Introduction
Breast carcinoma is the most common cause of
cancer-associated mortality in females and accounts for 6.9% of all
cancer-associated mortalities in Chinese females (1). The majority of breast cancer cases are
defined as invasive carcinoma of no special type (NST) (2). The global five-year survival rate of
invasive carcinoma of NST after diagnosis is 70–90% (3). Due to favorable prognosis, detection of
breast carcinoma at the early stage is crucial for successful
clinical treatment. Ultrasound (US) is a widely accepted technique
for differentiating between benign and malignant breast lesions
(BLs). In addition to B-mode US, elastography has been developed to
aid clinicians in the assessment of tissue stiffness. A previous
study demonstrated that malignant lesions tended to be harder,
while softer lesions comprised the bulk of benign lesions (4). In the case of invasive breast
carcinoma, disordered collagen makes BLs poorly compressible.
Conversely, even if benign BLs, e.g., fibroadenoma, are rich in
collagen, they are commonly less stiff due to the well-ordered
collagen. In addition, a previous study indicated that the
histopathological classification has a direct impact on the
five-year survival rate of females with invasive carcinoma of NST
(3).
The histopathological classification has a direct
impact on the therapeutic effect and prognosis. However, to the
best of our knowledge, few studies have reported on the association
between elastography and histopathology in breast cancers,
particularly in invasive carcinoma of NST. Therefore, the present
study aimed to investigate the correlation between the
classification based on pathologic histology and shear-wave
elastography in evaluating invasive carcinoma of NST.
Patients and methods
Clinical data
The present retrospective study involved 259
patients who had undergone conventional US at Pudong New Area
People's Hospital (Shanghai, China) between March 2016 and February
2018 and in whom BLs were detected. The Ethical Committee of Pudong
New Area People's Hospital approved the retrospective study of the
images and associated records of the enrolled patients. All data
were reported anonymously. Informed consent was obtained for the
publication of any images. In total, 84 BLs in 80 patients (age
range, 32–64 years; mean age, 46.87±7.82 years) were selected for
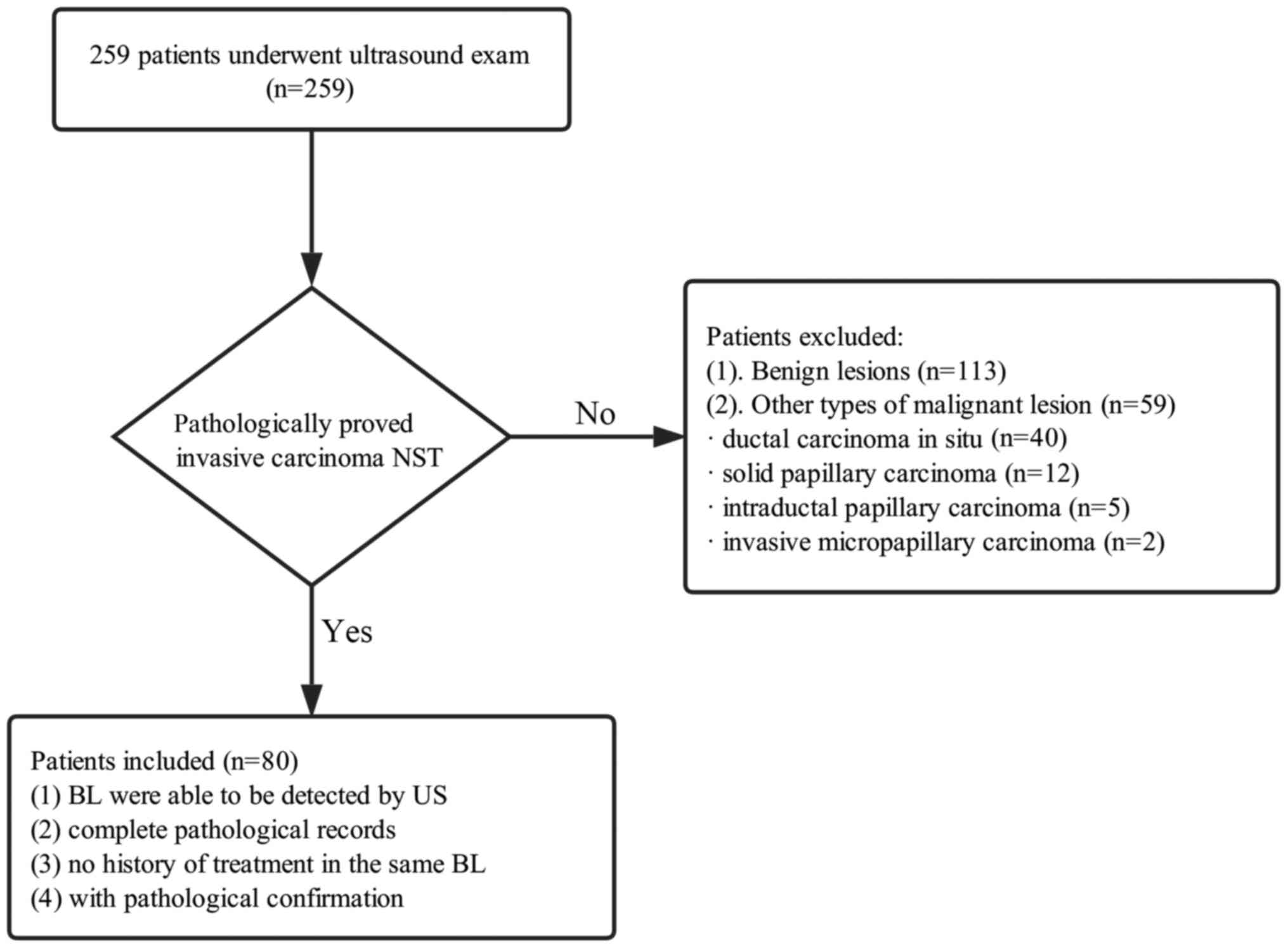
the present study. The selection flowchart is presented in Fig. 1, with the following inclusion
