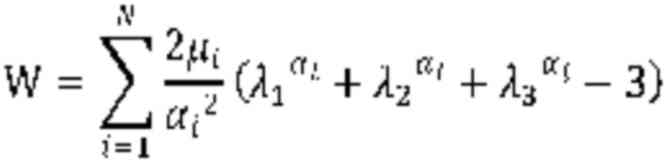Introduction
Extensive research on brain injury following the
onset of intracerebral hemorrhage (ICH) has focused on mass effect,
ischemia and release of clot components (1). However, these findings rarely involved
how to evaluate tissue's direct damage caused by the deformation of
tissue in ICH. Following a hemorrhagic stroke attack, the initial
bleeding causes physical injury to the brain's physiological
structure (2). As a result, the
formation of cracks by physical disruption provides space for
occurrence of hematoma, and the hematoma's mass compresses brain
tissues, exerting a direct force on white matter in particular
(3). With the expansion of the
hematoma, the extent of physical disruption and deformation of
surrounding tissues increases, thus the stretch caused by
deformation induces tissue damage (4). In fact, earlier research had
investigated soft tissue damage under mechanical loadings (5–9). For
instance, by comparing morphological injury and
electrophysiological impairment in an adult guinea pig, the
tissue-level mechanical thresholds for axonal injury were
determined in vivo (7). The
stress-stretch relationship of the human brain, within 12 h of
death, had been quantitatively studied, and the tissue deformation
had been described as similar to filled elastomers (8). Furthermore, the application of
computer-aided tools has been extended to evaluate tissue damage.
For example, a study by Cheng and Hannaford (9) used finite element analysis (FEA) to
predict tissue damage with in vivo uniaxial experimental
data of liver and compared the effect of damage evaluation between
a two-dimensional (2D) and three-dimensional (3D) model. Although
increased interests focused on the injury mechanisms of soft tissue
caused by external mechanical forces, there is currently no broadly
accepted indicator system for evaluating soft tissue damage, and
brain tissue is regarded as extremely soft tissue (10). To overcome this problem, the present
study introduced a novel method to evaluate the tensile damage of
white matter in ICH. Experimental tensile data of white matter from
former literature (8) was used as
the analysis object in the present study. The present work was
based on the energy conservation principle, strain energy theory
and hyperelastic theory in continuum mechanics.
Recently, research has used FEA as an effective tool
for simulating brain tissue deformation of different scales;
however, it is unable to sufficiently meet the needs of grading
brain tissue injury in ICH (11,12). In
addition, an accurate mechanical model is based on accurate
experimental data and reasonable assumption. The mechanical test
data of human brain is scarce, whether in vivo or in
vitro, because of ethics and scarcity of materials. However,
some researchers have obtained worthy experiment results. For
example, a study by Jin et al (13) tested 240 brain tissue specimens under
tension, compression and shear mode at varying strain rates, and
these experimental results provided useful information to develop
accurate constitutive equations for brain tissue. However,
unfortunately the maximum strains were set below 0.5 by Jin et
al (13), yet the majority of
tissue strains surrounding hematoma in ICH are over 0.5, according
to computed tomography (CT) or magnetic resonance imaging (MRI)
images (4). A study by Franceschini
et al (8) presented the whole
damage evolution and fracture process in a prismatic specimen of
white matter, and all these data met the requirements of the
present study.
Using a suitable mechanical model to fit the
experimental data, unknown material properties may be determined.
To predigest the solving process and promote model practicability,
brain tissue was treated as isotropic and homogenous in the
majority of studies, and this setting is also applicable to the
present study. In a common constitutive equation used to describe
brain tissue deformation, strain always changes with location and
time (14–16), which is called strain rate effect.
However, the deformation of brain tissue in ICH is a one-way
process before interventional therapy, and the deformation slowly
continues, with hematoma increasing following the onset of ICH
(3). It was reasonable to leave
relaxation effect and strain rate effect out of account in the
present work.
The objective of the present study was to identify
an effective evaluating method for grading white matter tensile
damage in ICH. On account of ignoring tissue damage in the
deformation process, an overwhelming majority of previous research
neglected detection of biological structure integrity following
experimental operation. Therefore, these results were not
applicable in present work. Furthermore, it is easier to understand
that tissue structural damage may lead to change of mechanical
properties. The mechanical properties of soft tissues are changing
continually when deformation exceeds strain threshold (17) and, up until now, no suitable
constitutive equation was able to describe this process. Research
has demonstrated that, in stretching the optic nerve in
vivo, the tissue-level strain thresholds for injury ranged from
0.09–0.47, with an average strain of 0.181 (7). Even if theories of fracture mechanics
make significant developments, after more than 15 years, no
suitable mathematical physics model has incorporated stretch with
structure damage. Therefore, the present study established a
grading evaluation criterion, which was obtained from comparing the
quantitative change of strain energy between ideal hyperelastic
deformation and actual deformation, and based on strain energy
theory and strain energy loss. From a biomechanical perspective for
white matter's tensile damage in ICH, this simple analytical model
may facilitate the improvement of clinical diagnosis and
treatment.
Materials and methods
Strain energy function
With strain energy loss in deformation as the
studied object, the mechanical behavior of white mater under
uniaxial tension (8) was the main
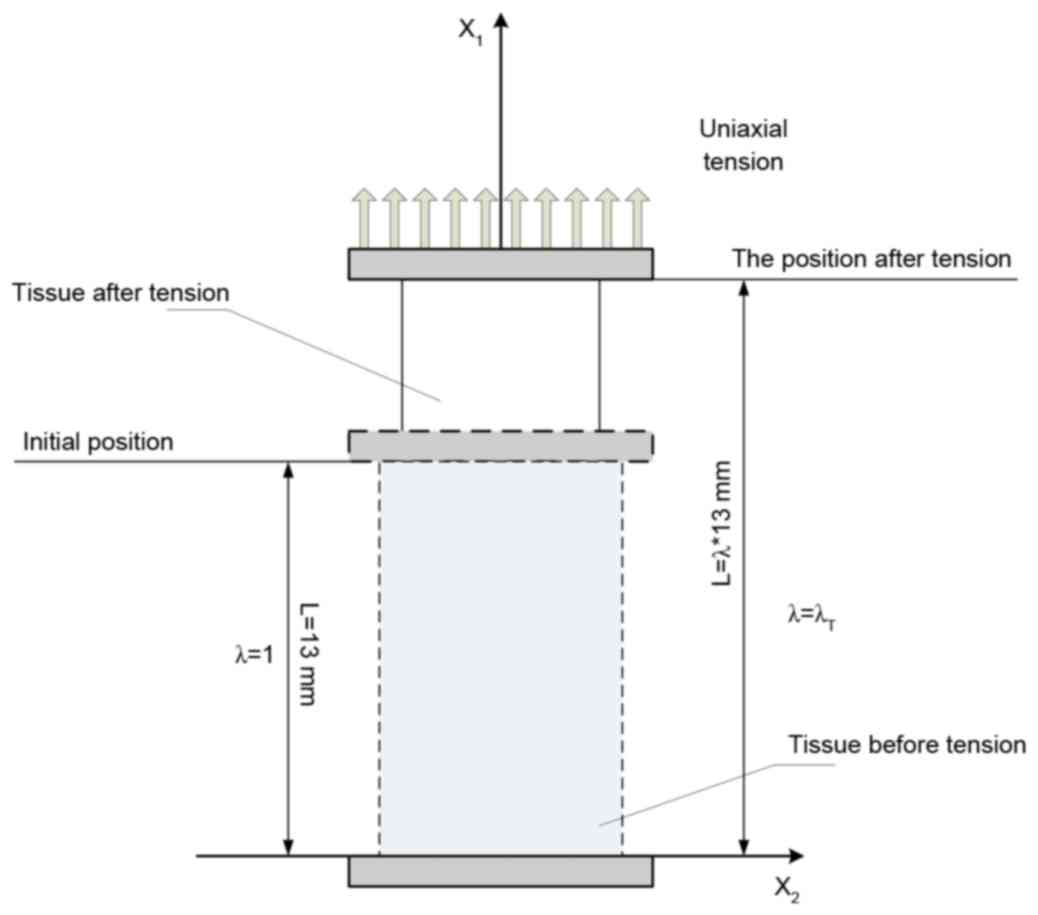
experimental data source. As demonstrated in Fig. 1, white matter stretched under the
uniaxial tension until structure failed, and the results of nominal
stress vs. stretch were analyzed by mathematical and mechanical
methods. Hyperelastic models and polynomial fitting models were
used to fit the mechanical properties, and FEA was used to
demonstrate the strain and stress distribution during stretching.
