Introduction
Infected abdominal aortic aneurysm (IAAA) is a
special type of abdominal aortic aneurysm (AAA), accounting for
0.7–3.0% of all AAA (1), and
approximately 1–3% of infected aneurysms (2). The disease is not easy to be found in
the early stage, and the missed diagnosis and misdiagnosis rate is
high. Once the rupture occurs, it risks the lives of patients
directly and brings huge economic burden to the patients as well as
their families. In some developed countries, the screening of
high-risk population of IAAA may effectively increase the survival
rate of patients (3–5). At present, the main molecular mechanism
of IAAA has not yet been identified, and most scholars believe that
the disease is caused by genetic, inflammation and other
factors.
Micro ribonucleic acids (miRNAs) are a class of
endo-genous, non-coding small molecule RNA, which regulate the
expression of target genes by inhibiting or promoting the lysis of
messenger RNA (mRNA) through specific translation of sequences,
resulting in the different impacts of biological behavior of tumors
(6). Many studies have found that
miRNA is expressed in other cancers and diseases. miRNA-141
(miR-141), a member of the miRNA-200 family, plays an important
role in the occurrence, development and physiological status of
diseases by regulating different signaling pathways (7). Research has shown that (8) miR-141 is closely related to tumor and
angiogenesis. In the study of Liu et al (9), through the analysis of 7 GEO chips, it
was found that miR-141 was differentially expressed in the tissues
of liver cancer patients. miRNA-183 (miR-183), located on human
7q32.2 chromosome, is one of the most important members of the
miR-183 family. In addition, research has shown that miR-183 is
closely related to tumor occurrence and recurrence (10). The study of Fan et al
(11) has shown that the
differential expression of miR-183 in breast cancer patients was
found through the analysis of GEO (GSE39093) chip. However, it is
not clear whether miR-141, miR-183 and IAAA are related. Some
studies have shown that (12,13)
miR-183 and miR-141 are highly expressed in TB patients, while TB
bacillus, as the main pathogenic microorganism of IAAA, has not
been reported in relevant studies before. The relationship between
miR-183, miR-141 and clinicopathological characteristics as well as
follow-up data were studied by detecting the expression levels of
miR-141 and miR-183 in IAAA to explore whether miR-183 and miR-141
were potential risk factors for the poor prognosis of patients with
IAAA.
Patients and methods
Sample source
Thirty-six patients with IAAA admitted and who
received vascular surgery in People's Hospital of Shenzhen
(Shenzhen, China) from June 2003 to June 2013 were collected and
selected, the average age of the patients was 41±16.1 years, and
they were diagnosed with IAAA by computerized tomography (CT)
scanning and abdominal magnetic resonance imaging (MRI) in the
imaging department of the hospital. There were 20 male patients and
16 female patients. The samples and adjacent tissues 1 cm away from
the aneurysm collected from surgical resection were stored in
liquid nitrogen within 5 min. None of the patients received
radiotherapy, chemotherapy or other anticancer therapies before
operation. The study was approved by the Ethics Committee of
People's Hospital of Shenzhen, and the family members and patients
were informed and signed the informed consent form.
Reagents and main equipment
TRIzol reagent and miRNA reverse transcriptase kit
were purchased from Invitrogen; Thermo Fisher Scientific, Inc.
(Waltham, MA, USA); SYBR Green Master Mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.) and ABI Prism 7900PCR instrument
was purchased from Applied Biosystems; Thermo Fisher Scientific,
Inc., and the reverse transcription primers and internal reference
primers were synthesized by Shanghai Biological Engineering Co.
Ltd. (Shanghai, China).
RNA extraction
The extraction procedure of the total RNA from the
collected aneurysm and adjacent tissues by TRIzol reagent was
carried out according to the instructions. The concentration and
purity of the extracted RNA were detected by ultraviolet
spectrophotometer (Bio Rad, Hercules, CA, USA), and the integrity
of RNA was detected by agarose gel electrophoresis.
Synthesis of complementary
deoxyribonucleic acid (cDNA)
The miRNAs of the total RNA were reversely
transcribed to synthesize cDNA following the instructions of the
miRNA reverse transcription kit. The reverse transcription
conditions of miRNA-183 and miRNA-141 were as follows: 37°C for 30
min, and then 95°C for 3 min. The synthesized cDNA solution was
stored at −20°C.
