Introduction
Gastric carcinoma usually arises from the gastric
mucosa (1). In the US, the incidence
of gastric carcinoma is 8.7–17.2 per 100,000 men and 9.7–43.1 per
100,000 women (2). In China, the
incidence of gastric carcinoma is high, with the age-standardized
incidence being 37.1 per 100,000 men and 17.4 per 100,000 women
(3). This high incidence is probably
due to the high prevalence of Helicobacter pylori infection
in China, which is a definite gastric carcinogen according to the
World Health Organization (3,4).
Endoscopic mucosal resection (EMR) and endoscopic
submucosal dissection (ESD) are widely used to treat early gastric
cancer (EGC) and gastric adenocarcinoma (5). In patients with EGC, the outcome of
endoscopic procedures with regard to survival is the same as that
of gastrectomy, but endoscopic procedures are associated with a
shorter hospital stay and decreased risk of post-operative
morbidity (6). Furthermore, ESD is
significantly better than EMR for removal of large lesions
(7–9).
One concern regarding ESD is the generation of
artificial ulcers, and studies describe delayed bleeding after ESD
as life-threatening with an incidence of ~5% (10,11).
Endoscopic hemostasis is effective during emergency endoscopy.
Therefore, it is necessary to determine the nature of delayed
bleeding and administer appropriate treatments. Previous studies
have suggested that the tumor site (middle and lower third of the
stomach), tumor size and ulcer formation are independent risk
factors for delayed bleeding (12–14), but
there is no consensus.
To further reduce the bleeding rate after ESD,
numerous hospitals in Europe and the US routinely perform
second-look endoscopy (SLE) to prevent delayed bleeding (15). The major purpose of SLE after ESD is
to inspect the non-bleeding visible blood vessels of the mucosal
defect that had bled recently or may eventually bleed (11,16).
When a bleeding or non-bleeding visible blood vessel is identified
by SLE, preventive hemostasis should be performed; hemostatic
clipping or thermocoagulation may be applied. However, it is
controversial whether SLE is able to prevent delayed bleeding. A
multicenter, prospective, randomized controlled non-inferiority
trial did not recommend SLE to prevent delayed bleeding after
gastric ESD (17), as supported by
certain other previous studies (16,18–20).
Therefore, the present study aimed to evaluate
whether SLE is able to prevent delayed bleeding, to assess the
clinical and pathological characteristics of patients with delayed
bleeding, and determine which specific lesions may require SLE. The
results of the present study may lead to the establishment of
improved guidelines to manage patients with EGC.
Materials and methods
Study design and patients
The present study was a retrospective analysis of a
prospective database of patients who were histologically diagnosed
with EGC and treated with ESD at the Center for Digestive Medicine
(key clinical entity of Jiangsu Province) of the Second Affiliated
Hospital of Nanjing Medical University (Nanjing, China) and at the
Department of Gastroenterology (key clinical entity of the Ministry
of Health, China) of the Qilu Hospital of Shandong University
(Jinan, China) between October 2014 and September 2016.
According to the guidelines of the Japan
Gastroenterological Endoscopy Society and the Japanese Gastric
Cancer Association (7), the
indications for ESD were lymph node-negative EGC, including the
following: i) Differentiated intramucosal carcinoma with a diameter
of ≥2 cm without ulcer; ii) differentiated intramucosal carcinoma
with a diameter of <3 cm and ulcer; and iii) undifferentiated
intramucosal carcinoma with a diameter of <2 cm without ulcer.
The diagnosis was made based on lesions identified on endoscopy,
chromoendoscopic biopsy or endoscopic ultrasonography. The
exclusion criteria were as follows: i) Digestive tract perforation
or ii) surgical specimens exhibiting submucosal invasion of ≥500
µm. The 3 treating gastroenterologists were all senior resident
physicians or deputy chief physicians, and all had a working
experience in ESD of >3 years and had performed >100 ESDs.
All operators had received training in narrow-band imaging for
detection of abnormal tumor vessels.
