).
However, ClC-7 may serve an important role during tooth eruption,
as it has been determined that ClC-7 knockout mice experience a
failure of tooth eruption (71,74,75,77);
however, further studies are required to explore the exact
mechanism of action. Thus, unlike its function in osteoclasts,
ClC-7 is not critical for ameloblast function (71).
NHE1 is a Na+/H+ exchanger located on
basolateral membrane of ameloblasts and is strongly immunoactivated
in the secretory and maturation stages of amelogenesis (42). It co-operates with other transporters
to transport H+ to the enamel matrix, thus altering pH.
Carbonic anhydrase (CA) is a zinc metalloenzyme required for the
survival of pro- and eukaryotic cells (38,78). It
is able to catalyze the reaction of carbon dioxide and water to
produce carbonic acid, which then rapidly dissociates into hydrogen
and bicarbonate ions (38,78,79).
Among >12 CA genes, it has been proven that CA II and CA VI are
expressed in maturation ameloblasts (38,78,79). CA
II is the most abundantly expressed isozyme in all major mammalian
organs and is localized in the cytoplasm. It can pump H+
into the enamel with the aid of H+-ATPase type V
(80). CA VI is a secreted enzyme
located in the enamel, which buffers local pH by providing
bicarbonate ions or recycling excess carbonic acid (38,78).
Generally speaking, CA II and CA VI participate in the pH
homeostasis of ameloblasts by transporting H+ and
HCO3−.
The effect of fluoride on pH regulation
during amelogenesis
Excessive fluoride may cause pH disturbance during
amelogenesis. As the pH balance is primarily maintained by
electrolyte transporters, fluoride may serve a role during
electrolyte transportation. Zheng et al (25) demonstrated that fluoride may
indirectly regulate ameloblasts in mice and humans. The
upregulation of NBCe1 during ameloblast maturation is not directly
stimulated by fluoride, as NBCe1 expression is unaffected following
the addition of fluoride. The exact mechanism of action of NBCe1
upregulation may be due to mineral deposition and matrix
acidification (25). Paine et
al (8) demonstrated that AE2 and
NBCe1 expression is upregulated when pH levels are low. A previous
study investigating the association between microRNA (miRNA) 224
expression and acidification indicated that acidification caused by
fluoride may downregulate miRNA 224 expression (81). Furthermore, miRNA 224 expression was
inversely correlated with the expression of SLC4A4 and CFTR
(81). Further studies are required
to investigate the direct effect of fluoride on their
expression.
The effect of fluoride on pH regulation has been
verified. A number of in vitro and in vivo studies
have proven that F− is able to accelerate crystal
formation and induce hypermineralized lines in secretory enamel
(6,61,82). The
process of crystal growth produces a large number of protons, which
can acidify the microenvironment. A decreased pH may induce a
series of changes in electrolyte transporters and may also affect
the toxicity of F− (4,8,25,29).
F− cannot enter the ameloblast directly and must be
converted to hydrogen fluoride (HF) beforehand (4). A low extracellular pH promotes this
conversion; >25 times HF is formed at pH 6.0 compared with at pH
7.4, as determined by the Henderson-Hasselbalch equation (4,29). Due
to the concentration gradient of pH, HF can diffuse easily into the
cytoplasm from the enamel matrix and revert to F− in the
neutral cytoplasm; it cannot consequently easily diffuse out of the
cell (4,29,83,84).
Increased F− concentration in the cytoplasm can induce
oxidative stress by reducing the activity of antioxidant enzymes,
which affects a variety of structures and processes of normal cells
due to reactive oxygen species accumulation (4,85–87).
Furthermore, fluoride ions in the cytoplasm may induce endoplasmic
reticulum (ER) stress, including the phosphorylation of eukaryotic
initiation factor 2, which may result in a decrease of overall
protein production, including secretion of the protease KLK4
(4,29,88,89).
Thus, F− induces more severe toxicity in ameloblasts
undergoing maturation (90–92).