Detection of expression levels of
miRNA-183 and miRNA-141 via reverse transcription polymerase chain
reaction (RT-PCR)
BI Prism 7900 PCR instrument was used for PCR. The
reaction system was 25 µl, including 12 µl SYBR Green Master Mix, 3
µl of 10X miRNA specific primers, 2.5 µl 10X universal primer, 2.5
µl diluted cDNA, and RNase-free double distilled water was added up
to 25 µl. For miRNA-183, upstream primer:
5′-CGTTGGATTCCTATGGCACTGGT-3′ and downstream primer:
5′-TTCAAGCAGGGTCCGAGGTATTC-3′; for miRNA-141, upstream primer:
5′-TTCCGATGGCGTAACACTGTCTG-3′ and downstream primer:
5′-TTCAAGCAGGGTCCGAGGTATTC-3′; the reaction conditions were as
follows: 95°C for 5 min, 95°C for 30 sec, 60°C for 45 sec, and 72°C
for 45 sec; a total of 40 cycles. U6 was used as reaction internal
reference. All samples were repeated in 3 wells, and the results
were analyzed by 2−∆∆Cq method (14).
Statistical analysis
These experimental data were analyzed by Statistical
Product and Service Solutions (SPSS) 19.0 software package (IBM
Corp., Armonk, NY, USA), measurement data were expressed as mean ±
standard deviation, enumeration data were tested by χ2
test, data between groups were compared by the independent sample
Student's t-test and survival data were analyzed by Cox regression
analysis. P<0.05 suggested that the difference was statistically
significant.
Results
Expression levels of miRNA-183 and
miRNA-141 in IAAA tissues and adjacent tissues
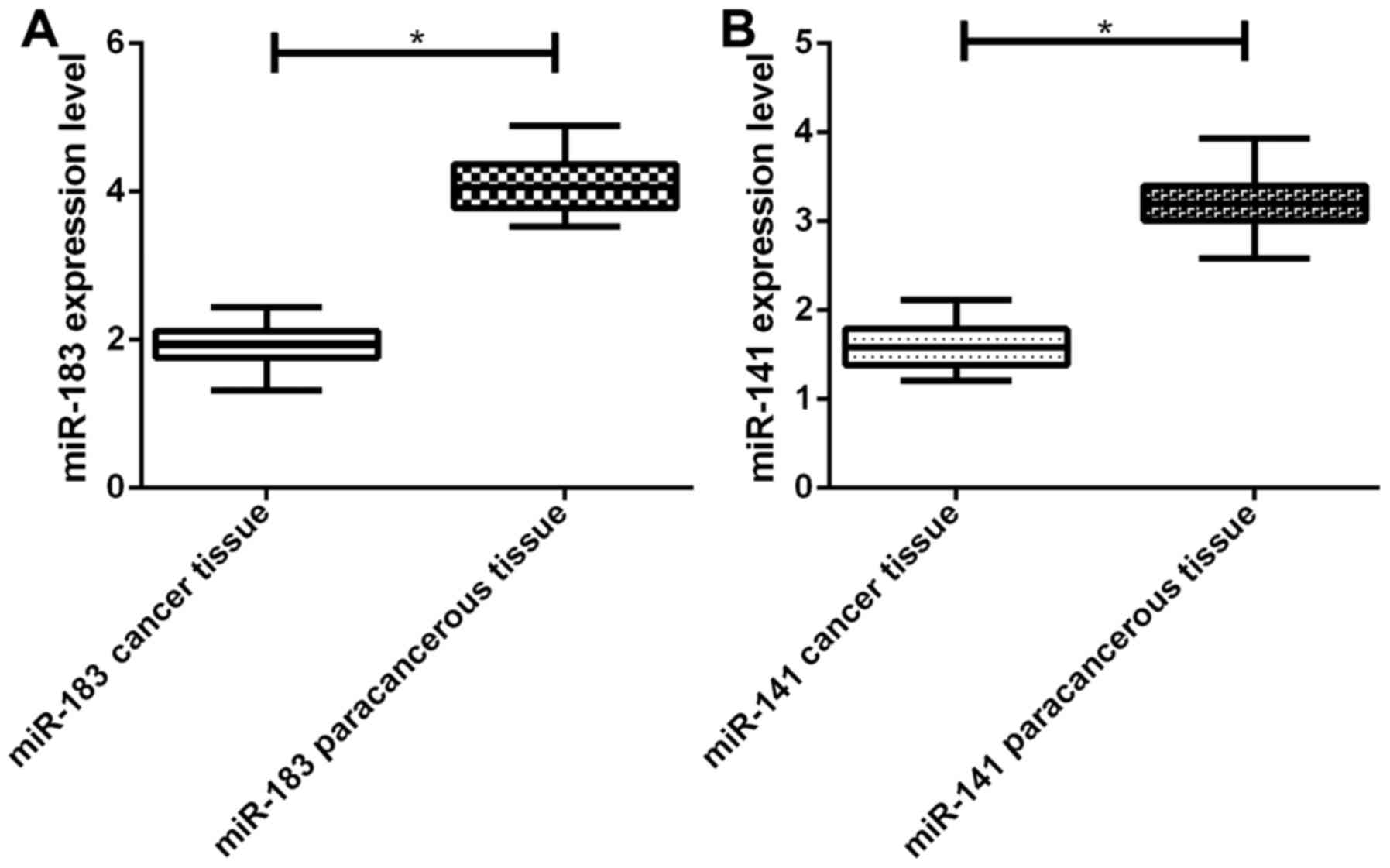
Detection of tissues in 36 patients with IAAA by
RT-qPCR revealed that the relative expression levels of RNA-183 and
miRNA-141 in IAAA tissues were significantly lower compared to
those in the adjacent tissues, and the difference was statistically
significant (P<0.05) (Fig.
1).
Analysis of correlation between IAAA
clinical factors and expression levels of miRNA-183 and
miRNA-141
Through the expression levels of miRNA-183 and
miRNA-141 and clinicopathological features, we found that there was
no statistical significance (P>0.05) between the expression
levels of miR-183 and miR-141 and sex, age, history of hypertension