criteria: i) BL was detectable by ultrasound; ii) BLs were
pathologically confirmed as invasive carcinoma of NST; iii)
complete pathological records were available; iv) the BL that was
examined had no history of treatment. The mean diameter of the
lesions was 24.47±10.09 mm (range, 10.2–48.3 mm) and the mean depth
was 24.93±11.30 mm (range, 8.1–47.3 mm).
Apparatus and methods
The patients first underwent B-mode US in the supine
position. An Acuson S2000™ Ultrasound System (Siemens
Healthcare, Munich, Germany), equipped with a 9L4 linear transducer
(4–9 MHz, 4.0 cm) was used. One radiologist (Y-CZ), who had >3
years of experience in breast sonography and had 4 months of
experience in virtual touch tissue quantification (VTQ)
measurement, performed the B-mode US examination. Once a lesion was
detected, the radiologist recorded a number of basic ultrasonic
features, including lesion size in diameter, lesion depth (vertical
distance from the skin to the bottom of the lesion), margin and
echotexture. Following the examination, the patients then underwent
shear-wave elastography, specifically VTQ diagnosis, using the same
machine. The same operator further observed the elasticity of the
BL using VTQ by reading the same imaging area of the B-mode US. The
transducer was strapped to the patient's chest lightly and patient
was asked to hold their breath for several seconds. The radiologist
measured the shear-wave velocity (SWV) of the lesion and the normal
breast tissues by using a quantification region of interest (ROI)
box (with fixed dimensions of 5×6 mm). The ROI box on the
shear-wave velocity (SWV) image included the mass and surrounding
breast tissue. Visible calcifications and cystic components were
avoided as far as possible and the ROI was placed in the solid
region during the investigation. The SWV values were obtained from
the ROI in the solid portion of the mass and the surrounding breast
tissue at the same depth. Absolute measurements of SWV, displayed
in meters per second, were generated by evaluating the peak
displacement at each transverse. The same depth, focus position and
gain setting were used for the same lesion. The operator performed
the measurement using the same methodology for seven times. The
rational of repeating the same methodology seven times was the
following: The rapid malignant proliferation led to insufficient
blood supply and eventually resulted in localized liquefaction and
necrosis. This further induced the stiffness of the breast tissue.