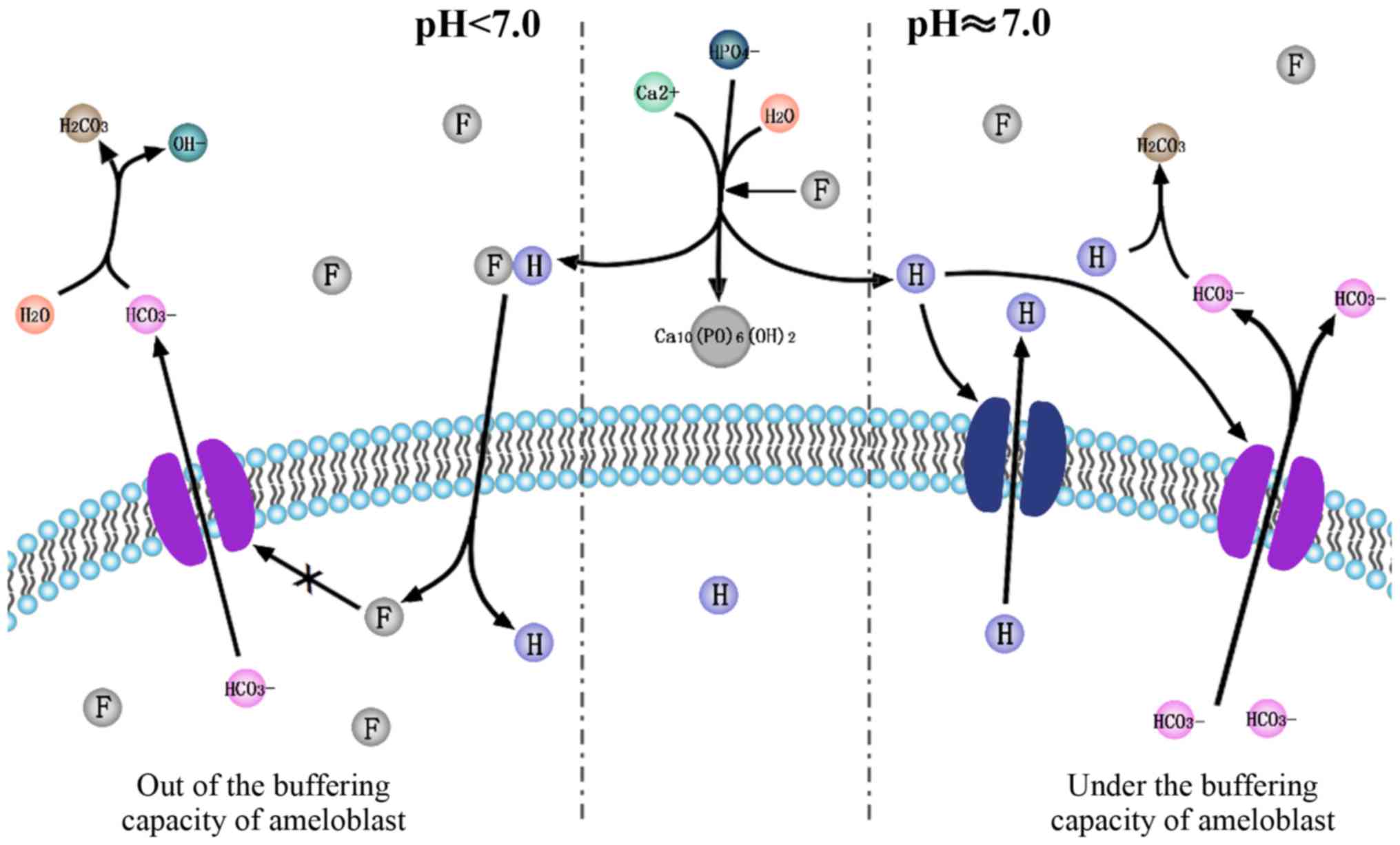
Based on the acidic hypothesis of F− and
the pH regulation in amelogenesis, the current review hypothesizes
that F− is associated with pH regulation during
amelogenesis. F− accelerates crystal formation in
ameloblasts in two different ways (Fig.