or alcoholism, but there was statistical significance in patients
with smoking history and aneurysm size (P<0.05) (Table I).
 | Table I.Analysis of clinical factors of IAAA
and expression levels of miRNA-183 and miRNA-141. |
Table I.
Analysis of clinical factors of IAAA
and expression levels of miRNA-183 and miRNA-141.
| Groups | n | Expression level of
miRNA-183 | χ2
value | P-value | Expression level of
miRNA-141 | χ2
value | P-value |
|---|
| Sex |
|
| 1.331 | 0.267 |
| 0.224 | 0.755 |
| Male | 20 | 1.825±0.417 |
|
| 1.247±0.389 |
|
|
|
Female | 16 | 1.617±0.674 |
|
| 1.384±0.247 |
|
|
| Age |
|
| 0.354 | 0.651 |
| 0.574 | 0.511 |
| ≥55 | 17 | 1.687±0.644 |
|
| 1.174±0.217 |
|
|
|
<55 | 19 | 1.715±0.347 |
|
| 1.089±0.373 |
|
|
| History of
hypertension |
|
| 2.219 | 0.212 |
| 0.667 | 0.493 |
| Yes | 21 | 1.574±0.734 |
|
| 1.294±0.241 |
|
|
| No | 15 | 1.674±0.684 |
|
| 1.188±0.299 |
|
|
| Alcoholism |
|
| 0.063 | 0.497 |
| 0.684 | 0.572 |
| Yes | 16 | 1.674±0.774 |
|
| 1.177±0.244 |
|
|
| No | 20 | 1.578±0.627 |
|
| 1.341±0.274 |
|
|
| Smoking history |
|
| 0.184 | 0.577 |
| 0.441 | 0.617 |
| Yes | 20 | 1.684±0.547 |
|
| 1.147±0.379 |
|
|
| No | 16 | 1.841±0.454 |
|
| 1.214±0.214 |
|
|
| Tumor size |
|
| 4.513 | 0.031 |
| 3.847 | 0.042 |
| ≥10
mm | 22 | 1.324±0.241 |
|
| 0.841±0.102 |
|
|
| <10
mm | 14 | 1.784±0.404 |
|
| 1.347±0.341 |
|
|
Cox regression survival analysis
In this experiment, the 3-year overall survival rate
after surgery of 36 patients was 41.6% (15/36). Univariate analysis
of the collected clinicopatho-logical features found that sex, age,
history of hypertension, alcoholism, smoking history, and aneurysm
location were not related to prognosis (P>0.05), but low
expression of miR-183, low expression of miR-141, and aneurysm size
in patients were significantly related to prognosis (Table II). The results of subsequent Cox
multivariate analysis showed that low expression of miR-183 [hazard
ratio (HR)=3.587, 95% confidence interval (95% CI): 2.641–6.541,
P=0.014] and low expression of miR-141 (HR=3.841 95% CI:
2.894–5.981, P=0.016) could be used as independent prognostic
factors for survival rate (Table
III).