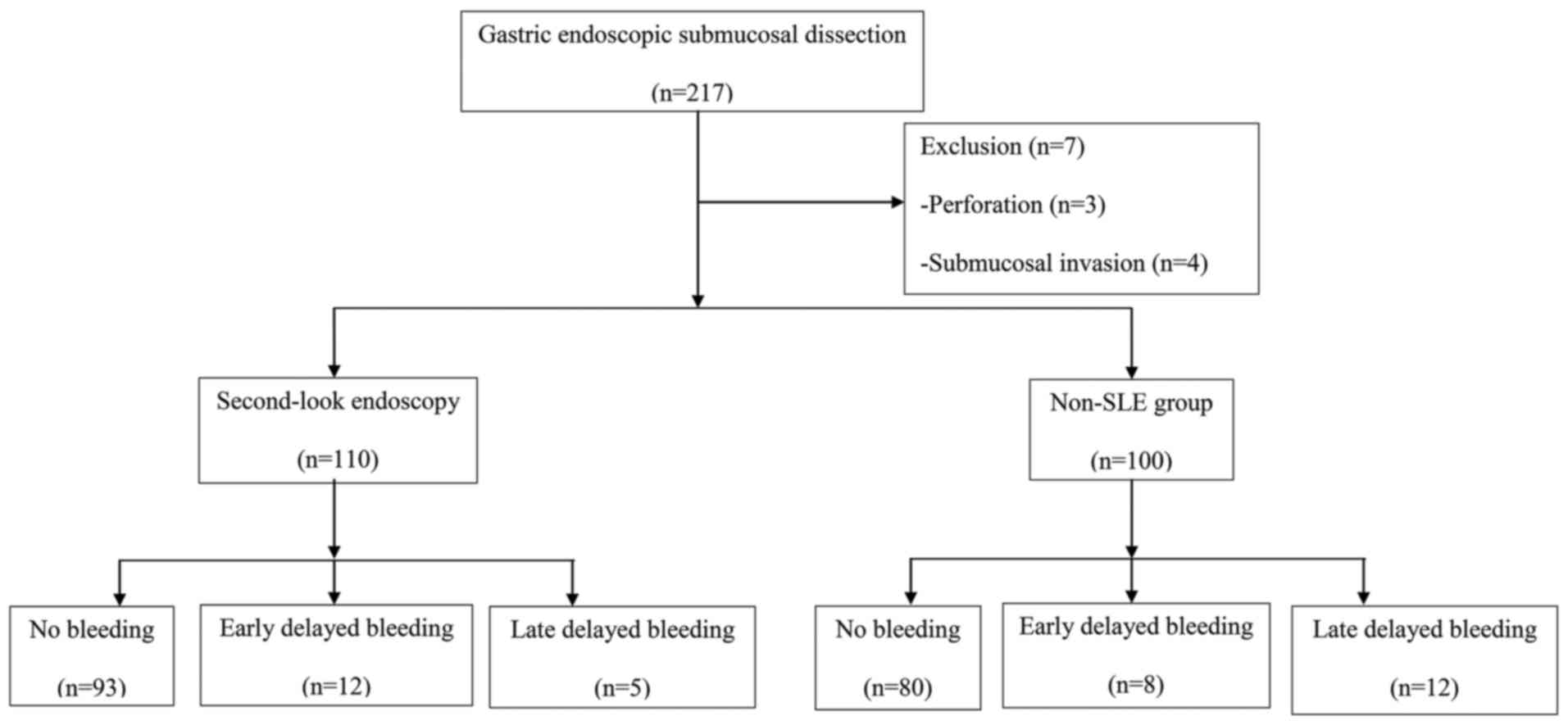
Of the 217 gastric neoplasm patients, 3 were
excluded due to perforation during ESD, and 4 were excluded as an
additional surgery was required for submucosal invasion. Finally,
210 patients were randomly divided into 2 groups: The non-SLE group
(n=100) and the SLE group (n=110). Of the 210 patients, 172 were
diagnosed with gastric high-grade intraepithelial neoplasia and 38
were diagnosed with gastric low-grade intraepithelial neoplasia.
The present study was approved by the ethics committee of the
Second Affiliated Hospital of Nanjing Medical University and Qilu
Hospital of Shandong University. All patients provided written
informed consent for inclusion in the database.
ESD strategy
All patients were required to provide written
informed consent prior to treatment. ESD was performed as
previously described (21). The
patients fasted from the morning on the operation day and underwent
surgery under conscious sedation. Argon plasma coagulation (PSD-60;
Olympus, Tokyo, Japan) was used for marking, and the marking points
were 5 mm away from the tumor edge. A submucosal injection of
1:10,000 epinephrine (0.01 mg/ml) + saline solution equal to a
total of 10.0–15.0 ml was performed around the lesion. The mucosa
was cut 5 mm away from the outer edge of the marking. After mucosal
incision, the lesion was dissected using an IT knife (KD-612L or
Dual knife (KD-650Q; both Olympus). Electrocoagulation of all
visible vessels on the ulcer surface was performed using hot biopsy
forceps (FD-410LR; Olympus). Sodium hyaluronate was used when
saline: Epinephrine (1:100,000) was not able to completely lift the
tumor. After the lesion was dissected from the stomach,
conventional electrocoagulation of non-bleeding visible vessels and
infiltration were performed using hot biopsy forceps.
SLE or emergency endoscopy
SLE was performed on the second day after ESD.