Model solutions were obtained by using ABAQUS/Standard finite
element v. 6.12 software (Dassault Systems Simulia Corp., Johnston,
RI, USA).
The strain energy function may either be a direct
function of the principal stretch ratios, W=W
(λ1, λ2, λ3), or a function of the
strain invariants, W=W (I1, I2,
I3). Let the deformation gradient tensor as:
F_=dx_dX_
X is the reference position of a material
element and X is the current position of the same element.
The tension deformation, as demonstrated in Fig. 1, may be written as:
x1=λ1X1,x2=λ2X2,x3=λ3X3
Where λi are the principal stretch
ratios of tension in the Rectangular Cartesian coordinate
system:
F_=[λ1000λ2000λ3]
We assumed that tissue kept a fixed volume and
initial form in tension deformation. Thus:
λ1λ2λ3=1andλ1=λT,λ2=λ3=λT–12
Where λT is the principal stretch
ratio in the stretching direction. Then:
F_=[λT000λT–12000λT–12]andF_T=[λT000λT–12000λT–12]
From equation (ii), the right Cauchy-Green
deformation tensor (18) maybe be
determined as follows:
C_=F_TF_=[λT2000λT–1000λT–1]
In general, an isotropic hyperelastic incompressible
material is characterized by a strain-energy density function, W,
which is a function of two principal strain invariants only: W=W
(I1, I2), where I1 and
I2 are defined as (19):
I1=trC_=λT2+2λT–1,I2=12(I12–trC_2)=λT–2+2λT
So that:
W=W(I1,I2)=W(λT2+2λT–1,λT–2+2λT)=W(λT)
During uniaxial tension tests, the tension stress
was generally evaluated as σ11=T/A, where
T is the tension force and A is the area of a
cross-section of the specimen. Thus:
∫σ11dλT=W(λT)
Hyperelastic models
Neo-Hookean strain energy
function
The Neo-Hookean model (20) is often used for the modeling of
nonlinear elastic material. It is based on the statistical
thermodynamics of cross-linked polymer chains and depends on the
first invariant of the right Cauchy-Green deformation tensor. The
strain energy function for an incompressible Neo-Hookean material
under uniaxial mode is given:
W(λT)=C1(I1–3)
It yields the following uniaxial tension stress
component σ11 along the x1-axis:
σ11=W′(λT)=2C1(–1λT2+λT)
Where C1 is a material
constant.
Ogden strain energy function
The Ogden model has been used previously to
describe the nonlinear stress-strain behavior of complex materials,
such as rubbers, biological tissue and brain tissues (20) The Ogden hyperelastic function is
given by:
In uniaxial tension, the following equation
applies:
W(λT)=∑i=1N2μiαi2(λTαi+2λT–12αi–3)
It yields the following uniaxial tension stress
component, σ11, along the x1-axis:
σ11=W′(λT)=∑i=1N2μiαi2(λTαi–1–λT–12αi–1)
For N=1:
σ11=W′(λT)=2μ1α1(–λT–1–α12+λT–1+α1)
For N=2:
σ11=W′(λT)=2μ1α1(–λT–1–α12+λT–1+α1)+2μ2α2(–λT–1–α22+λT–1+α2)
Where μi and αi
are material constants, and λi are the principal
stretch ratios.
Mooney-Rivlin strain energy
function
The strain energy function for an incompressible
Mooney-Rivlin material (20) is:
W=C1(I1–3)+C2(I2–3)
Its strain energy depends on the first and second
strain invariants. In uniaxial tension:
W(λT)=C1(λT2+2λT–1–3)+C2(λT–2+2λT–3)
So that:
σ11=W′(λT)=(2C1+2C2λT)(λT2–λT–1)
Where the C1 and
C2 are material constants.
Assumptions
The present study was based on the following
assumptions: White matter was considered to be an incompressible
material, and were demonstrated to be isotropic and homogenous
before structures failing; under a small range of stretch
(λ≤1.15) loading, the mechanical response of white matter,
particularly under tension load, could be described by hyperelastic
models; with the premise of assumption that no structural damage
occurred in white matter during the whole stretch process, the
hyperelastic model and material parameters mentioned above could be
extended to use in a wider range (λ≤2.24); and, without
consideration of energy loss, such as heat transfer, during the
actual deformation process under tension loading, the transmission
of energy was just restricted to energy storage, which was
expressed as strain energy, and structural failure.