 | Table II.Univariate analysis. |
Table II.
Univariate analysis.
|
| Single factor |
|---|
|
|
|
|---|
| Factors | HR (95% CI) | P-value |
|---|
| Age | 1.541
(1.122–3.895) | 0.062 |
| Sex | 1.714
(1.399–1.654) | 0.788 |
| History of
hypertension | 0.813
(0.375–1.727) | 0.174 |
| Alcoholism | 2.143
(1.288–2.755) | 0.034 |
| Smoking
history | 1.241
(0.355–2.943) | 0.237 |
| Tumor size | 2.064
(1.282–5.723) | 0.064 |
| Low expression of
miR-183 | 2.124
(1.557–4.479) | 0.002 |
| Low expression of
miR-141 | 2.315
(1.231–3.524) | 0.004 |
 | Table III.Multivariate analysis. |
Table III.
Multivariate analysis.
|
| Multiple
factor |
|---|
|
|
|
|---|
| Factors | HR (95% CI) | P-value |
|---|
| Tumor size | 0.974
(0.384–1.894) | 0.074 |
| Low expression of
miR-183 | 3.587
(2.641–6.541) | 0.014 |
| Low expression of
miR-141 | 3.841
(2.894–5.981) | 0.016 |
Discussion
The current traditional therapy for IAAA is surgical
operation, including local excision of aneurysm tissues,
debridement at a large area, and then vascular reconstruction in
the outside bypass of in situ disease (13). However, IAAA patients have a sudden
onset of disease, and the majority of patients are admitted to
hospital due to rupture of aneurysm and hemorrhage. As the
condition is relatively serious and there are a variety of
complications, the disease is difficult to treat with traditional
surgery. The mortality rate during operation is high and the
survival rate is not ideal (15).
miRNAs have been found closely related to the
occurrence, development, invasion and metastasis of tumors. They
are a kind of molecular biological target. miRNAs match the target
by the principle of complementary base pairing to degrade the
target mRNA and inhibit the translation process to achieve the
target gene differential expression (6,16,17).
Therefore, to the best of our knowledge, we studied the expression
of miRNA in IAAA patients for the first time, hoping to explore the
expression of miRNAs and adverse prognosis in IAAA patients.
miRNA-183 is a member of the miRNA-183 family
(mainly including miRNA-183, miRNA-182 and miRNA-96), located on
the human 7q32.3 chromosome, involved in the occurrence and
development of a variety of tumors and closely related to their
physiological processes (18,19).