Delayed bleeding was characterized by the presence of melena,
hematochezia or hematemesis within 24 h after ESD, and mucosal
defects and bleeding were observed during emergency endoscopy
(22). Delayed bleeding was
classified as early (hematemesis or melena occurring in the time
interval between ESD and SLE or active or possible bleeding at the
time of the SLE) and late (hematemesis, hematochezia or melena
occurring after SLE) delayed bleeding. If there was significant
bleeding, hemostasis of the bleeding points or non-bleeding visible
vessels was performed under emergency endoscopy, mainly including
hemostatic clamps or thermocoagulation. Patients who had
hematochezia, hematemesis or hypotension and met the criteria were
given component blood transfusion. After ESD, continuous
intravenous esomeprazole administration (40 mg/day) was performed
for 2 days. On the third day, administration was changed to oral
esomeprazole (20 mg twice per day). Most patients started eating
food after SLE. The patients were discharged from the hospital at 6
days after surgery unless bleeding complications were noted. If
hematochezia or hematemesis occurred after discharge, the patients
were required to contact their physicians. When perforation or
delayed bleeding occurred, food intake and discharge plans were
changed depending on the patient's condition. The patients were
routinely followed up for 60 days in the first, second, fourth and
eighth week after discharge. The results of routine blood tests and
fecal occult blood test were recorded. The resection was considered
curative when the lesion was resected en bloc, was <2 cm
in diameter, was predominantly of the differentiated type,
demonstrated macroscopically intramucosal differentiated carcinomas
(pT1a), was absent of ulcers (UL-), lymphatic invasion (ly-) and
venous invasion (v-) (7). Expanded
criteria for curative resection were en bloc resection of
the lesion and a diameter of ≥2 cm, a predominantly differentiated
type, pT1a and UL(−); a diameter of <3 cm, a predominantly
differentiated type, pT1a and UL(+); a diameter of <2 cm, a
predominantly undifferentiated type, pT1a and UL(−); or a diameter
of <3 cm, a predominantly differentiated type, pT1b (SM1), ly(−)
and v(−); and negative surgical margins applied to all of the above
(7).
Data collection
The following information was recorded: Age, sex,
comorbidities (hypertension, heart disease, type 2 diabetes and
acute cerebrovascular disease), use of anti-coagulants or
anti-platelet drugs (patient-associated factors), H. pylori
infection, longitudinal axis position (upper, middle or lower third
of the stomach), cross-sectional position (anterior gastric wall,
posterior gastric wall, lesser curvature or greater curvature),
gross type of EGC, lesion diameter (cm), diameter of the resected
specimen (cm), histological type (differentiation degree), ESD
time, bleeding condition under emergency endoscopy (pulsatile
bleeding, active permeating bleeding, vessel exposure, bloodstain
or blood clot) and post-operative blood transfusion. The rates of
delayed bleeding with and without SLE were used as the endpoints to
determine the effectiveness of SLE.
Statistical analysis
SPSS 18.0 (SPSS, Inc., Chicago, IL, USA) was used
for statistical analysis. The Student's t-test or Fisher's exact
test was used to analyze differences in patient age, tumor size,
specimen size and ESD operative time between the two groups. The
chi-square test was used to analyze differences in sex,
complications, use of anti-coagulation or anti-platelet drugs,
longitudinal axis position, cross-sectional position, gross type
and degree of differentiation. If more than one predictive index
was significantly different on the Cox proportional hazards model
was used to determine the independent risk factors. Optimum cut-off
values for risk factors were determined using receiver operating
characteristic analysis. A two-sided P<0.05 was considered to
indicate a statistically significant difference.
Results
Characteristics of the surgeries
Fig. 1 presents the
patient flow chart. Table I presents
the characteristics of the 2 groups at baseline. The en bloc
resection rate was 100%. All resection margins were negative. No
gastrointestinal perforation, death or severe complication
occurred. The median time interval between ESD and SLE was 2 days
after surgery (range, 1–3 days). For patients in the SLE group
(n=110) and the non-SLE group (n=100), the mean operative time was
69±38 and 89±35 min, the mean lesion diameter was 2.8±0.9 and
2.5±1.2 cm and the number of specimens sized >40 mm was 8 and
10, respectively. There were no significant differences between the
2 groups with regard to the abovementioned parameters (P>0.05).
Late delayed bleeding was observed 2 days following ESD in the
non-SLE group. The incidence of late delayed bleeding occurring
after SLE was significantly different (4.5 vs. 12.0%, respectively;
P<0.05; Table I). Table I also provides information on the
occurrence of late delayed bleeding.
 | Table I.Characteristics of patients with
delayed bleeding after gastric endoscopic submucosal dissection in
the SLE and non-SLE groups. |
Table I.
Characteristics of patients with
delayed bleeding after gastric endoscopic submucosal dissection in
the SLE and non-SLE groups.
| Characteristic | SLE group
(n=110) | Non-SLE group
(n=100) | P-value |
|---|
| Age (years, mean ±
SD) | 61.5±10.6 | 65.5±10.2 | 0.557 |
| Male sex | 83 (75.5) | 71 (71.0) | 0.283 |
| Location of
lesion |
|
|
|
| Upper
third | 28 (25.5) | 18 (18.0) | 0.192 |
| Middle
third | 32 (29.0) | 36 (36.0) | 0.285 |
| Lower
third | 50 (45.5) | 46 (46.0) | 0.937 |
| Gross type |
|
|
|
|
Elevated | 57 (51.8) | 56 (56.0) | 0.544 |
|
Flat | 12 (10.9) | 9 (9.0) | 0.645 |
|
Depressed | 41 (37.3) | 35 (35.0) | 0.732 |
| Ulcer |
|
|
|
| Surface
redness | 73 (66.4) | 66 (66.0) | 0.956 |
| Surface
erosion | 21 (19.1) | 23 (23.0) | 0.487 |
|
Submucosal fibrosis | 16 (14.5) | 11 (11.0) | 0.443 |
| Degree of
differentiation |
|
|
|
|
Well-differentiated
carcinoma | 90 (81.8) | 82 (82.0) | 0.973 |
| Poorly
differentiated carcinoma | 20 (18.2) | 18 (18.0) |
|
| Lesion diameter
(cm, mean ± SD) | 2.8±0.9 | 2.5±1.2 | 0.178 |
| Artificial ulcer
diameter (cm, mean ± SD) | 4.0±1.3 | 2.6±1.2 | 0.954 |
| Helicobacter
pylori infection | 96 (87.3) | 81 (81.0) | 0.256 |
| Abdominal pain | 40 (36.4) | 31 (31.0) | 0.412 |
| Early delayed
bleeding | 12 (10.9) | 8 (8.0) | 0.316 |
| Late delayed
bleeding | 5 (4.5) | 12 (12.0) | 0.028 |
| Operation time
(min, mean ± SD) | 69.4±38.2 | 89.3±34.7 | 0.130 |
| Specimens sized
>40 mm | 8 (7.2) | 10 (10.0) | 0.323 |
Early delayed bleeding
Among the 210 patients, 20 (9.5%) demonstrated early
delayed bleeding following ESD. However, no statistically
significant differences were identified between patients with SLE
and non-SLE groups (P>0.05). The flat gross type (P<0.01),
ulcer (P<0.01) and specimen size >40 mm (4.6 vs. 30%;
P<0.001) was associated with an increased risk of early delayed
bleeding. Furthermore, artificial ulcer diameter (2.61±1.20 vs.