1). Firstly, it stimulates the release of protons and lowers
the pH of the cell microenvironment. This upregulates the
expression of ion transporters, which transport H+ and
HCO3−. Yet through this complicated
regulation of H+ and HCO3− in and
out of ameloblasts by different ion transporters mentioned above,
the net efflux of HCO3− exceeds
H+. Secondly, it triggers unknown signaling pathways and
upregulates bicarbonate transporters, including NBCe1, AE2, CA2,
CA6, CFTR. Upregulated bicarbonate transporters release a large
amount of bicarbonate from the ameloblasts, which may neutralize
the pH to form a microenvironment that favors crystal nucleation.
By contrast, following a decrease in the pH, more F−
diffuses into cytoplasm of amelobalsts along the concentration
gradient formed by the release of protons. The retention of
F− induces a series of pathological changes, including
oxidative and ER stress. Under the buffering capacity of
ameloblasts facing F− toxicity, normal mineralization
occurs; however, if the buffering capacity of ameloblasts is
overwhelmed by excessive F−, hypomineralization occurs,
which may cause dental fluorosis. Further studies are required to
investigate the signaling pathways involved and the exact process
by which ions are transported.
Conclusions
Amelogenesis is a complicated process that involves
crystal formation, the removal of matrix proteins and ions
transportation. The pH of the enamel matrix fluctuates during
different stages of ameloblast development. Under normal
conditions, pH levels are regulated and ameloblasts may perform
their normal function and stimulate normal mineralization. CFTR,
AE2, NBCe1, ClC-5, ClC-7, NHE1, CA2, CA6 and H+-ATPase
type V are all involved in the regulation of pH. However, under
high fluoride concentrations, pH regulation becomes dysregulated.
This causes the malfunction of ameloblasts, resulting in
hypomineralization and dental fluorosis. The effect of fluoride on
ameloblasts is due to its impact on electrolyte transporters and
its direct diffusion into the cytoplasm in an acidic
environment.
Acknowledgements
Not applicable.
Funding
The current review was supported by the Natural
Science Foundation of Shandong Province, China (grant no.
ZR2014HQ067).
Availability of data and materials
Not applicable.
Authors' contributions
This review is the result of joint efforts. DZ
designed the study, MJ was responsible for writing the manuscript,
LXu constructed the figure, LXia analyzed and summarized the
literature. SH helped with analysis of the literature, assisted in
providing constructive discussions and revised the manuscript. All
authors read and approve the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Aoba T and Fejerskov O: Dental fluorosis:
Chemistry and biology. Crit Rev Oral Biol Med. 13:155–170. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beltrán-Aguilar ED, Barker L and Dye BA:
Prevalence and severity of dental fluorosis in the United States,
1999–2004. NCHS Data Brief. 1–8. 2010.
|
|
3
|
Denbesten P and Li W: Chronic fluoride
toxicity: Dental fluorosis. Monogr Oral Sci. 22:81–96. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sierant ML and Bartlett JD: Stress
response pathways in ameloblasts: Implications for amelogenesis and
dental fluorosis. Cells. 1:631–645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mohamed AR, Thomson WM and Mackay TD: An
epidemiological comparison of Dean's index and the developmental
defects of enamel (DDE) index. J Public Health Dent. 70:344–347.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lyaruu DM, Medina JF, Sarvide S, Bervoets
TJ, Everts V, Denbesten PK, Smith CE and Bronckers AL: Barrier
formation: Potential molecular mechanism of enamel fluorosis. J
Dent Res. 93:94–102. 2014. View Article : Google Scholar
|
|
7
|
Smith CE: Cellular and chemical events
during enamel maturation. Crit Rev Oral Biol Med. 9:128–161. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paine ML, Snead ML, Wang HJ, Abuladze N,
Pushkin A, Liu W, Kao LY, Wall SM, Kim YH and Kurtz I: Role of
NBCe1 and AE2 in secretory ameloblasts. J Dent Res. 87:391–395.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lacruz RS, Nanci A, Kurtz I, Wright JT and
Paine ML: Regulation of pH during amelogenesis. Calcif Tissue Int.