A slower SWV was associated with softer BLs. Furthermore, the
entire lesion did not exhibit a consistent hardness due to the
inhomogeneous proliferation rates of the cancer cells.
Proliferation increased fibrosis and decrease necrosis, thus areas
with high proliferation rates were stiffer compared with those with
low proliferation rates (5).
Therefore, it was necessary to repeat the same process for seven
times. The SWV values and the percentage of successful measurements
were recorded and examined. The scale for the SWV was 0–9.1
m/sec.
In terms of pathological examination, one
pathologist with 10 years of experience (DW) applied a
semi-quantitative method to classify invasive carcinoma of NST
according to the grading system developed by the World Health
Organization (WHO) (6). A numerical
scoring system with a scale of 1–3 was used to assess three
features in total, namely tubule and gland formation, nuclear
pleomorphism and mitotic counts. The cut-off points for the first
feature were 75 and 10 for score allocation: One point was assigned
on the condition that >75% of the mass area consisted of
definite tubules, and tumors that exhibited between 10 and 75%
tubule formation were scored as 2 points. With regard to nuclear
pleomorphism, a score of 1 referred to those small, regular uniform
cells that were highly similar in size with that of the benign
pre-existing epithelial cells. A score of 2 was assigned for mild
to moderate pleomorphism and inconspicuous nucleoli, while a score
of 3 demonstrated that the nuclei were larger in size with marked
variation. Mitotic counts were determined as the number of mitotic
figures observed in 10 consecutive high-power fields (0.42 mm) in
the most mitotically active part of the tumor (the leading edge).
The cut-off points for this characteristic were 5 and 10: The
presence of 0–5 mitotic figures was rated as 1; 6–10 mitotic
figures were scored as 2 and >10 mitotic figures were scored as
3. A final grading was calculated as the sum of the three
aforementioned values (7). Grade I
(well-differentiated) referred to those with a score of 3–5, Grade
II (moderately differentiated) was assigned to those with a score
of 6 or 7, and those with a score of >7 were rated as Grade III
(poorly differentiated).
Statistical analysis
The Chi-square test or Fisher's exact test were
applied in order to evaluate differences between groups in terms of
categorical variables, while analysis of variance was used to
compare continuous variables between the groups. P<0.05 was
considered to indicate a statistically significant difference. All
data were analyzed using SPSS 25.0 (IBM Corp., Armonk, NY,
USA).
Results
Ultrasonic features
Of the 84 BLs, 14 (16.7%) were rated as Grade I, 29
(34.5%) as Grade II and 41 (48.8%) as Grade III. The results
demonstrated that there were three ultrasonic characteristics that
were significantly associated with the histological grade (Table I). First, the size of the BL
increased with the increase in histological grade (P<0.001). The
median diameter of the Grade-I BLs was 12.90±0.59 mm, whilst the
size of the Grade III BLs was twice of that (25.90±1.56 mm).
Furthermore, 29.3% of the Grade-III BLs exhibited acoustic
enhancement, while the majority of the Grade-I BLs (78.6%)
exhibited acoustic shadowing (P=0.002). Finally, the Grade-III BLs
tended to display acoustic enhancement; however, this was
relatively rare in the Grade-I and Grade-II BLs (7.1 and 3.4%,
respectively). In total, 2 out of 43 (4.7%) Grade-I and Grade-II
BLs exhibited acoustic enhancement, whereas 15 out of 41 (36.6%)
Grade-III BLs exhibited the same feature (P=0.002). Other
ultrasonic features of the invasive carcinoma of NST of different
grades, including the margin, echotexture and halo sign, were not
significantly associated with the histopathologic classification
(all P>0.05).
 | Table I.Ultrasonic features of all of the
invasive carcinomas of NST of different histopathological
grades. |
Table I.