Damage evaluation
As previously mentioned, the present study assumed
that brain tissue could be treated as a hyperelastic material in a
small range of deformation under tension. These limited data could
be fitted by using common hyperelastic constitutive models, and the
constitutive relation could be generalized to present an
‘undamaged’ stress-stretch relationship in large deformation in
tension. Then, WUDG was defined as strain energy
for the ‘undamaged’ stress-stretch curve and WEPT
as strain energy for the experimental curve. Therefore, it was
evident that the difference between WUDG and
WEPT, which could be defined as:
WDMG=WUDG–WEPT
Quantified the degree of tissue damage
in stretching
According to equation (ix), the
WUDG and WEPT could be obtained
by getting the definite integral of fitting functions. Considering
white matter as a soft and vulnerable material, a traditional
polynomial function, rather than common mechanical constitutive
models, were used to describe the relationship between experimental
tension stress values and the corresponding amount of stretch, and
the parameters were given by nonlinear least-square fitting. The
regression equation was as follows:
σ11=B0+∑i=1NBiλTi
Where σ11 is tension stress,
λT is the principal stretch ratio in the
stretching direction and Bi is the coefficient in
each term. The fitting procedure was performed by using the
nonlinear fitted module in OriginPro 8.0 (OriginLab, Northampton,
MA, USA) and the quality of fit for each model was assessed based
on R2. Table I
demonstrated the coefficients of the regression equation, and
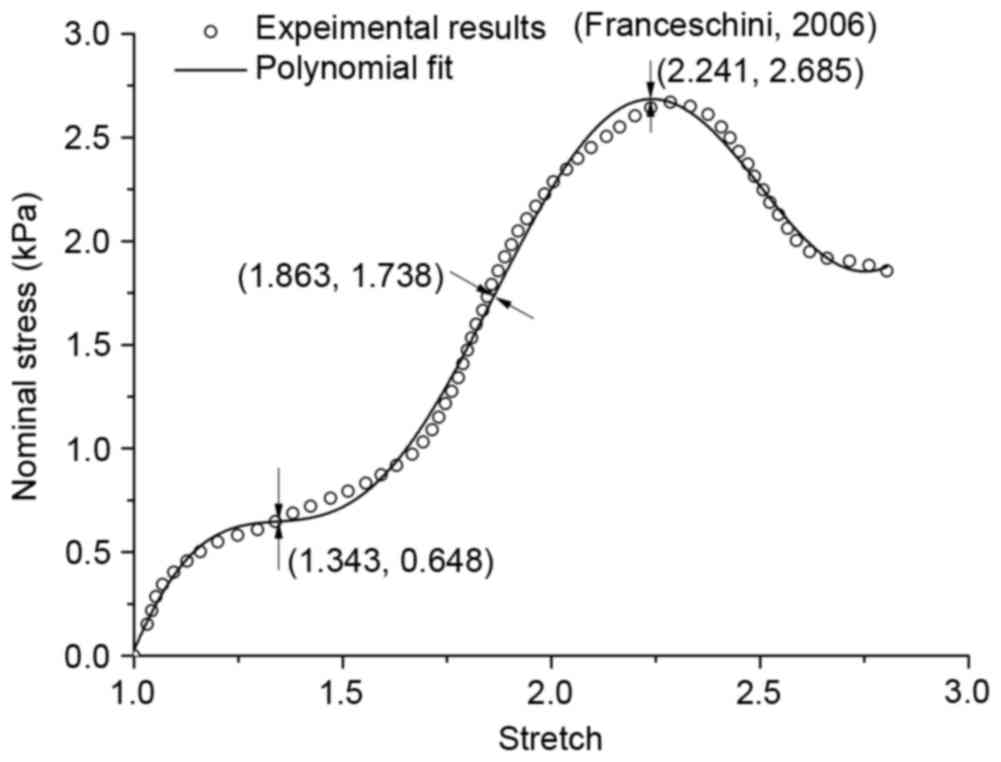
inflexion points have been sought through high order derivation, as
demonstrated in Fig. 2. The first
inflexion point appeared when stretch reached 1.343, and it marked
the beginning of tissue mechanical property change. In the present
study, it was enough to ensure an ideal hyperelastic deformation if
the stretch was not >1.15.
 | Table I.Coefficients of the regression
equation. |
Table I.
Coefficients of the regression
equation.
| Parameters | Value | Standard Error |
|---|
| Intercept | 162.54421 | 83.33519 |
| B1 | −813.54332 | 342.58013 |
| B2 | 1629.70556 | 591.66707 |
| B3 | −1710.6673 | 556.7951 |
| B4 | 1023.37927 | 308.54665 |
| B5 | −350.50895 | 100.75664 |
| B6 | 63.9283 | 17.96696 |
| B7 | −4.81159 | 1.35075 |
| R2 | 0.9954 | – |
If a small stretch (λ≤1.15) of brain white matter
could be regarded as ‘undamaged’ deformation, we could have the
hyperelastic constitutive function based on strain energy function
by fitting these experimental data, and if the above constitutive
function could be used to describe an ‘undamaged’ deformation of
brain white matter in the whole process of tension load, we could
have the ‘undamaged’ constitutive equation, which presented the
different stress-strain relationship with the experimental curve.
It was clear that WDMG was a relation of function
dependent on stretch, and the increment in WDMG
expressed the intensity of structural failure.
Finite element analysis
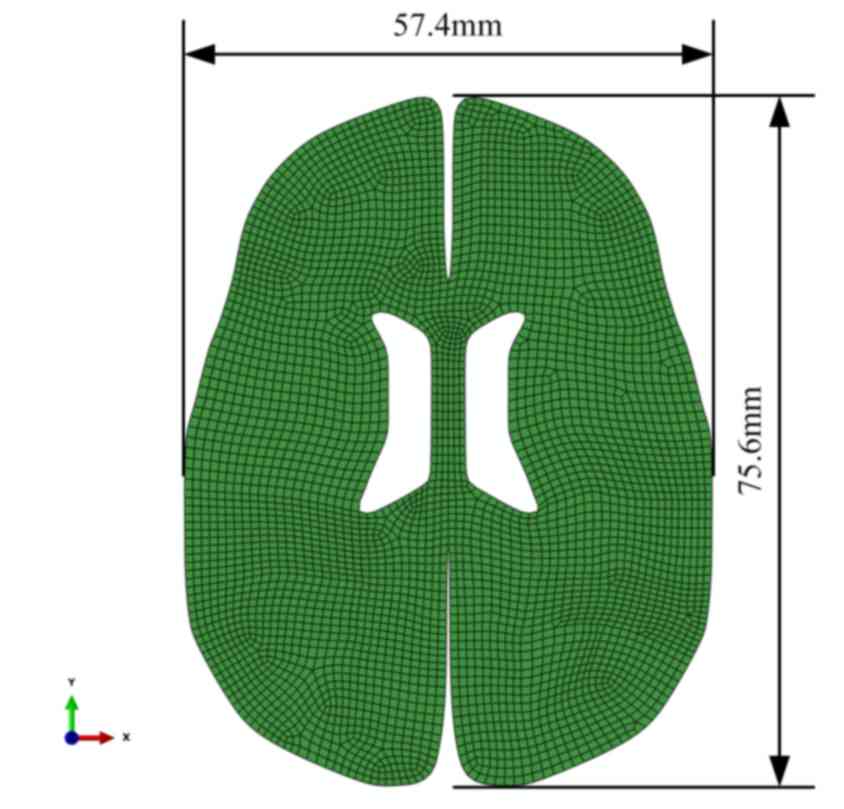
The 3D model from the white matter stretch
experiment with geometry and displacement by Franceschini et
al (8) was applied in Fig. 1. To simplify simulation, a finite
element model that only included tested tissue had been set up. The
tensile process was divided into three stages according to protrude
and concave of the polynomial fitted curve, as demonstrated in
Fig. 2. The 2D model of brain tissue
deformation with geometry and displacement for bleeding in ICH was
demonstrated in Fig. 3. The model
comprised 4313 8-node quadrilateral elements of type CPE8RP (ABAQUS
v. 6.12 Standard,), and 13,373 nodes. All nodes on the outer edge
in all directions constrained except for the gap edge between the
left and right hemisphere. Elements were detached along the
direction of the hematoma's common position on the cross section.