86:91–103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Simmer JP and Fincham AG: Molecular
mechanisms of dental enamel formation. Crit Rev Oral Biol Med.
6:84–108. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sasaki S, Takagi T and Suzuki M: Cyclical
changes in pH in bovine developing enamel as sequential bands. Arch
Oral Biol. 36:227–231. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takagi T, Ogasawara T, Tagami J, Akao M,
Kuboki Y, Nagai N and LeGeros RZ: pH and carbonate levels in
developing enamel. Connect Tissue Res. 38:181–205. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bawden JW, Crenshaw MA, Wright JT and
LeGeros RZ: Consideration of possible biologic mechanisms of
fluorosis. J Dent Res. 74:1349–1352. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Robinson C, Connell S and Kirkham J:
Dental enamel-a biological ceramic: Regular substructures in enamel
hydroxyapatite crystals revealed by atomic force microscopy. J
Mater Chem. 14:2242–2248. 2004. View Article : Google Scholar
|
|
15
|
Robinson C, Connell S, Kirkham J, Brookes
SJ, Shore RC and Smith AM: The effect of fluoride on the developing
tooth. Caries Res. 38:268–276. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bronckers AL, Lyaruu DM and DenBesten PK:
The impact of fluoride on ameloblasts and the mechanisms of enamel
fluorosis. Crit Rev Oral Biol Med. 88:877–893. 2009.
|
|
17
|
Yan Q, Zhang Y, Li W and Denbesten PK:
Micromolar fluoride alters ameloblast lineage cells in vitro. J
Dent Res. 86:336–340. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei W, Gao Y, Wang C, Zhao L and Sun D:
Excessive fluoride induces endoplasmic reticulum stress and
interferes enamel proteinases secretion. Environ Toxicol.
28:332–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Li W, Chi HS, Chen J and
Denbesten PK: JNK/c-Jun signaling pathway mediates the
fluoride-induced down-regulation of MMP-20 in vitro. Matrix Biol.
26:633–641. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jacinto-Alemán LF, Hernández-Guerrero JC,
Trejo-Solís C, Jiménez-Farfán MD and Fernández-Presas AM: In vitro
effect of sodium fluoride on antioxidative enzymes and apoptosis
during murine odontogenesis. J Oral Pathol Med. 39:709–714. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang T, Zhang Y, Li Y, Hao Y, Zhou M, Dong
N and Duan X: High amounts of fluoride induce apoptosis/cell death
in matured ameloblast-like LS8 cells by downregulating Bcl-2. Arch
Oral Biol. 58:1165–1173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsuo S, Inai T, Kurisu K, Kiyomiya K and
Kurebe M: Influence of fluoride on secretory pathway of the
secretory ameloblast in rat incisor tooth germs exposed to sodium
fluoride. Arch Toxicol. 70:420–429. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hannas AR, Pereira JC, Granjeiro JM and
Tjäderhane L: The role of matrix metalloproteinases in the oral
environment. Acta Odontol Scand. 65:1–13. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Denbesten PK and Heffernan LM: Enamel
proteases in secretory and maturation enamel of rats ingesting 0
and 100 PPM fluoride in drinking water. Adv Dent Res. 3:199–202.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng L, Zhang Y, He P, Kim J, Schneider
R, Bronckers AL, Lyaruu DM and DenBesten PK: NBCe1 in mouse and
human ameloblasts may be indirectly regulated by fluoride. J Dent
Res. 90:782–787. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duan X, Mao Y, Wen X, Yang T and Xue Y:
Excess fluoride interferes with chloride-channel-dependent
endocytosis in ameloblasts. J Dent Res. 90:175–180. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nanci A: Ten Cate's oral histology:
Development, structure, and function. Ebook, MosbyElsevier. 1–432.