Ultrasonic features of all of the
invasive carcinomas of NST of different histopathological
grades.
| Characteristic | Grade I (n=14) | Grade II (n=29) | Grade III (n=41) | P-value |
|---|
| Age (years) | 49.57±8.15 | 48.59±7.99 | 44.73±7.15 | 0.045 |
| Position |
|
|
| 0.605 |
|
Right | 8 (57.1) | 12 (41.4) | 18 (43.9) |
|
| Left | 6 (42.9) | 17 (58.6) | 23 (56.1) |
|
| Lesion size (mm) | 12.90
(10.90–15.65) | 22.90
(18.25–31.9) | 25.90
(19.25–34.85) | <0.001 |
| Margin |
|
|
| 0.089 |
|
Well-defined | 0 (0.0) | 2 (6.9) | 8 (19.5) |
|
| Poorly
defined | 14 (100.0) | 27 (93.1) | 33 (80.5) |
|
| Echotexture |
|
|
| 0.944 |
|
Heterogeneous | 10 (71.4) | 22 (75.9) | 31 (75.6) |
|
|
Homogeneous | 4 (28.6) | 7 (24.1) | 10 (24.4) |
|
| Halo sign |
|
|
| 0.692 |
|
Present | 4 (28.6) | 11 (37.9) | 17 (41.5) |
|
|
Absent | 10 (71.4) | 18 (62.1) | 24 (58.5) |
|
| Calcification |
|
|
| 0.443 |
| No | 6 (42.9) | 11 (37.9) | 11 (26.8) |
|
| Yes | 8 (57.1) | 18 (62.1) | 30 (73.2) |
|
| Posterior acoustic
feature |
|
|
| 0.002 |
| None | 2 (14.3) | 7 (24.1) | 7 (17.1) |
|
|
Shadowing | 11 (78.6) | 14 (48.3) | 12 (29.3) |
|
|