The load was acted on the detached free edge, and the load
simulated the pressure applying on the edge by the mass effect of
hematoma in ICH.
Results
Model validation
The fitting results for stretch no greater than
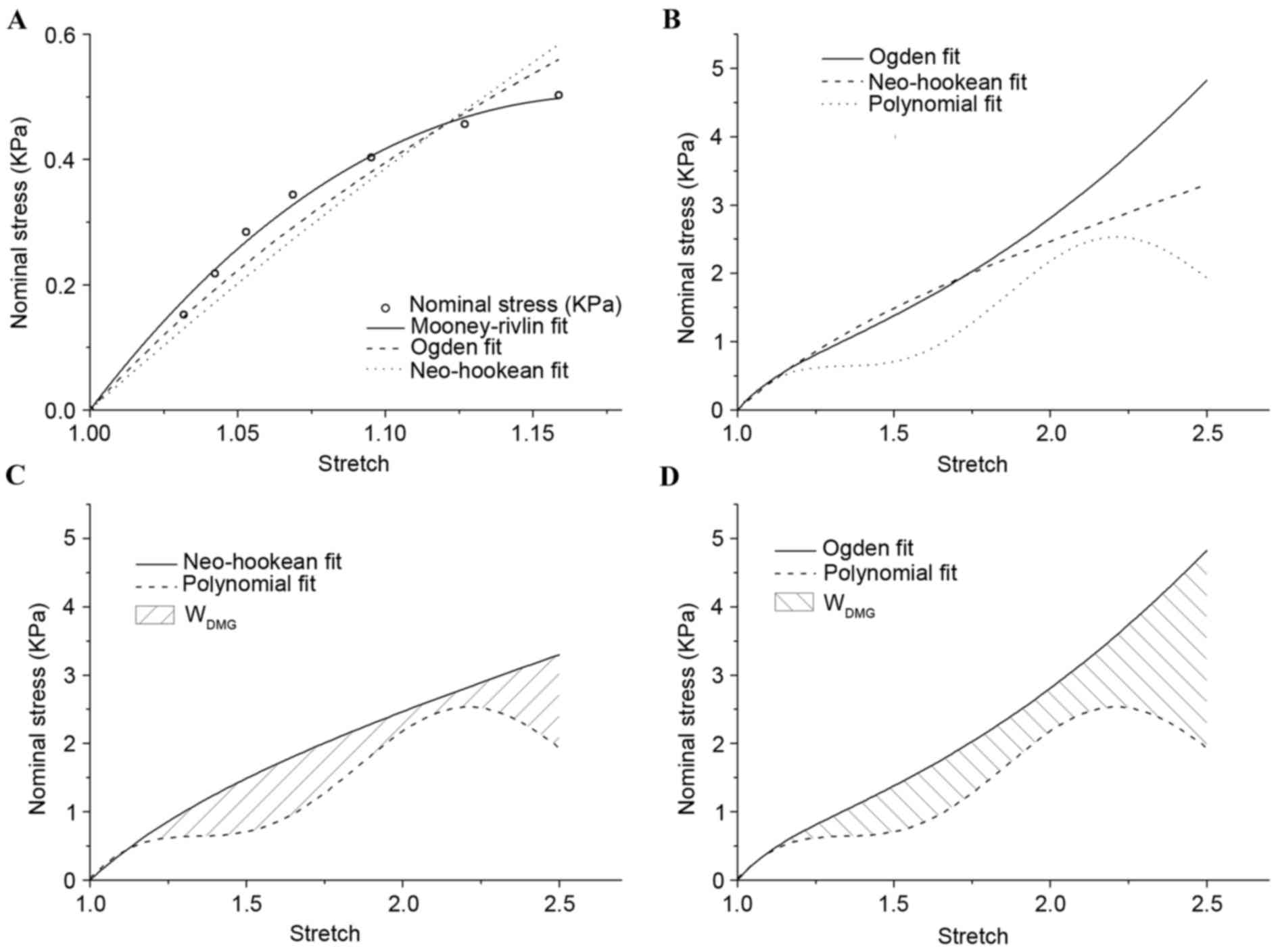
1.15 was plotted in Fig. 4, and all
hyperelastic models demonstrated good agreement with experimental
results (8). On the premise of
λ≤1.15, the material constants for white matter were
indicated in Table II. The
Mooney-Rivlin model demonstrated an improved fitting effect
compared with the other models. The Ogden and Neo-Hookean models
were not stable for extending to a larger scale. When the stretch
was >1.18, the changing curve of stress declined in the
Mooney-Rivlin model. This result was not consistent with the
stress-strain relationship in this simple tension experiment, and
so the Mooney-Rivlin model was eliminated.
 | Table II.List of material constants for white
matter on the premise of the stretch at λ≤1.15. |
Table II.
List of material constants for white
matter on the premise of the stretch at λ≤1.15.
|
| Model |
|---|
|
|
|
|---|
| Material
constant | Neo-Hookean | Ogden | Mooney-Rivlin |
|---|
|
C1 | 762.75 | – | −2097.043 |
|
C2 | – | – | 3134.634 |
|
µ1 | – | 1799.094 | – |
| α1 | – | −7.0557 | – |
In Fig. 4B, the
results of nominal stress vs. stretch for the Ogden and Neo-Hookean
models were demonstrated, which were extrapolated to a larger scale
by material constants on the premise of λ≤1.15 and polynomial
fitting. The majority of the Ogden and Neo-Hookean fitting curves
were located above the polynomial fitting curve. The area under the
curves demonstrated corresponding strain energy, and the shaded
area in Fig. 4C and D indicated
WDMG: The degree of tissue damage in comparison
with the Ogden and Neo-Hookean fitting curves, respectively.