2007.
|
|
28
|
Hu JC, Chun YH, Al Hazzazzi T and Simmer
JP: Enamel formation and amelogenesis imperfecta. Cells Tissues
Organs. 186:78–85. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sharma R, Tsuchiya M, Skobe Z, Tannous BA
and Bartlett JD: The acid test of fluoride: How pH modulates
toxicity. PLoS One. 5:e108952010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Varga G, Kerémi B, Bori E and Földes A:
Function and repair of dental enamel-potential role of epithelial
transport processes of ameloblasts. Pancreatology. 15 Suppl
4:S55–S60. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nanci A and Smith CE: Development and
calcification of enamel. Calcification in biological systems.
313–343. 1992.
|
|
32
|
Bartlett JD and Simmer JP: Proteinases in
developing dental enamel. Crit Rev Oral Biol Med. 10:425–441. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Smith CE, Issid M, Margolis HC and Moreno
EC: Developmental changes in the pH of enamel fluid and its effects
on matrix-resident proteinases. Adv Dent Res. 10:159–169. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Simmer JP, Fukae M, Tanabe T, Yamakoshi Y,
Uchida T, Xue J, Margolis HC, Shimizu M, DeHart BC, Hu CC and
Bartlett JD: Purification, characterization, and cloning of enamel
matrix serine proteinase 1. J Dent Res. 77:377–386. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Smith CE, Chong DL, Bartlett JD and
Margolis HC: Mineral acquisition rates in developing enamel on
maxillary and mandibular incisors of rats and mice: Implications to
extracellular acid loading as apatite crystals mature. J Bone Miner
Res. 20:240–249. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Damkier HH, Josephsen K, Takano Y, Zahn D,
Fejerskov O and Frische S: Fluctuations in surface pH of maturing
rat incisor enamel are a result of cycles of H(+)-secretion by
ameloblasts and variations in enamel buffer characteristics. Bone.
60:227–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bronckers AL, Lyaruu DM, Guo J, Bijvelds
MJ, Bervoets TJ, Zandieh-Doulabi B, Medina JF, Li Z, Zhang Y and
DenBesten PK: Composition of mineralizing incisor enamel in
CFTR-deficient mice. Eur J Oral Sci. 123:9–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Smith CE, Nanci A and Moffat P: Evidence
by signal peptide trap technology for the expression of carbonic
anhydrase 6 in rat incisor enamel organs. Eur J Oral Sci. 114 Suppl
1:(S147): S153 Discussion 164–165. 380–381. 2006.
|
|
39
|
Hou J, Situ Z and Duan X: ClC chloride
channels in tooth germ and odontoblast-like MDPC-23 cells. Arch
Oral Biol. 53:874–878. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Su X, Yang F, Duan X, Yuan L, Li Y and Wu
L: Expression of CLC-7 during mouse tooth development. J Pract
Stomatol. 24:342–345. 2008.(In Chinese).
|
|
41
|
Duan X, Mao Y, Yang T, Wen X, Wang H, Hou
J, Xue Y and Zhang R: ClC-5 regulates dentin development through
TGF-beta1 pathway. Arch Oral Biol. 54:1118–1124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Josephsen K, Takano Y, Frische S,
Praetorius J, Nielsen S, Aoba T and Fejerskov O: Ion transporters
in secretory and cyclically modulating ameloblasts: A new
hypothesis for cellular control of preeruptive enamel maturation.
Am J Physiol Cell Physiol. 299:C1299–C1307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lacruz RS, Nanci A, White SN, Wen X, Wang
H, Zalzal SF, Luong VQ, Schuetter VL, Conti PS, Kurtz I and Paine
ML: The sodium bicarbonate cotransporter (NBCe1) is essential for
normal development of mouse dentition. J Biol Chem.
285:24432–24438. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Okumura R, Shibukawa Y, Muramatsu T,
Hashimoto S, Nakagawa K, Tazaki M and Shimono M: Sodium-calcium
exchangers in rat ameloblasts. J Pharmacol Sci. 112:223–230. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bronckers AL, Guo J, Zandieh-Doulabi B,
Bervoets TJ, Lyaruu DM, Li X, Wangemann P and DenBesten P:
Developmental expression of SLC26A4 (Pendrin) during amelogenesis
in developing rodent teeth. Eur J Oral Sci. 119 Suppl 1:S185–S192.