miRNA-141 is a member of the miRNA-200 family, which plays a major
role in the stable direction of epithelial cells. When the
expression disorders occur, it leads to cell epithelial-mesenchymal
transition increase, resulting in proliferation and metastasis of
epithelial tumors (20,21). This study on the detection of the
expression levels of miRNA-183 and miRNA-141 in aneurysm and
adjacent tissues in 36 cases of IAAA patients revealed that the
expression of miRNA-183 and miRNA-141 in IAAA patients was low, and
compared with the expression levels in the adjacent tissues, there
was statistical significance (P<0.05). This may indicate that
these two kinds of miRNA are associated with the clinicobiological
behavior of IAAA patients. The downregulation of miRNA-183
expression in hepatocellular carcinoma is mentioned in the study of
Li et al (22), and the
downregulation of miRNA-141 in gastric cancer is also noted by Du
et al (23), which may
indicate that different miRNAs have the same expression in
different cancers. The correlation analysis of the clinical factors
of IAAA patients showed that there was no statistical significance
between the expression of miR-183 and miR-141 and sex, age, history
of hypertension, alcoholism or aneurysm location (P>0.05), but
there was statistical significance in patients with smoking
history, and the aneurysm size (P<0.05). Finally, Cox regression
analysis showed the low expression levels of miRNA-183 and
miRNA-141 could be used as independent prognostic factors for IAAA
patients. The main treatments of IAAA are antibiotic therapy and
thorough debridement for in situ vascular reconstruction.
However, as the course and the causes of IAAA are not clear at
present, the location of the lesions is deep and the adjacent
organs are difficult to handle when they are affected, resulting in
the rupture of the aneurysm, which is mostly fatal (24). Therefore, we detected miRNA in IAAA
patients and found better detection methods through this study to
prevent the occurrence of the disease.
However, this study has certain limitations. As
samples were difficult to collect and the number of samples was
small, there was bias on the results, and there were also
differences among different races. The current clinical trials are
not sufficient on this subject. We hope to increase the collection
of samples and increase animal experiments to support our results
in future studies.
Collectively, miRNA is related to the occurrence and
development of IAAA, but specific impact needs to be proven by test
and study in the future. The results of this study indicate that
downregulation of miRNA-183 and miRNA-141 is one of the
pathogenesis of IAAA patients, and the lower the expression level
is, the worse the prognosis gets, which can be used as a marker of
prognosis in patients with IAAA. Thus, a new method is provided for
the detection of IAAA, laying the foundation for future study of
IAAA patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CM and ZG conceived and designed the study. CM, DL,
HL and JZ were responsible for the collection and analysis of the
data. CM, DW and BL interpreted the data and drafted the
manuscript. ZG and DL revised the manuscript critically for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
People's Hospital of Shenzhen (Shenzhen, China). Signed informed
consents were obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maeda H, Umezawa H, Goshima M, Hattori T,
Nakamura T, Umeda T and Shiono M: Primary infected abdominal aortic
aneurysm: Surgical procedures, early mortality rates, and a survey
of the prevalence of infectious organisms over a 30-year period.
Surg Today. 41:346–351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laser A, Baker N, Rectenwald J, Eliason
JL, Criado-Pallares E and Upchurch GR Jr: Graft infection after
endovascular abdominal aortic aneurysm repair. J Vasc Surg.
54:58–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Greenhalgh RM, Brown LC, Powell JT,
Thompson SG, Epstein D and Sculpher MJ; United Kingdom EVAR Trial
Investigators, : Endovascular versus open repair of abdominal
aortic aneurysm. N Engl J Med. 362:1863–1871. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moll FL, Powell JT, Fraedrich G, Verzini
F, Haulon S, Waltham M, van Herwaarden JA, Holt PJ, van Keulen JW,
Rantner B, et al: European Society for Vascular Surgery: Management
of abdominal aortic aneurysms clinical practice guidelines of the
European society for vascular surgery. Eur J Vasc Endovasc Surg. 41
Suppl 1:S1–S58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moulakakis KG, Dalainas I, Mylonas S,
Giannakopoulos TG, Avgerinos ED and Liapis CD: Conversion to open
repair after endografting for abdominal aortic aneurysm: A review
of causes, incidence, results, and surgical techniques of
reconstruction. J Endovasc Ther. 17:694–702. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mestdagh P, Hartmann N, Baeriswyl L,
Andreasen D, Bernard N, Chen C, Cheo D, D'Andrade P, DeMayo M,
Dennis L, et al: Evaluation of quantitative miRNA expression
platforms in the microRNA quality control (miRQC) study. Nat
Methods. 11:809–815. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siddiqui S, Zlock L, Jun D, Bonser LR,
Finkbeiner W, Erle DJ and Woodruff PG: The role of miR-141 in
IL-13-mediated mucus production. A40. Epithelial Regulation of
Inflammation. American Thoracic Society. A1489. 2016.https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2016.193.1_MeetingAbstractsA1489.