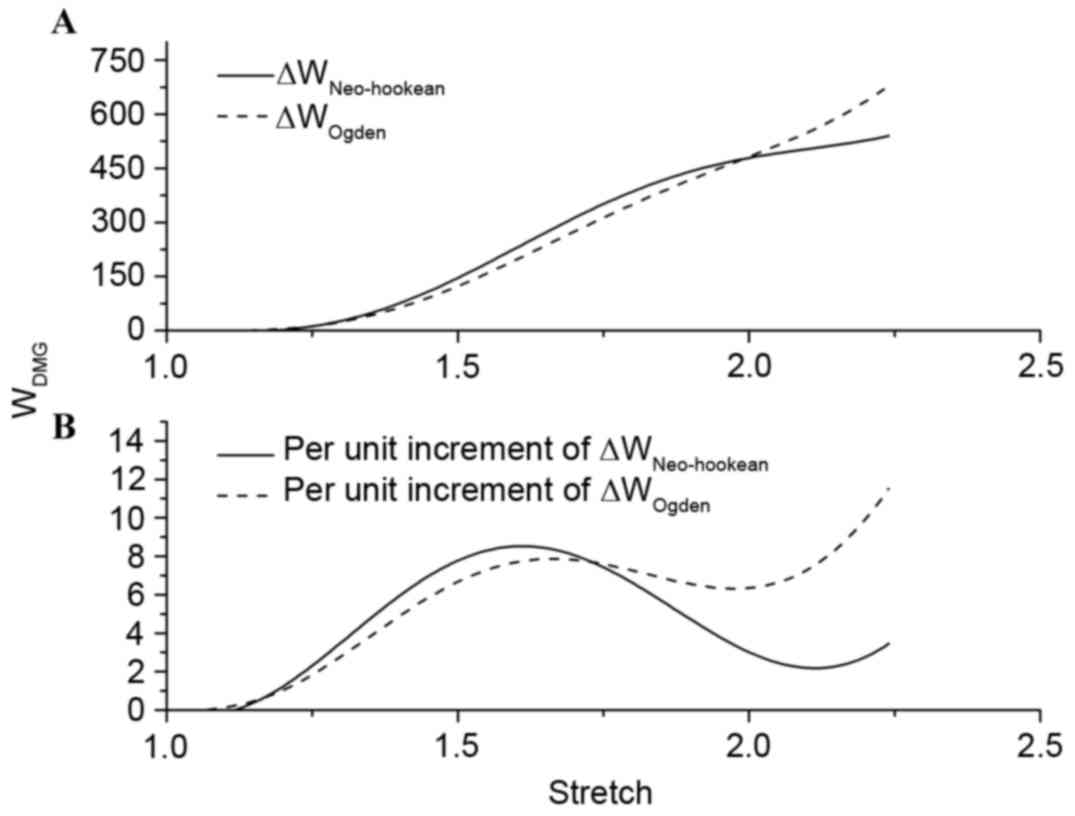
ΔWNeo-Hookean and ΔWOgden were
used to represent the difference of strain energy between the
hyperelastic model and the polynomial fitting model with increasing
stretch. Fig. 5A demonstrated that
there was no distinction in the trend of strain energy loss during
the tensile process. By setting fix-stretch-increment=0.01, per
unit increment of ΔWNeo-Hookean and
ΔWOgden had been calculated and compared
(Fig. 5B). The change of per unit
increment of strain energy loss in the two groups demonstrated a
similar trend; however, the maximum value appeared at stretch=1.61
in ΔWNeo-Hookean and at stretch=2.24 in
ΔWOgden. Combining this result with the inflexion
points on the polynomial fitting curve in the present study, the
level of damage in stretch could be divided into four phases:
Undamaged (λ≤1.343); slight damage (1.343≤λ≤1.863); serious damage
(1.863≤λ≤2.241); and fracture (λ≥2.241).
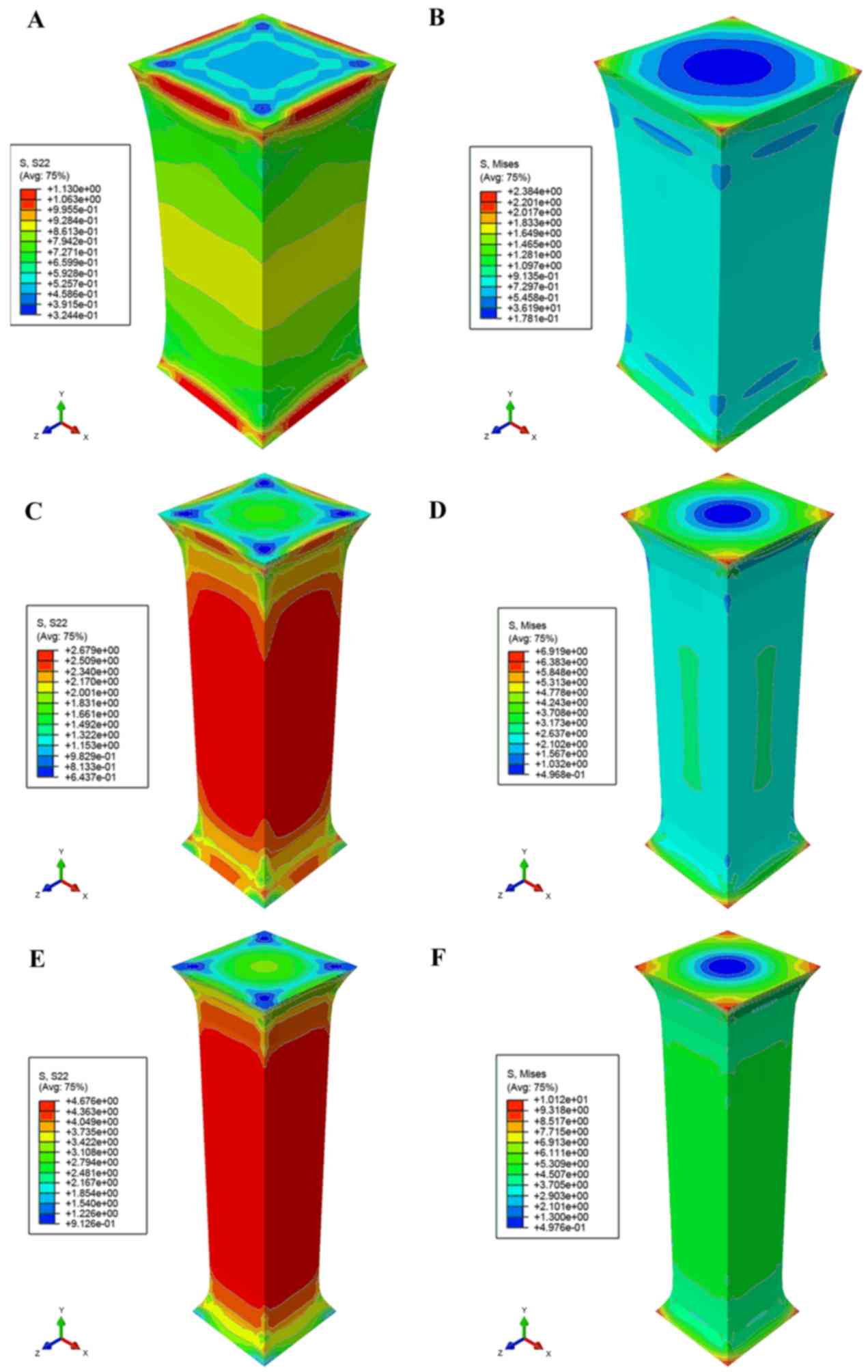
Taking the polynomial fitting model (equation xxi)
as a constitutive function, Fig. 6
demonstrated Mises- and S22-stress distribution under the different
stretch lengths based on the study by Franceschini et al
(8). The simulation demonstrated a
strong agreement with the experimental results that both sides of a
specimen had been fixed on a stretching device so that the
deformation of both ends was less than the mid part in the
stretching direction. The concentration Mises stresses were located
in the corner regions in contact with the stretching device, and
tensile stress was concentrated in the mid part. The tensile load
on the end of the white matter agreed with experimental data in
checkpoints (λ=1.343, 1.863 and 2.241), as demonstrated in Fig. 6A, C and E. Results also indicated
that the tissue broke in experiments described by Franceschini
et al (8), when the S22
reached ~4.676 kPa.