4.06±1.73 cm; P<0.001) was associated with significantly higher
early delayed bleeding rates (Table
II). Univariate analysis (Table
III) revealed that early delayed bleeding was associated with
ulcer, flat gross type, artificial ulcer diameter (>3 cm) and
the resected tumor size (>40 mm). Multivariate analysis
(Table IV) revealed that the flat
gross type (OR=16.315; 95% CI: 2.874–92.625; P<0.01), ulcer
(OR=1.052; 95% CI: 1.011–1.094; P<0.05), the resected tumor size
>40 mm (OR=1.189; 95% CI: 1.111–1.272; P<0.01) and artificial
ulcer diameter (>3 cm; OR=1.226; 95% CI: 1.118–1.345;
P<0.001) were independently associated with early delayed
bleeding.
 | Table II.Analysis of risk factors related to
early delayed bleeding. |
Table II.
Analysis of risk factors related to
early delayed bleeding.
| Variable | No bleeding
(N=173) | Early delayed
bleeding (N=20) | P-value |
|---|
| Age (years, mean ±
SD) | 57.9±9.5 | 61.7±10.1 | 0.290 |
| Male sex | 120 (69.4) | 18 (90.0) | 0.066 |
| Location |
|
| 0.571 |
| Upper
1/3 | 35 (20.3) | 6 (30.0) |
|
|
Middle1/3 | 62 (35.8) | 2 (10.0) |
|
| Lower
1/3 | 76 (43.9) | 12 (60.0) |
|
| Ulcer | 18 (10.4) | 7 (35.0) | 0.007 |
| Gross type |
|
| 0.030 |
|
Elevated | 98 (56.6) | 5 (25.0) |
|
|
Flat | 12 (6.9) | 6 (30.0) |
|
|
Depressed | 63 (36.4) | 9 (45.0) |
|
| Lesion diameter
(cm, mean ± SD) | 2.29±1.24 | 3.61±1.43 | 0.221 |
| Artificial ulcer
diameter (cm, mean ± SD) | 2.61±1.20 | 4.06±1.73 | 0.000 |
| The resected tumor
of >40 mm | 8 (4.6) | 6 (30.0) | 0.000 |
| Operation time
(min, mean ± SD) | 61.57±18.58 | 78.82±24.40 | 0.240 |
| Pathology |
|
| 0.537 |
| Well-differentiated
carcinoma | 141(81.5) | 18 (90.0) |
|
| Poorly
differentiated carcinoma | 32 (18.5) | 2 (10.0) |
|
| Depth of
invasion |
|
| 0.787 |
| Mucous layer | 64 (37.0) | 4 (20.0) |
|
| Mucosal muscular
layer | 99 (57.2) | 11 (55.0) |
|
| Submucosa
layer | 10 (5.8) | 5 (25.0) |
|
| Helicobacter
pylori infection | 149 (86.1) | 18 (90.0) | 0.631 |
 | Table III.Univariate analysis of factors
associated with early delayed bleeding after endoscopic submucosal
dissection. |
Table III.
Univariate analysis of factors
associated with early delayed bleeding after endoscopic submucosal
dissection.
| Variable | OR (95% CI) | P-value |
|---|
| Age (>60 vs. ≤60
years) | 0.993
(0.957–1.030) | 0.698 |
| Sex (male vs.
female) | 4.038
(0.903–18.065) | 0.068 |
| Location of lesion
(upper third vs. other locations) | 0.394
(0.050–3.134) | 0.379 |
| Ulcer (yes vs.
no) | 4.631
(1.592–13.476) | 0.005 |
| Gross type (flat
gross type vs. other types) | 8.500
(1.856–38.938) | 0.006 |
| Lesion diameter
(>2 vs. ≤2 cm) | 0.973
(0.878–1.079) | 0.607 |
| Artificial ulcer
diameter (>3 vs. ≤3 cm) | 1.123
(1.020–1.237) | 0.019 |
| The resected tumor
size (>40 vs. ≤40 mm) | 8.455
(2.553–27.998) | <0.001 |
| Operation time
(>60 vs. ≤60 min) | 1.018
(0.991–1.045) | 0.190 |
| Degree of
differentiation (poorly. vs. well differentiated carcinoma) |
0.272(0.056–1.328) | 0.107 |
| Depth of
infiltration (mucous layer vs. all others) | 0.321
(0.089–1.163) | 0.084 |
| Helicobacter
pylori infection (yes vs. no) | 1.008
(0.210–4.835) | 0.992 |
 | Table IV.Multivariate analysis of factors
associated with early delayed bleeding after endoscopic submucosal
dissection. |
Table IV.