Enhancement | 1 (7.1) | 1 (3.4) | 15 (36.6) |
|
|
Mixed | 0 (0.0) | 7 (24.1) | 7 (17.1) |
|
Association of VTQ results with the
histopathological grading
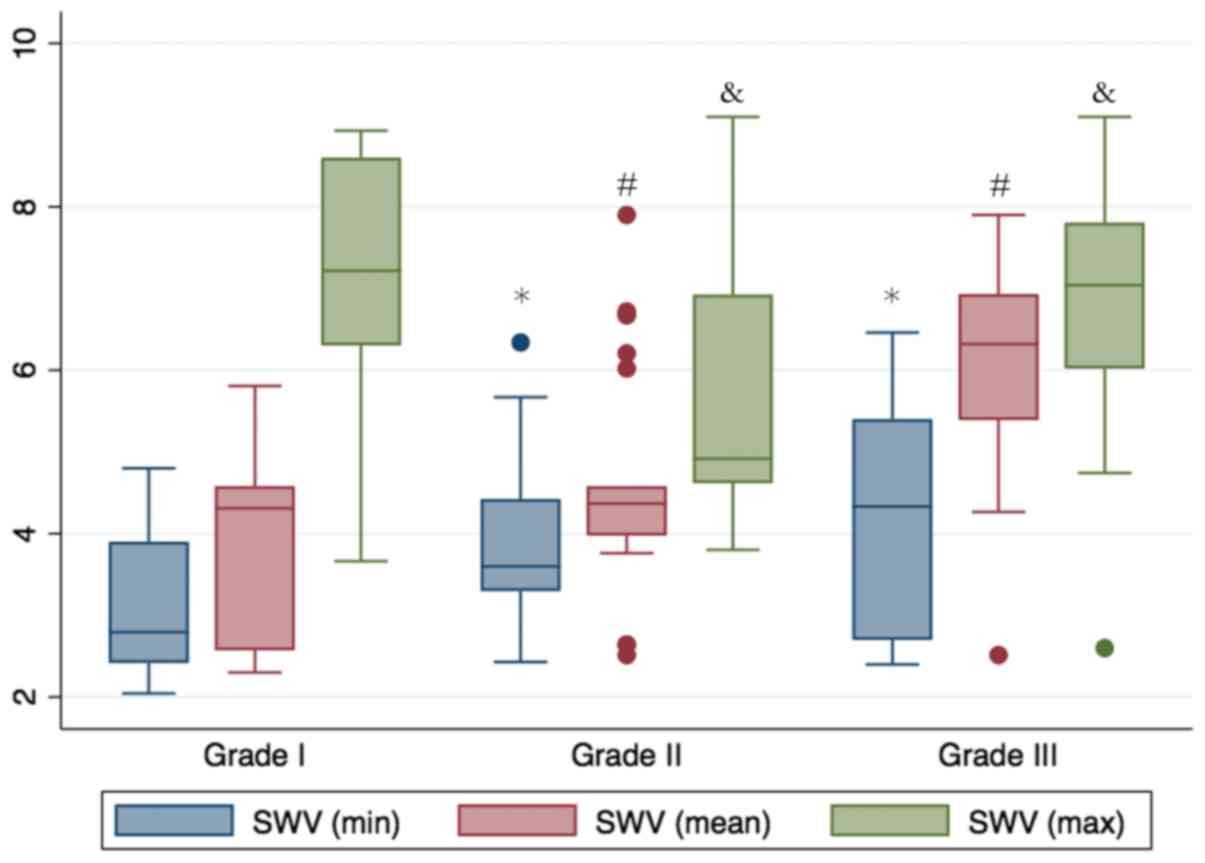
The results demonstrated that a higher
histopathological grade was closely associated with a higher
minimum, mean and maximum SWV value (Figs. 2 and 3). The Grade-III BLs exhibited the highest
mean SWV value of 6.32 m/sec [interquartile range (IQR), 5.35–6.98
m/sec], followed by those of Grade II (mean, 4.37 m/sec; IQR,
3.98–4.58 m/sec) and Grade I (mean, 4.31 m/sec; IQR, 2.56–4.54
m/sec; P=0.032). However, the minimum, mean and maximum SWV values
were not significantly associated with the lesion size (Table II). For instance, as a
representative case, a 61-year-old female patient presented at
Pudong New Area People's Hospital with a large palpable mass (data
not shown). The 48.3-mm BL exhibited a high mitotic frequency and
central necrosis, and was histologically classified as Grade III
(data not shown). The BL was pathologically confirmed as
pleomorphic carcinoma, a rare variant of high-grade invasive
carcinoma of NST (data not shown).