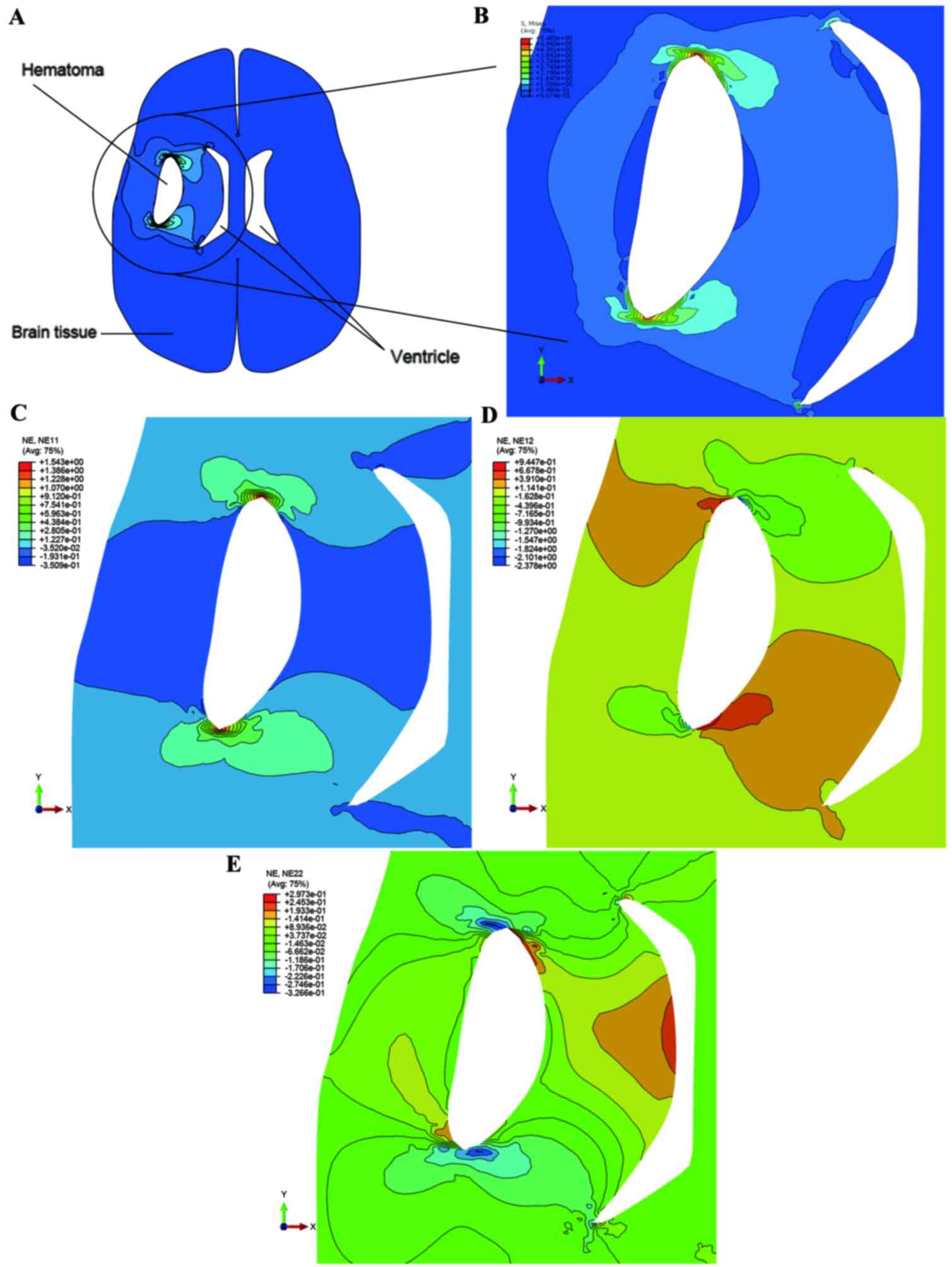
Stress distribution
Fig. 7 demonstrated
the Mises stress distributions of the loading caused by the mass
effect of the hematoma. Stress increased around the crack as the
pressure was applied on the crack edges, and maximum stress was
located in both ends of the crack. As demonstrated in Fig. 6F, the maximum tolerance of Mises
stress was set to 4.908 kPa (mean of Mises stress on the mid part).
The area of ventricle close to the hematoma was evidently reduced
when the loading was set to 5.96 kPa, caused by the mass effect of
the hematoma. Apart from at both ends of the crack, the stress was
concentrated at regions located in the ventricle side. Fig. 7C-E, respectively, demonstrated the
strain distribution in the X-axis, Y-axis and X-Y-axis directions.
The maximum value of strain derived from numerical simulation was
1.543 and the experimental expectation value was 1.241.
Discussion
Spontaneous intracerebral hemorrhages commonly
occur in the white matter, which is described as a particular
location where axons gather and interact with each other by fibers
(21). Due to hemorrhage, the effect
of a tear on white matter is the direct physical injury; however,
the potential damage, which is caused by tissue deformation and
mass effect, cannot be ignored. Within a small deforming range, the
deformation behavior of brain tissue is considered to obey
nonlinear hyperelastic law, as does the majority of biological soft
tissues (20). However, within a
larger deforming range, the deformation has been indicated to be
entirely different due to structural damage of the biological
tissue itself (7), which may be
predominantly attributed to the failure of cellular structure and
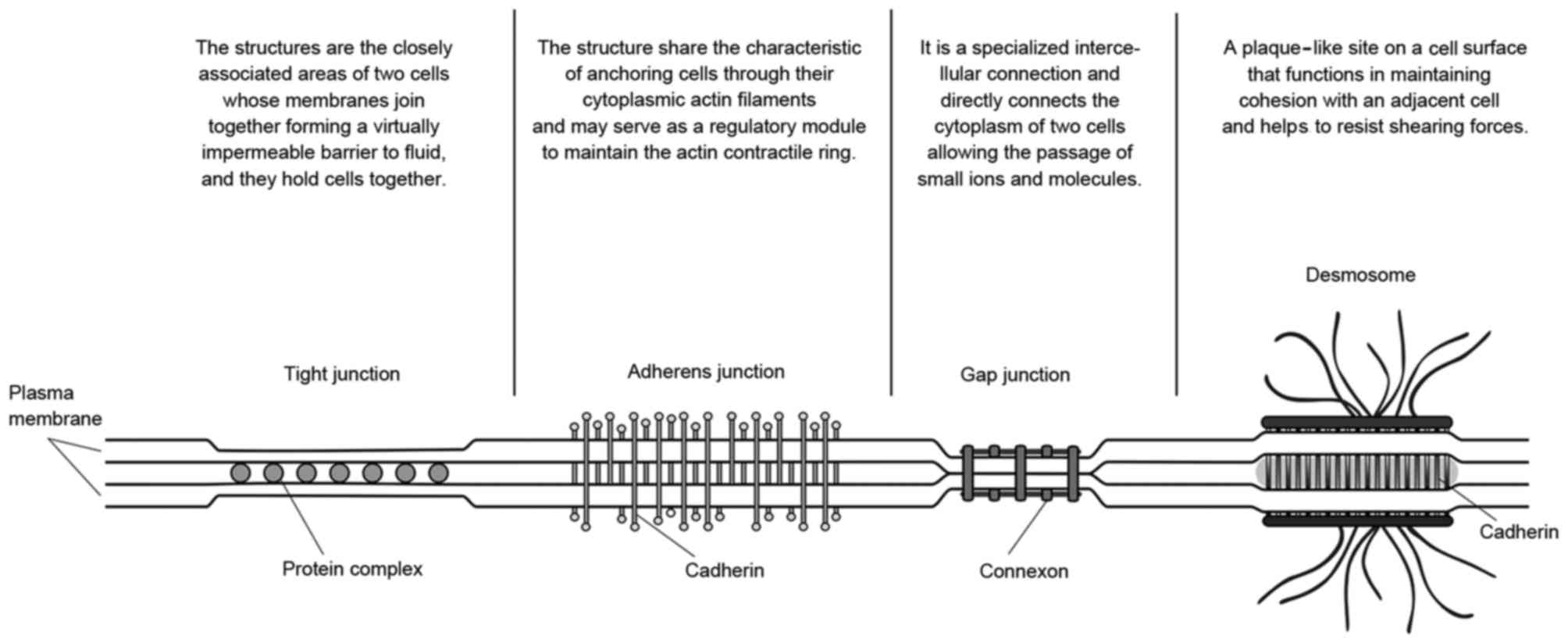
cell junctions. As demonstrated in Fig.