Multivariate analysis of factors
associated with early delayed bleeding after endoscopic submucosal
dissection.
| Parameter | OR (95% CI) | P-value |
|---|
| Flat gross type
(flat gross vs all other gross types) | 16.315
(2.874–92.625) | 0.002 |
| Ulcer (yes vs.
no) | 1.052
(1.011–1.094) | 0.012 |
| The resected tumor
size (>40 vs. ≤40 mm) | 1.189
(1.111–1.272) | 0.007 |
| Artificial ulcer
diameter (>3 vs. ≤3 cm) | 1.226
(1.118–1.345) | <0.001 |
Late delayed bleeding
Among the 210 patients, 17 (8.1%) had late delayed
bleeding after ESD and statistically significant differences were
identified between the patients with and without SLE (P<0.05).
The median interval between bleeding and ESD among these 17
patients was 1 day (range, 1–10 days) and operation time
(61.57±18.58 vs. 78.82±24.40 min P=0.001) increased the risk of
late delayed bleeding (Table V).
Bleeding was successfully stopped in all patients during SLE and
none of the patients required re-operation. No re-bleeding occurred
during the follow-up in the 173 patients without delayed bleeding.
Among the 17 patients with delayed bleeding, 2 (11.8%) required
blood transfusion.
 | Table V.Analysis of risk factors related to
late delayed bleeding. |
Table V.
Analysis of risk factors related to
late delayed bleeding.
| Variable | No bleeding
(N=173) | Late delayed
bleeding (N=17) | P-value |
|---|
| Age (years, mean ±
SD) | 57.9±9.5 | 57.1±10.6 | 0.740 |
| Male sex | 120 (69.4) | 16 (94.1) | 0.045 |
| Location |
|
| 0.119 |
| Upper
1/3 | 35 (20.3) | 5 (29.4) |
|
|
Middle1/3 | 62 (35.8) | 4 (23.5) |
|
| Lower
1/3 | 76 (43.9) | 8 (47.1) |
|
| Ulcer | 18 (10.4) | 8 (47.1) | 0.000 |
| Gross type |
|
| 0.011 |
|
Elevated | 98 (56.6) | 10 (58.8) |
|
|
Flat | 12 (6.9) | 3 (17.6) |
|
|
Depressed | 63 (36.4) | 4 (23.5) |
|
| Lesion diameter
(cm, mean ± SD) | 2.29±1.24 | 3.61±1.43 | 0.006 |
| Artificial ulcer
diameter (cm, mean ± SD) | 2.61±1.20 | 4.06±1.73 | 0.595 |
| The resected tumor
of >40 mm | 8 (4.6) | 4 (23.5) | 0.014 |
| Operation time
(min, mean ± SD) | 61.57±18.58 | 78.82±24.40 | 0.001 |
| Pathology |
|
| 0.535 |
| Well-differentiated
carcinoma | 141 (81.5) | 13 (76.5) |
|
| Poorly
differentiated carcinoma | 32 (18.5) | 4 (23.5) |
|
| Depth of
invasion |
|
| 0.182 |
| Mucous layer | 64 (37.0) | 3 (17.6) |
|
| Mucosal muscular
layer | 99 (57.2) | 8 (47.1) |
|
| Submucosa
layer | 10 (5.8) | 6 (35.3) |
|
| Helicobacter
pylori infection | 149 (86.1) | 10 (58.8) | 0.009 |
Seventeen cases of late delayed bleeding were
divided into 3 types: Pulsatile bleeding (n=8; Forrest grade I),
active permeating bleeding (n=6; Forrest grade IIa) and vessel
exposure (n=3; Forrest grade IIb). One patient underwent SLE on the
second day after ESD, but delayed bleeding occurred on the tenth
day after ESD. The patient had a remnant stomach with the lesion
located on the anterior wall of the gastric antrum, and the size of
the excised lesion was 1.5×1.5 cm.
Univariate analysis (Table VI) revealed that late delayed
bleeding was associated with male sex, ulcer, flat gross type,
lesion diameter (>2 cm), specimen size of >40 mm, longer
operative time (>60 min; 61.57±18.58 vs. 78.82±24.40 min;
P<0.01) and H. pylori infection (86.1 vs. 58.8%;
P<0.01). Multivariate analysis (Table VII) revealed that ulcer [odds ratio
(OR)=3.752, 95% confidence interval (CI): 3.202–7.052, P<0.05],
flat gross type (OR=4.229, 95% CI: 1.355–14.258, P<0.05), lesion
diameter (OR=1.470, 95% CI: 1.047–2.064, P<0.05), specimen size
of >40 mm (OR=1.139, 95% CI: 0.988–1.314, P<0.01) and H.
pylori infection (OR=1.112, 95% CI: 0.309–1.225, P<0.01)
were independently associated with the occurrence of late delayed
bleeding.
 | Table VI.Univariate analysis of the
association of various clinicopathological and surgical parameters
with the incidence of late delayed bleeding after endoscopic
submucosal dissection. |
Table VI.