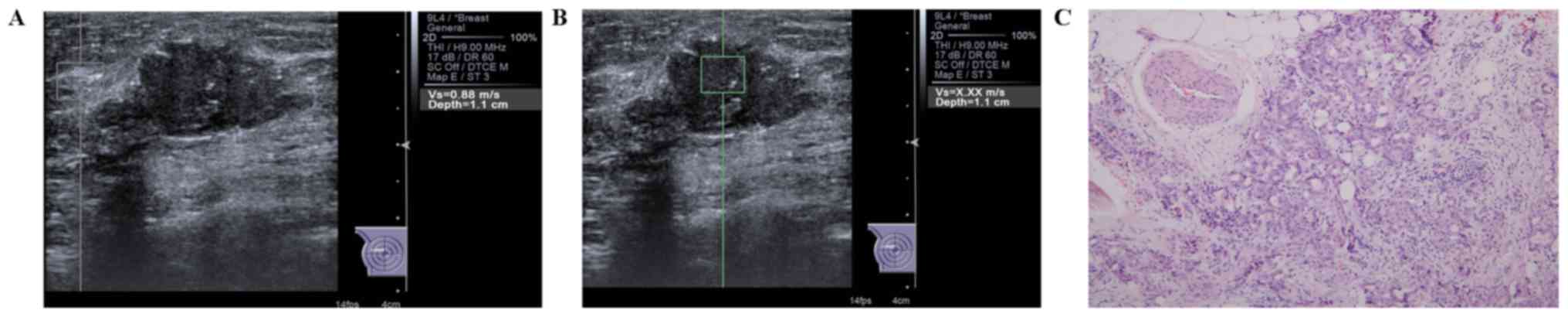 | Figure 3.Evaluation of SWV values in a case of
invasive carcinoma of no special type in the right breast of a
58-year-old female patient. (A) The SWV of the surrounding breast
tissue was 0.88 m/sec, while (B) the SWV of the mass was X.XX
m/sec. The non-applicable situation occurred when the screen
displayed ‘X.XX m/sec’, which may be interpreted as the tissue
being either extremely soft or hard as it exceeded the range of the
SWV value. The value was then denoted as either 9.1 or 0 m/sec,
depending on whether it was the solid portion or the cystic
portion, respectively, once technical failures or other potential
factors, including the patient's breathing, were ruled out. In this
case, the two interpreters agreed to denote the value as 9.1 m/sec.
(C) In terms of histologic grading, the lesion was classified as
Grade II (H&E; magnification, ×100). SWV, shear-wave
velocity. |
 | Table II.SWV values in correlation with
histological grade and lesion size. |
Table II.
SWV values in correlation with
histological grade and lesion size.
|
| SWV |
|---|
|
|
|
|---|
| Value | Minimum | Mean | Maximum |
|---|
| Histologic grade |
|
|
|
| 1 | 3.43 (2.43–4.03) | 4.31 (2.56–4.54) | 4.84 (2.69–5.40) |
| 2 | 3.60 (3.40–4.42) | 4.37 (3.98–4.58) | 4.84 (4.56–5.70) |
| 3 | 5.60 (4.62–6.10) | 6.32 (5.35–6.98) | 7.04 (6.06–7.81) |
| P-value | 0.011 | 0.032 | 0.032 |
| Size (mm) |
|
|
|
|
<20 | 3.30 (2.43–4.43) | 4.71 (3.76–6.72) | 7.37 (6.20–8.60) |
| ≥20 | 3.90
(3.30–5.05) | 6.02
(4.92–7.28) | 5.24
(4.37–6.38) |
| P-value | 0.411 | 0.525 | 0.482 |
Discussion
Although the morbidity of breast carcinoma and the
mortality of affected patients are not excessively high among
Chinese females, the incidence rate in China has been increasing
over the past decades (8). Early
diagnosis is vital for increasing the five-year survival rate and
prognosis, particularly for females with invasive carcinoma of NST.
According to the WHO classification, breast carcinomas may be
classified into three histopathological grades. These grades have
significance for prognostic implications, as breast cancer
encompasses a great diversity of subtypes, grades, risk of
metastasis and prognosis. The histopathological classification
integrates three components that are crucial for breast cancer
development. First, the cell morphology indicates pleomorphism of
the nuclei. Nuclei vary in size and shape; larger and abnormal
nuclei frequently have multiple nucleoli and moderate vesicular
nuclei exhibit visible nucleoli. Second, the percentage of tubule
formation is accepted for quantifying the proliferation. The third
indicator is mitotic activity; the higher the mitotic activity, the
more rapid the proliferation. This well-established grading system
is applied for the unified evaluation of tumor differentiation
together with additional objective criteria. The histopathological
grade acknowledges molecular events that are detected in
histological morphology, which further assists in the
identification of breast carcinoma growth and thus the adoption of
the most appropriate treatment. In general, high levels of
proliferation are commonly observed in poorly-differentiated
masses, while lower well-differentiated masses have lower
proliferation indexes. A number of studies have confirmed the role
of histological prognostic factors in guiding adequate therapies
(9–13).