2011. View Article : Google Scholar
|
|
46
|
Hu P, Lacruz RS, Smith CE, Smith SM, Kurtz
I and Paine ML: Expression of the sodium/calcium/potassium
exchanger, NCKX4, in ameloblasts. Cells Tissues Organs.
196:501–509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lacruz RS, Smith CE, Moffatt P, Chang EH,
Bromage TG, Bringas P Jr, Nanci A, Baniwal SK, Zabner J, Welsh MJ,
et al: Requirements for ion and solute transport, and pH regulation
during enamel maturation. J Cell Physiol. 227:1776–1785. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lacruz RS, Brookes SJ, Wen X, Jimenez JM,
Vikman S, Hu P, White SN, Lyngstadaas SP, Okamoto CT, Smith CE and
Paine ML: Adaptor protein complex 2 (ap-2) mediated, clathrin
dependent endocytosis, and related gene activities, are a prominent
feature during maturation stage amelogenesis. J Bone Miner Res.
28:672–687. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lacruz RS, Smith CE, Kurtz I, Hubbard MJ
and Paine ML: New paradigms on the transport functions of
maturation-stage ameloblasts. J Dent Res. 92:122–129. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Duan X: Ion channels, channelopathies, and
tooth formation. J Dent Res. 93:117–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bronckers AL, Lyaruu D, Jalali R, Medina
JF, Zandieh-Doulabi B and DenBesten PK: Ameloblast modulation and
transport of Cl-, Na+, and K+ during amelogenesis. J Dent Res.
94:1740–1747. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wright JT, Kiefer CL, Hall KI and Grubb
BR: Abnormal enamel development in a cystic fibrosis transgenic
mouse model. J Dent Res. 75:966–973. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sui W, Boyd C and Wright JT: Altered pH
regulation during enamel development in the cystic fibrosis mouse
incisor. J Dent Res. 82:388–392. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bronckers AL, Kalogeraki L, Jorna HJ,
Wilke M, Bervoets TJ, Lyaruu DM, Zandieh-Doulabi B, Denbesten PK
and de Jonge H: The cystic fibrosis transmembrane conductance
regulator (CFTR) is expressed in maturation stage ameloblasts,
odontoblasts and bone cells. Bone. 46:1188–1196. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH,
Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ and Muallem S:
Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell
Biol. 6:343–350. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mount DB and Romero MF: The SLC26 gene
family of multifunctional anion exchangers. Pflugers Arch.
447:710–721. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ishiguro H, Steward MC, Naruse S, Ko SB,
Goto H, Case RM, Kondo T and Yamamoto A: CFTR functions as a
bicarbonate channel in pancreatic duct cells. J Gen Physiol.
133:315–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Shcheynikov N, Kim KH, Kim KM, Dorwart MR,
Ko SB, Goto H, Naruse S, Thomas PJ and Muallem S: Dynamic control
of cystic fibrosis transmembrane conductance regulator
Cl(−)/HCO3(−) selectivity by external Cl(−). J Biol Chem.