Univariate analysis of the
association of various clinicopathological and surgical parameters
with the incidence of late delayed bleeding after endoscopic
submucosal dissection.
| Variable | OR (95% CI) | P-value |
|---|
| Age (>60 vs. ≤60
years) | 0.963
(0.910–1.019) | 0.189 |
| Sex (male vs.
female) | 8.210
(1.055–63.869) | 0.044 |
| Location of lesion
(upper third vs. other locations) |
1.676(0.446–6.302) | 0.445 |
| Ulcer (yes vs.
no) | 6.791
(1.963–23.496) | 0.002 |
| Gross type (flat
gross type vs. other types) | 6.012
(1.338–27.006) | 0.019 |
| Lesion diameter
(>2 vs. ≤2 cm) | 1.174
(1.064–1.295) | 0.001 |
| Artificial ulcer
diameter (>3 vs. ≤3 cm) | 0.992
(0.933–1.055) | 0.800 |
| The resected tumor
size (>40 vs. ≤40 mm) | 8.040
(2.003–32.273) | 0.003 |
| Operation time
(>60 vs. ≤60 min) | 1.034
(1.010–1.058) | 0.005 |
| Degree of
differentiation (poorly vs. well differentiated) | 1.023
(0.261–4.006) | 0.973 |
| Depth of
infiltration (mucous layer vs. all others) | 0.343
(0.075–1.570) | 0.168 |
| Helicobacter
pylori infection (yes vs. no) | 0.022
(0.003–0.182) | <0.001 |
 | Table VII.Multivariate analysis of factors
associated with late delayed bleeding after endoscopic submucosal
dissection. |
Table VII.
Multivariate analysis of factors
associated with late delayed bleeding after endoscopic submucosal
dissection.
| Parameter | OR (95% CI) | P-value |
|---|
| Sex (male vs.
female) | 0.007
(0.202–3.013) | 0.775 |
| Ulcer (yes vs.
no) | 3.752
(3.202–7.052) | 0.031 |
| Flat gross type
(vs. all other gross types) | 4.229
(1.355–14.258) | 0.013 |
| Lesion diameter
(>2 vs. ≤2 cm) | 1.470
(1.047–2.064) | 0.026 |
| Operation time
(>60 vs. ≤60 min) | 1.099
(0.976–1.238) | 0.119 |
| The resected tumor
size (>40 vs. ≤40 mm) | 1.139
(0.988–1.314) | 0.002 |
| Helicobacter
pylori infection (yes vs. no) | 1.112
(0.309–1.225) | 0.002 |
Discussion
It is controversial whether SLE is able to prevent
delayed bleeding after ESD for gastric cancer. Therefore, the
present study aimed to evaluate whether SLE is able to prevent
delayed bleeding after ESD and clarified the types of lesions that
require SLE. The results suggest that SLE was effective in
preventing delayed bleeding after ESD, particularly within 48 h
after ESD. Lesion diameter (>2 cm), ulcer, flat gross type, the
resected tumor >40 mm, H. pylori infection and operative
time (>60 min) were independently associated with late delayed
bleeding after ESD, while flat gross type, ulcer, the resected
tumor >40 mm and artificial ulcer diameter (>3 cm) were
independently associated with early delayed bleeding.
Certain studies have indicated that
procedure-associated bleeding is not associated with age, sex,
tumor size and tumor location (21).
In addition, the preventive coagulation of non-bleeding visible
vessels in SLE following gastric ESD may do little to prevent late
delayed bleeding (20), SLE for
preventing delayed bleeding after ESD may be excessively performed
at present and unnecessary in certain patients (16,18,19).
However, the present study suggested that SLE has an important role
after gastric ESD, as it is able to identify and treat potential
bleeding foci. Takizawa et al (23) suggested that coagulation of visible
vessels during ESD prevented delayed bleeding. Therefore, different
approaches may be used to prevent bleeding and avoid a second
endoscopy.
In the present study, the lesions were completely
excised during ESD in all 210 patients who met the criteria for SLE
after ESD. The results indicated that late delayed bleeding was
markedly more common in the non-SLE group, and numerous patients
had H. pylori infection, which may cause a greater local
inflammatory response and further influence the gastric mucosal
blood flow during healing of ESD-induced ulcer, resulting in injury
to the vessel walls. Flat gross type is another risk factor; as
such lesions are frequently rich in vascularity and are mostly
reddish due to the existence of more vessels in the submucosal
layer compared with that in the elevated or depressed type. The
presence of more vessels may increase the risk of post-ESD bleeding
(11). There was no significant
association between age and ESD-associated hemorrhage, which was
inconsistent with the results reported by Takahashi et al
(18). Regarding the post-operative
complications of ESD, the rates of perforation, bleeding, and
lymphatic vessel invasion were lower than those reported in
previous studies (11,14,16–20,24–26),
which may be due to the improvements in therapeutic instruments and
techniques, as well as the absence of positive margins in the 210
patients.