With regard to conventional ultrasonography, the
results of the present study indicated that the histologic grade
was highly associated with the lesion size and posterior acoustic
features. First, a higher the histologic grade of a lesion was
associated with a larger lesion size. The size of Grade-III BLs was
nearly twice of that of Grade-I lesions. Furthermore, the posterior
acoustic characteristics were identified to be closely associated
with the histologic grade of the lesion. BLs that exhibited
acoustic shadowing were more commonly identified in patients with
grade I and II invasive carcinoma of NST. In total, 78.6% of the
Grade-I BLs and 48.3% of the Grade-II BLs, buy only 29.3% of the
Grade-III BLs featured acoustic shadowing. This result suggests
that different histological grades of BLs had distinct attenuation
characteristics. In the lesions that displayed acoustic shadowing,
a clear growth of fibrous tissue was observed and this growth was
normally accompanied with sclerotic stroma. This may be the reason
why invasive carcinoma of NST often presents with a scirrhous and
stellate appearance. Blaichman et al (14) suggested a strong link between
posterior acoustics and histologic grade after reviewing 299
invasive breast carcinomas of NST of Breast Imaging Reporting and
Data System 4 and 5. The results indicated that only 13 out of 108
Grade-III BLs (12.0%) exhibited acoustic shadowing, while 22
(57.9%) and 67 (43.8%) of Grade-I and -II BLs, respectively, had
the same characteristic. The Grade-III BLs had a greater tendency
to display posterior enhancement (53/108; 49.1%). In addition, the
results of the present study were consistent with those of Rotstein
and Neerhut (15), who determined
that high-grade invasive ductal carcinomas were less prone to
exhibiting acoustic shadowing compared to low- and
intermediate-grade carcinomas. The amount of connective tissue
volume was suggested to be one of the factors that influences
acoustic shadowing. Scirrhous carcinomas frequently consist of rich
connective tissues, which bring about attenuation in neoplastic
tissue and therefore cause acoustic shadowing. Furthermore,
attenuation is also determined by tissue organization, as reported
by Gozzi et al (16). With
regard to acoustic enhancement, Blaichman et al (14) identified that the cellular content
was proportional to acoustic enhancement. Higher-grade BLs
demonstrated a tendency to exert marked desmoplastic effects on
peritumoral tissues, which led to intensive cellularity (14). Therefore, Grade-III BLs have a higher
potential to exhibit posterior enhancement (36.6% of cases in the
present study), whereas intermediate- and low-grade BLs have a
higher probability of exhibiting posterior shadowing (48.3 and
78.6% of cases in the present study, respectively).
The VTQ technique is a quantitative tool for
evaluating tissue stiffness. The basic working principle is
shear-wave propagation, which is induced by a specific acoustic
impulse. Advanced VTQ is able to directly and mechanically detect
the velocities of transverse waves to provide quantified
information on tissue deformations. The SWV is therefore generated
through interference of waves with the tissue and is proportional
to tissue stiffness. In contrast to static elastography, absolute
SWV values expressed in m/sec are obtained by evaluating the peak
displacement at each transverse wave. A number of studies have
evaluated the diagnostic performance of the measurement of
quantitative elasticity values of thyroid nodules (17), metastatic and non-metastatic cervical
lymph nodes (18) and acute
pancreatitis (19). The
elastographic standard deviation was rated as a valuable means of
measuring heterogeneity in the aforementioned studies.