|
|
8
|
Tamagawa S, Beder LB, Hotomi M, Gunduz M,
Yata K, Grenman R and Yamanaka N: Role of miR-200c/miR-141 in the
regulation of epithelial-mesenchymal transition and migration in
head and neck squamous cell carcinoma. Int J Mol Med. 33:879–886.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu CZ, Ye ZH, Ma J, He RQ, Liang HW, Peng
ZG and Chen G: A qRT-PCR and gene functional enrichment study
focused on downregulation of miR-141-3p in hepatocellular carcinoma
and its clinicopathological significance. Technol Cancer Res Treat.
Jan 1–2017.(Epub ahead of print). View Article : Google Scholar
|
|
10
|
Kundu ST, Byers LA, Peng DH, Roybal JD,
Diao L, Wang J, Tong P, Creighton CJ and Gibbons DL: The miR-200
family and the miR-183~96~182 cluster target Foxf2 to inhibit
invasion and metastasis in lung cancers. Oncogene. 35:173–186.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan J, Jia L, Li Y, Ebrahim S, May-Simera
H, Wood A, Morell RJ, Liu P, Lei J, Kachar B, et al: Maturation
arrest in early postnatal sensory receptors by deletion of the
miR-183/96/182 cluster in mouse. Proc Natl Acad Sci USA.
114:E4271–E4280. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cui JY, Liang HW, Pan XL, Li D, Jiao N,
Liu YH, Fu J, He XY, Sun GX, Zhang CL, et al: Characterization of a
novel panel of plasma microRNAs that discriminates between
Mycobacterium tuberculosis infection and healthy individuals. PLoS
One. 12:e01841132017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou M, Yu G, Yang X, Zhu C, Zhang Z and
Zhan X: Circulating microRNAs as biomarkers for the early diagnosis
of childhood tuberculosis infection. Mol Med Rep. 13:4620–4626.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lai CH, Luo CY, Lin PY, Kan CD, Chang RS,
Wu HL and Yang YJ: Surgical consideration of in situ prosthetic
replacement for primary infected abdominal aortic aneurysms. Eur J
Vasc Endovasc Surg. 42:617–624. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Volinia S, Calin GA, Liu C-G, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng ZM and Wang X: Regulation of
cellular miRNA expression by human papillomaviruses. Biochim
Biophys Acta. 1809:668–677. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li H, Kloosterman W and Fekete DM:
MicroRNA-183 family members regulate sensorineural fates in the
inner ear. J Neurosci. 30:3254–3263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang M, Liu R, Li X, Liao J, Pu Y, Pan E,
Yin L and Wang Y: miRNA-183 suppresses apoptosis and promotes
proliferation in esophageal cancer by targeting PDCD4. Mol Cells.
37:873–880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao G, Wang B, Liu Y, Zhang JG, Deng SC,
Qin Q, Tian K, Li X, Zhu S, Niu Y, et al: miRNA-141, downregulated
in pancreatic cancer, inhibits cell proliferation and invasion by
directly targeting MAP4K4. Mol Cancer Ther. 12:2569–2580. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee JW, Park YA, Choi JJ, Lee YY, Kim CJ,
Choi C, Kim TJ, Lee NW, Kim BG and Bae DS: The expression of the
miRNA-200 family in endometrial endometrioid carcinoma. Gynecol
Oncol. 120:56–62. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Fu H, Xu C, Tie Y, Xing R, Zhu J,
Qin Y, Sun Z and Zheng X: miR-183 inhibits TGF-beta1-induced
apoptosis by downregulation of PDCD4 expression in human
hepatocellular carcinoma cells. BMC Cancer. 10:3542010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Du Y, Xu Y, Ding L, Yao H, Yu H, Zhou T
and Si J: Down-regulation of miR-141 in gastric cancer and its
involvement in cell growth. J Gastroenterol. 44:556–561. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kan CD, Lee HL and Yang YJ: Outcome after
endovascular stent graft treatment for mycotic aortic aneurysm: A
systematic review. J Vasc Surg. 46:906–912. 2007. View Article : Google Scholar : PubMed/NCBI
|