Certain studies have examined the risk factors for
delayed bleeding after ESD. Choi et al (24) determined that surface erosion,
location of the lesion and high-risk ulcer were independently
associated with the risk of delayed bleeding. In a study by Kim
et al (16), a large tumor
size (>20 mm) was the only independent risk factor for delayed
bleeding. Nakamura et al (27) reported that low platelets and
positive lateral margins were associated with delayed bleeding.
Other risk factors include wide resection (14,18,22), no
post-ESD coagulation (23), tumor
located in the lower third of the stomach (22,23),
tumor located in the L segment (18), large tumor size (18), histological ulcer (14), long ESD procedure (14), age of <65 years (26) and use of anti-thrombotic drugs
(26). Ryu et al (19) reported that no specific factor was
associated with delayed bleeding after ESD. In the present study,
ulcer, flat gross type, lesion diameter (>2 cm), the resected
tumor size of >40 mm and H. pylori infection were
independently associated with late delayed bleeding after ESD,
while flat gross type, ulcer, the resected tumor size of >40 mm
and artificial ulcer diameter (>3 cm) were independently
associated with early delayed bleeding. Of note, the present study
is not without limitations. It was a retrospective study, with all
of the inherent limitations, and the sample size was small.
In conclusion, based on the present retrospective
study, SLE after ESD has a certain value in the prevention of
delayed bleeding in patients with gastric cancer after treatment
with ESD, particularly within 48 h after the surgery. Ulcer, flat
gross type, lesion diameter (>2 cm), the resected tumor size of
>40 mm and H. pylori infection were used to identify
those high-risk patients who should ideally be subjected to SLE to
prevent late delayed bleeding.
Acknowledgements
Part of the data of the present study has been
presented as a poster at the 17th Congress of Gastroenterology
China, (Xi'an, China), 14–16 September 2017 (28).
Funding
No funding received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZG wrote the manuscript. LM and HH contributed to
project development and data collection. LC and ZG recorded and
analyzed the results. LC and YX performed the statistical analysis.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of the Second Affiliated Hospital of Nanjing Medical
University and Qilu Hospital of Shandong University. All patients
provided written informed consent for inclusion in the
database.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EGC
|
early gastric cancer
|
|
EMR
|
endoscopic mucosal resection
|
|
ESD
|
endoscopic submucosal dissection
|
|
SLE
|
second-look endoscopy
|
References
|
1
|
Thrumurthy SG, Chaudry MA, Hochhauser D
and Mughal M: The diagnosis and management of gastric cancer. BMJ.
347:f63672013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kohler BA, Ward E, McCarthy BJ, Schymura
MJ, Ries LA, Eheman C, Jemal A, Anderson RN, Ajani UA and Edwards
BK: Annual report to the nation on the status of cancer, 1975–2007,
featuring tumors of the brain and other nervous system. J Natl
Cancer Inst. 103:714–736. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang L: Incidence and mortality of gastric
cancer in China. World J Gastroenterol. 12:17–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salih BA: Helicobacter pylori infection in
developing countries: The burden for how long? Saudi J
Gastroenterol. 15:201–207. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
NCCN Clinical Practice Guidelines in
Oncology (NCCN Guidelines), . Gastric Cancer. Version 2.2017.
National Comprehensive Cancer Network; Fort Washington: 2017
|
|
6
|
Wang S, Zhang Z, Liu M, Li S and Jiang C:
Endoscopic resection compared with gastrectomy to treat early
gastric cancer: A systematic review and meta-analysis. PLoS One.
10:e01447742015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ono H, Yao K, Fujishiro M, Oda I, Nimura
S, Yahagi N, Iishi H, Oka M, Ajioka Y, Ichinose M and Matsui T:
Guidelines for endoscopic submucosal dissection and endoscopic
mucosal resection for early gastric cancer. Dig Endosc. 28:3–15.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Inoue H, Ikeda H, Hosoya T, Yoshida A,
Onimaru M, Suzuki M and Kudo SE: Endoscopic mucosal resection,
endoscopic submucosal dissection, and beyond: Full-layer resection
for gastric cancer with nonexposure technique (CLEAN-NET). Surg
Oncol Clin N Am. 21:129–140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Facciorusso A, Antonino M, Di Maso M and
Muscatiello N: Endoscopic submucosal dissection vs endoscopic
mucosal resection for early gastric cancer: A meta-analysis. World
J Gastrointest Endosc. 6:555–563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park CH, Shin S, Park JC, Shin SK, Lee SK,
Lee YC and Lee H: Long-term outcome of early gastric cancer after
endoscopic submucosal dissection: Expanded indication is comparable
to absolute indication. Dig Liver Dis. 45:651–656. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goto O, Fujishiro M, Kodashima S, Ono S,
Niimi K, Hirano K, Yamamichi N and Koike K: A second-look endoscopy
after endoscopic submucosal dissection for gastric epithelial
neoplasm may be unnecessary: A retrospective analysis of
postendoscopic submucosal dissection bleeding. Gastrointest Endosc.