279:21857–21865. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Pushkin A and Kurtz I: SLC4 base
(HCO3−, CO32−)
transporters: Classification function, structure, genetic diseases,
and knockout models. Am J Physiol Renal Physiol. 290:F580–F599.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Jalali R, Guo J, Zandieh-Doulabi B,
Bervoets TJ, Paine ML, Boron WF, Parker MD, Bijvelds MJ, Medina JF,
DenBesten PK and Bronckers AL: NBCe1 (SLC4A4) a potential pH
regulator in enamel organ cells during enamel development in the
mouse. Cell Tissue Res. 358:433–442. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bronckers AL, Lyaruu DM, Jansen ID, Medina
JF, Kellokumpu S, Hoeben KA, Gawenis LR, Oude-Elferink RP and
Everts V: Localization and function of the anion exchanger Ae2 in
developing teeth and orofacial bone in rodents. J Exp Zool B Mol
Dev Evol. 312B:1–387. 2009. View Article : Google Scholar
|
|
62
|
Arquitt CK, Boyd C and Wright JT: Cystic
fibrosis transmembrane regulator gene (CFTR) is associated with
abnormal enamel formation. J Dent Res. 81:492–496. 1999. View Article : Google Scholar
|
|
63
|
Gawenis LR, Ledoussal C, Judd LM, Prasad
V, Alper SL, Stuart-Tilley A, Woo AL, Grisham C, Sanford LP,
Doetschman T, et al: Mice with a targeted disruption of the AE2
Cl-/HCO3- exchanger are achlorhydric. J Biol Chem. 279:30531–30539.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Dinour D, Chang MH, Satoh J, Smith BL,
Angle N, Knecht A, Serban I, Holtzman EJ and Romero MF: A novel
missense mutation in the sodium bicarbonate cotransporter
(NBCe1/SLC4A4) causes proximal tubular acidosis and glaucoma
through ion transport defects. J Biol Chem. 279:52238–52246. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Inatomi J, Horita H, Braverman N, Sekine
T, Yamada H, Suzuki Y, Kawahara K, Moriyama N, Kudo A, Kawakami H,
et al: Mutational and functional analysis of SLC4A4 in a patient
with proximal renal tubular acidosis. Pflugers Arch. 448:438–444.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Royaux IE, Belyantseva IA, Wu T, Kachar B,
Everett LA, Marcus DC and Green ED: Localization and functional
studies of pendrin in the mouse inner ear provide insight about the
etiology of deafness in pendred syndrome. J Assoc Res Otolaryngol.
4:394–404. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wall SM, Hassell KA, Royaux IE, Green ED,
Chang JY, Shipley GL and Verlander JW: Localization of pendrin in
mouse kidney. Am J Physiol Renal Physiol. 284:F229–F241. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wangemann P, Kim HM, Billings S, Nakaya K,
Li X, Singh R, Sharlin DS, Forrest D, Marcus DC and Fong P:
Developmental delays consistent with cochlear hypothyroidism
contribute to failure to develop hearing in mice lacking
Slc26a4/pendrin expression. Am J Physiol Renal Physiol.
297:F1435–F1447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang SS, Devuyst O, Courtoy PJ, Wang XT,
Wang H, Wang Y, Thakker RV, Guggino S and Guggino WB: Mice lacking
renal chloride channel, CLC-5, are a model for Dent's disease, a
nephrolithiasis disorder associated with defective receptormediated
endocytosis. Hum Mol Genet. 9:2937–2945. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Duan X: Spatial-temporal distribution of
CLC-5 in rat tooth germ development. J Dent Res. 83:27412004.
|
|
71
|
Guo J, Bervoets TJ, Henriksen K, Everts V
and Bronckers AL: Null mutation of chloride channel 7 (Clcn7)
impairs dental root formation but does not affect enamel
mineralization. Cell Tissue Res. 363:361–370. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Leisle L, Ludwig CF, Wagner FA, Jentsch TJ
and Stauber T: ClC-7 is a slowly voltage-gated
2Cl(−)/1H(+)-exchanger and requires Ostm1 for transport activity.
EMBO J. 30:2140–2152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lange PF, Wartosch L, Jentsch TJ and
Fuhrmann JC: ClC-7 requires Ostm1 as a beta-subunit to support bone
resorption and lysosomal function. Nature. 440:220–223. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kornak U, Kasper D, Bösl MR, Kaiser E,
Schweizer M, Schulz A, Friedrich W, Delling G and Jentsch TJ: Loss
of the ClC-7 chloride channel leads to osteopetrosis in mice and
man. Cell. 104:205–215. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kasper D, Planells-Cases R, Fuhrmann JC,
Scheel O, Zeitz O, Ruether K, Schmitt A, Poët M, Steinfeld R,
Schweizer M, et al: Loss of the chloride channel ClC-7 leads to
lysosomal storage disease and neurodegeneration. EMBO J.