8, common cell junctions may have a role in linking cells with
other cells or the surrounding matrix, regardless of what
physiological functions or connection strength they had. The degree
of irreversible distortion determines whether the cell junctions
have been damaged (22). In former
literature, according to nonlinear mechanical law, the
stretch-stress curves obtained in the present study may be divided
into five parts: An initial stiff response; hardening behavior;
locking behavior; hardening; and softening (8). However, before white matter suffers
irreversible structural damage, it is a reasonable condition to
treat white matter as a simple homogeneous material, although it
will not be sufficient to describe mechanical properties of white
matter under large deformation. It is reasonable to infer that each
part corresponds to a certain degree of failure of cell or tissue
structure and junctions. With the deformation intensified, the
mechanical behavior of brain tissue may change constantly, and it
is difficult to abstract mechanical parameters according to
mechanical law, such as continuum mechanics or fracture mechanics
(23). Therefore, in the present
study, the method, which combines strain or stretch with strain
energy loss, should be an effective way to gauge the damage degree
of brain white matter.
During uniaxial stretching of white matter, the
curve of nominal stretch vs. nominal stress demonstrated mechanical
behavioral changes at different stages, and the entire process
could be divided into three parts: 1) nondestructive deformation;
2) destructive deformation; and 3) fracture. The axonal
microstructure in the optic nerve demonstrated an undulated
appearance (22), which possesses
the ability to withstand some degree of distortion for deformation,
and individual axons in white matter demonstrate a similar
appearance (2). While deforming, if
the degree of deformation exceeds the limit, cell junctions and
cytoskeletons will fracture, and cell structure will collapse
(22). When structural damage
reaches the critical level, severe tear ictus may occur (24). It was comparatively easy to match
nondestructive deformation and fracture with a section of the
stress-stretch curve; however, the degree of structure damage under
destructive deformation is difficult to measure. The fitting curves
for nominal stretch to nominal stress had been studied for the
correlation between the degree of structure damage and the
concavity and convexity of fitting function. In the present study,
regarding deformation before the point (1.343, 0.648) as
nondestructive deformation, and after the point (2.241, 2.685) as
fracture, the inversion point (1.863, 1.738) was likely to
partition destructive deformation into two sub-phases, which
corresponds to certain structural failure. A study by Bain and
Meaney (7) investigated tissue-level
thresholds for axonal damage in central nervous system white matter
injury, and their results, which agreed with the result in the
present study, demonstrated that the 90% probability value strain
of 0.34 defined the liberal threshold for morphological injury.
In this simple tension experiment, when the stretch
was <1.15, the stress-strain relationship may be presented by
Mooney-Rivlin, Ogden and Neo-Hookean models (20), which were commonly used for modeling
nonlinear elastic material and brain tissue (11,16,25,26). The
Mooney-Rivlin model was not stable for extending to a larger scale
despite demonstrating an improved fitting effect compared with the
other models. The present study indicated that there was not only a
negligible difference of WDMG between the Ogden
and Neo-Hookean fittings, but also between
ΔWNeo-Hookean and ΔWOgden;
however, per unit increment of ΔW demonstrated different
trends in the late tension stage. The increment of
ΔWOgden increased with increasing stretch, and
the increment of ΔWNeo-Hookean demonstrated a
trend of fluctuations. The former implied that stretch increase
aggravated structural failure, while the latter declined gradually
after reaching its maximum. It may be inferred that structural
failure of white matter is not an irregular linear process, but a
process controlled by multiple structures, and the failure of
different structures is not synchronized.
3D FEA results demonstrated that Franceschini et
al (8) experimental data cannot
expound the stress distribution. The maximal von Mises stress
focused on both fixed surfaces, and it highlighted the influence of
the fixation method on the results. S22 stress distribution
demonstrated that the stresses were concentrated in the mid part
rather than at both ends of the specimen. In the experiment by
Franceschini et al (8), the
fracture took place in the region near the ends where the large
range of tension stress was distributed; this result was in
conformity with previous FEA results. The polynomial fitting
function could be reliably used as a constitutive function to model
the mechanical behavior of white matter under tension. The present
study indicated that deformation caused by hematoma compression is
predominantly distributed around the hematoma, and made the
ipsilateral ventricle smaller. After ICH ictus, the space occupied
by hematoma, which is called mass effect, causes direct tearing
injury and will produce deformation on the region of stress
concentration, resulting in varying degrees of structural failure
(3). The distribution of stress and
strain demonstrate the potential damage distribution, but stress,
which may be calculated with constitutive function and the variable
of strain, depend on the material itself and cannot be directly
measured, particularly in vivo. Therefore, it is reasonable
to use strain as an evaluating standard for brain tissue damage in
ICH.
Uniaxial tension experimental data (8) was analyzed and evaluation criterion of
tissue damage based on strain was discussed in the present paper.
However, the deformation of brain tissue is a complex process that
simultaneously includes tension, compression and shear (10). The lack of similar experimental data
under simple shear makes it difficult to obtain a revised
evaluation criterion. Nevertheless, biological tissues are so
complex and specific that it is difficult to ensure the result
reproducibility of experiments, even if the test tissues are taken
from the same sample (19).