71:241–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kataoka Y, Tsuji Y, Sakaguchi Y, Minatsuki
C, Asada-Hirayama I, Niimi K, Ono S, Kodashima S, Yamamichi N,
Fujishiro M and Koike K: Bleeding after endoscopic submucosal
dissection: Risk factors and preventive methods. World J
Gastroenterol. 22:5927–5935. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang KJ, Kim KM, Min BH, Lee JH and Kim
JJ: Endoscopic submucosal dissection of early gastric cancer. Gut
Liver. 5:418–426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ono S, Ono M, Nakagawa M, Shimizu Y, Kato
M and Sakamoto N: Delayed bleeding and hemorrhage of mucosal
defects after gastric endoscopic submucosal dissection on
second-look endoscopy. Gastric Cancer. 19:561–567. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akintoye E, Obaitan I, Muthusamy A, Akanbi
O, Olusunmade M and Levine D: Endoscopic submucosal dissection of
gastric tumors: A systematic review and meta-analysis. World J
Gastrointest Endosc. 8:517–532. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim JS, Chung MW, Chung CY, Park HC, Ryang
DY, Myung DS, Cho SB, Lee WS and Joo YE: The need for second-look
endoscopy to prevent delayed bleeding after endoscopic submucosal
dissection for gastric neoplasms: A prospective randomized trial.
Gut Liver. 8:480–486. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mochizuki S, Uedo N, Oda I, Kaneko K,
Yamamoto Y, Yamashina T, Suzuki H, Kodashima S, Yano T, Yamamichi
N, et al: Scheduled second-look endoscopy is not recommended after
endoscopic submucosal dissection for gastric neoplasms (the SAFE
trial): A multicentre prospective randomised controlled
non-inferiority trial. Gut. 64:397–405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takahashi F, Yoshitake N, Akima T, Kino H,
Nakano M, Tsuchida C, Tsuchida K, Tominaga K, Sasai T, Masuyama H
and Hiraishi H: A second-look endoscopy may not reduce the bleeding
after endoscopic submucosal dissection for gastric epithelial
neoplasm. BMC Gastroenterol. 14:1522014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ryu HY, Kim JW, Kim HS, Park HJ, Jeon HK,
Park SY, Kim BR, Lang CC and Won SH: Second-look endoscopy is not
associated with better clinical outcomes after gastric endoscopic
submucosal dissection: A prospective, randomized, clinical trial
analyzed on an as-treated basis. Gastrointestl Endosc. 78:285–294.
2013. View Article : Google Scholar
|
|
20
|
Na S, Ahn JY, Choi KD, Kim MY, Lee JH,
Choi KS, Kim DH, Song HJ, Lee GH, Jung HY and Kim JH: Delayed
bleeding rate according to the Forrest classification in
second-look endoscopy after endoscopic submucosal dissection. Dig
Dis Sci. 60:3108–3117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Isomoto H, Shikuwa S, Yamaguchi N, Fukuda
E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J and Kohno
S: Endoscopic submucosal dissection for early gastric cancer: A
large-scale feasibility study. Gut. 58:331–336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okada K, Yamamoto Y, Kasuga A, Omae M,
Kubota M, Hirasawa T, Ishiyama A, Chino A, Tsuchida T, Fujisaki J,
et al: Risk factors for delayed bleeding after endoscopic
submucosal dissection for gastric neoplasm. Surg Endosc. 25:98–107.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takizawa K, Oda I, Gotoda T, Yokoi C,
Matsuda T, Saito Y, Saito D and Ono H: Routine coagulation of
visible vessels may prevent delayed bleeding after endoscopic
submucosal dissection-an analysis of risk factors. Endoscopy.
40:179–183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi CW, Kim HW, Kang DH, Hong YM, Kim SJ,
Park SB, Cho M, Kim DJ and Hong JB: Clinical outcomes of
second-look endoscopy after gastric endoscopic submucosal
dissection: Predictive factors with high risks of bleeding. Surg
Endosc. 28:2213–2220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fujishiro M, Abe N, Endo M, Kawahara Y,
Shimoda R, Nagata S, Homma K, Morita Y and Uedo N: Current
managements and outcomes of peptic and artificial ulcer bleeding in
Japan. Dig Endosc. 22 Suppl 1:S9–S14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tano S, Horiki N, Omata F, Tanaka K,
Hamada Y, Katsurahara M, Ninomiya K, Nishikawa K, Nojiri K, Yamada
R, et al: Second and third-look endoscopy for the prevention of
post-ESD bleeding. Medicine (Baltimore). 94:e4912015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakamura M, Nishikawa J, Hamabe K,
Nishimura J, Satake M, Goto A, Kiyotoki S, Saito M, Fukagawa Y,
Shirai Y, et al: Risk factors for delayed bleeding from endoscopic
submucosal dissection of gastric neoplasms. Scand J Gastroenterol.
47:1108–1114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo Z, Xin Y, Dai H and Cao J: Second-look
endoscopy could be effective in preventing delayed bleeding after
gastric endoscopic submucosal dissection. Abstract No: PO-937. In:
The 17th Congress of Gastroenterology China (CGC). 2017.
|