24:1079–1091. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Steinberg BE, Huynh KK, Brodovitch A, Jabs
S, Stauber T, Jentsch TJ and Grinstein S: A cation counterflux
supports lysosomal acidification. J Cell Biol. 189:1171–1186. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wen X, Lacruz RS and Paine ML: Dental and
cranial pathologies in mice lacking the Cl(−)/H(+)-exchanger ClC-7.
Anat Rec (Hoboken). 298:1502–1508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tripp BC, Smith K and Ferry JG: Carbonic
anhydrae: New insights for an ancient enzyme. J Biol Chem.
276:48615–48618. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chegwidden WR and Carter ND: Introduction
to the carbonic anhydrases. EXS. 90:14–28. 2000.
|
|
80
|
Lin HM, Nakamura H, Noda T and Ozawa H:
Localization of H(+)-ATPase and carbonic anhydrase II in
ameloblasts at maturation. Calcif Tissue Int. 55:38–45. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Fan Y, Zhou Y, Zhou X, Sun F, Gao B, Wan
M, Zhou X, Sun J, Xu X, Cheng L, et al: MicroRNA 224 regulates ion
transporter expression in ameloblasts to coordinate enamel
mineralization. Mol Cell Biol. 35:2875–2890. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Brown WE, Edelman N and Tomzaic BB:
Octacalcium phosphate as precursors in biomineral formation. Adv
Dent Res. 1:306–313. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kawase T and Suzuki A: Studies on the
transmembrane migration of fluoride and its effects on
proliferation of L-929 fibroblasts (L cells) in vitro. Arch Oral
Biol. 34:103–107. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
He H, Ganapathy V, Isales CM and Whitford
GM: pH-dependent fluoride transport in intestinal brush border
membrane vesicles. Biochim Biophys Acta. 1372:244–254. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Mittal M and Flora SJ: Effects of
individual and combined exposure to sodium arsenite and sodium
fluoride on tissue oxidative stress, arsenic and fluoride levels in
male mice. Chem Biol Interact. 162:128–139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Jin XQ, Xu H, Shi HY, Zhang JM and Zhang
HQ: Fluoride-induced oxidative stress of osteoblasts and protective
effects of baicalein against fluoride toxicity. Biol Trace Elem
Res. 116:81–89. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Varol E, Icli A, Aksoy F, Bas HA, Sutcu R,
Ersoy IH, Varol S and Ozaydin M: Evaluation of total oxidative
status and total antioxidant capacity in patients with endemic
fluorosis. Toxicol Ind Health. 29:175–180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Sharma R, Tsuchiya M and Bartlett JD:
Fluoride induces endoplasmic reticulum stress and inhibits protein
synthesis and secretion. Environ Health Perspect. 116:1142–1146.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kubota K, Lee DH, Tsuchiya M, Young CS,
Everett ET, Martinez-Mier EA, Snead ML, Nguyen L, Urano F and
Bartlett JD: Fluoride induces endoplasmic reticulum stress in
ameloblasts responsible for dental enamel formation. J Biol Chem.
280:23194–23202. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lyaruu DM, de Jong M, Bronckers AL and
Wöltgens JH: Ultrastructure of in-vitro recovery of mineralization
capacity of fluorotic enamel matrix in hamster tooth germs
pre-exposed to fluoride in organ culture during the secretory phase
of amelogenesis. Arch Oral Biol. 32:107–115. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Smith CE, Nanci A and Denbesten PK:
Effects of chronic fluoride exposure on morphometric parameters
defining the stages of amelogenesis and ameloblast modulation in
rat incisors. Anat Rec. 237:243–258. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhou R, Zaki AE and Eisenmann DR:
Morphometry and autoradiography of altered rat enamel protein
processing due to chronic exposure to fluoride. Arch Oral Biol.
41:739–747. 1996. View Article : Google Scholar : PubMed/NCBI
|