Additionally, the test tissues cannot be intact after suffering any
mechanical load greater than the maximum tolerance. Fortunately,
FEA software, such as ABAQUS and ANSYS, are able to evaluate
mechanical parameters of material by analyzing a single type of
strain-stress data if soft tissues are assumed to be hyperelastic
material in a certain range of deformation, and the uniaxial
tension test is a common way of obtaining parameters. Through these
methods, the model has a limited application area, for instance, it
cannot evaluate damage caused by compression and shear. In the
stage of this research, damage evaluation under compression and
shear will be investigated.
White matter is anisotropic and heterogeneous, and
each independent sample demonstrates different mechanical
parameters (13). The evaluation in
the present study was only able to accurately evaluate the damage
degree of a single specimen and would be unable to obtain an
accurate and universal assessment standard unless we knew precisely
the stress-stretch relationship of each evaluation object. In the
present study, the strain rate dependency was ignored because the
majority of medical images are acquired 4 h after ICH ictus. The
deformation of brain tissue in ICH may be treated as a one-way
process if the process of cerebral hemorrhage is simplified to the
process of hematoma increasing continuously; however, in reality,
cerebral hemorrhage is a complex biochemical and physical
process.
With further simulation studies, animal experiments
and tissue-level mechanical experiments, direct evidence for tissue
damage may be obtained. Despite gaining understanding of the
relationship between stress and strain, the keystone of future work
may be to design a device that is able to realize the quantitative
deformation of brain tissue, including tension, compression and
shear in vitro. An ICH tensile damage evaluation method
based on CT or MRI images may be developed by composing digital
image processing technology with the results of the present
study.
Acknowledgements
The authors would like to acknowledge Professor
Zhan-fang Liu, College of Aerospace Engineering, Chongqing
University, for the use of ABAQUS in the present study and Dr
Ju-Chou, Associate Professor of Chemistry Department of Chemistry
and Physics, Florida Gulf Coast University, for copyediting the
manuscript for language and grammar.
Funding
The present study was supported by the National
Basic Research Program of China (973 program; grant no.
2014CB541600) and by the Visiting Scholar Foundation of Key
Laboratory of Biorheological Science and Technology (Chongqing
University) and the Ministry of Education (grant no.
CQKLBST-2018-019).
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors' contributions
PR performed the modeling, the finite element
analysis and was a major contributor in writing the manuscript.
BCW, YZW and SLH performed the simulation analyses. TWG and XFL
performed the simulations and contributed in the preparation of the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Keep RF, Hua Y and Xi G: Intracerebral
haemorrhage: Mechanisms of injury and therapeutic targets. Lancet
Neurol. 11:720–731. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xi G, Strahle J, Hua Y and Keep RF:
Progress in translational research on intracerebral hemorrhage: Is
there an end in sight? Prog Neurobiol. 115:45–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu G, Xi G and Huang F: Spontaneous
intracerebral hemorrhage in humans: Hematoma enlargement, clot
lysis, and brain edema. Acta Neurochir Suppl. 96:78–80. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Strik HM, Borchert H, Fels C, Knauth M,
Rienhoff O, Bähr M and Verhey JF: Three-dimensional reconstruction
and volumetry of intracranial haemorrhage and its mass effect.
Neuroradiology. 47:417–424. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morrison B III, Meaney DF, Margulies SS
and McIntosh TK: Dynamic mechanical stretch of organotypic brain
slice cultures induces differential genomic expression:
Relationship to mechanical parameters. J Biomech Eng. 122:224–230.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cater HL, Sundstrom LE and Morrison B III:
Temporal development of hippocampal cell death is dependent on
tissue strain but not strain rate. J Biomech. 39:2810–2818. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bain AC and Meaney DF: Tissue-level
thresholds for axonal damage in an experimental model of central
nervous system white matter injury. J Biomech Eng. 122:615–622.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Franceschini G, Bigoni D, Regitnig P and
Holzapfel GA: Brain tissue deforms similarly to filled elastomers
and follows consolidation theory. J Mechanics Physics Solids.
54:2592–2620. 2006. View Article : Google Scholar
|
|
9
|
Cheng L and Hannaford B: Finite element
analysis for evaluating liver tissue damage due to mechanical
compression. J Biomech. 48:948–955. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goriely A, Geers MG, Holzapfel GA,
Jayamohan J, Jérusalem A, Sivaloganathan S, Squier W, van Dommelen
JA, Waters S and Kuhl E: Mechanics of the brain: Perspectives,
challenges, and opportunities. Biomech Model Mechanobiol.
14:931–965. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rashid B, Destrade M and Gilchrist MD:
Mechanical characterization of brain tissue in tension at dynamic
strain rates. J Mech Behav Biomed Mater. 33:43–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Javid S, Rezaei A and Karami G: A
micromechanical procedure for viscoelastic characterization of the
axons and ECM of the brainstem. J Mech Behav Biomed Mater.
30:290–299. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin X, Zhu F, Mao H, Shen M and Yang KH: A
comprehensive experimental study on material properties of human
brain tissue. J Biomech. 46:2795–2801. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miller K and Chinzei K: Constitutive
modelling of brain tissue: Experiment and theory. J Biomech.
30:1115–1121. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miller K: Constitutive model of brain
tissue suitable for finite element analysis of surgical procedures.
J Biomech. 32:531–537. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kohandel M, Sivaloganathan S, Tenti G and
Drake JM: The constitutive properties of the brain parenchyma Part
1. Strain energy approach. Med Eng Phys. 28:449–454. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gonzalez-Rodriguez D, Guevorkian K,
Douezan S and Brochard-Wyart F: Soft matter models of developing
tissues and tumors. Science. 338:910–917. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bonet J and Wood RD: Nonlinear continuum
mechanics for finite element analysis. Nuclear Fusion.
38:1062008.
|
|
19
|
Ogden RW: Large deformation isotropic
elasticity-correlation of theory and experiment for incompressible
rubberlike solids. Proceed R Soc London Series A. 326:565–583.
1972. View Article : Google Scholar
|
|
20
|
Fung YC: Biomechanics: Mechanical
properties of living tissues. Springer Science & Business
Media. 2013. View Article : Google Scholar
|
|
21
|
Trobe JD: Youmans neurological surgery,
5th Edition. J Neuro Ophthalmol. 25:3342005. View Article : Google Scholar
|
|
22
|
Bain AC, Shreiber DI and Meaney DF:
Modeling of microstructural kinematics during simple elongation of
central nervous system tissue. J Biomech Eng. 125:798–804. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miller K: Biomechanics of the Brain, in
Biological and Medical Physics, Biomedical Engineering. 2011.
Springer; New York, NY: 2011, View Article : Google Scholar
|
|
24
|
Brouwers HB and Greenberg SM: Hematoma
expansion following acute intracerebral hemorrhage. Cerebrovas Dis.
35:195–201. 2013. View Article : Google Scholar
|
|
25
|
Kyriacou SK, Mohamed A, Miller K and Neff
S: Brain mechanics For neurosurgery: Modeling issues. Biomech Model
Mechanobiol. 1:151–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dutta-Roy T, Wittek A and Miller K:
Biomechanical modelling of normal pressure hydrocephalus. J
Biomech. 41:2263–2271. 2008. View Article : Google Scholar : PubMed/NCBI
|