Heterogeneity has been widely accepted as a crucial indicator in
distinguishing between benign and malignant lesions. In the case of
evaluating breast masses, there is an increasing trend of applying
VTQ measurement (20,21). For instance, Tozaki et al
(22) concluded that the mean SWV
value of the malignant neoplasms was larger than that of benign
masses (P<0.01). In addition, Golatta et al (23) examined 104 BLs in 103 patients via
VTQ and concluded that malignant lesions are stiffer than benign
masses in terms of the mean maximum velocity. In the present study,
the association between VTQ parameters and the histological grade
of carcinoma of NST was investigated. The results indicated that
invasive carcinoma of NST of a higher grade tend to be stiffer.
Previous studies investigating the association between
semi-quantitative elastography measurements and histopathological
grading of invasive breast carcinoma have provided similar results
(24,25). In addition to the elastographic
standard deviation, higher minimum and maximum SWV values were also
obtained for higher-grade BLs in the present study. This result may
be highly associated with regulatory factors. With the increasing
histopathological grade, the mitotic figures became more active and
the mitosis was more pronounced. In other words, the malignant
proliferation index was higher. In the present study, it was
demonstrated that higher-degree BLs exerted excessive desmoplastic
effects on peritumoral tissues. Abundant cells conglomerated, which
resulted in larger tension of the lesions and a greater increase in
the synthesis of collagen fibers in the stroma. Consequently, a
higher histopathological grade was associated with a stiffer
lesion. In turn, in the high-grade BLs, the SWV value was greater
than that of the lower-grade BLs.
The present study had a number of limitations.
First, the sample size was not sufficiently large, as the study was
performed at only one hospital. Therefore, further studies with a
larger sample size are necessary to validate the present results.
Furthermore, selection bias was not avoidable due to the
retrospective nature of the study and future prospective study is
required. Owing to its retrospective nature, the present study did
not explore any associations between stiffness and other important
tumor characteristics, including the vascularity of the lesions,
which is considered as an important feature for increasing the
diagnostic specificity.
In conclusion, tissue stiffness of invasive
carcinoma of NST is highly associated with the histopathological
grade. The higher the pathological grade, the stiffer the BL.
Lesion size, posterior acoustic characteristics and tissue
stiffness together with histopathological grade are important
complementary prognostic factors for evaluating invasive carcinoma
of NST. A better understanding of histopathological grading and
tissue stiffness are essential in order to avoid unnecessary
surgery. Therefore, shear-wave elastography may enhance the meaning
of histological grading and provide an important clinical reference
value.
Acknowledgements
Not applicable.
Funding
The present study was supported by Pu Dong New Area
Health and Family Planning Commission Subject Leader Course Project
(grant no. PWRd 2017-06) and Pu Dong New Area Health and Family
Planning Commission Important Vulnerable Course Project (grant no.
PWzbr 2017-10).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Ethics approval and consent to
participate
The Ethics Committee of Shanghai Pudong New Area
People's Hospital (Shanghai, China) approved the retrospective
study of the images and associated records of the enrolled
patients.
Authors' contributions
YCZ designed the study DSW ollected and analyzed the
patients' data regarding pathology. SHD collected the patients'
data regarding tissue stiffness. YZ, QJ and SHD interpreted the
data. YCZ was a major contributor in writing the manuscript. All
authors read and approved the final manuscript.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zuo TT, Zheng RS, Zeng HM, Zhang SW and
Chen WQ: Female breast cancer incidence and mortality in China,
2013. Thorac Cancer. 8:214–218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schatten H: Cell and molecular biology of
breast cancer. Humana Press; 2013, View Article : Google Scholar
|
|
3
|
Reed McCart AE, Kalaw E, Reid A and
Lakhani SR: Breast Cancer—Pathology and GeneticsReference Module in
Biomedical Sciences. Elsevier; 2018, View Article : Google Scholar
|
|
4
|
Luo J, Cao Y, Nian W, Zeng X, Zhang H, Yue
Y and Yu F: Benefit of shear-wave elastography in the differential
diagnosis of breast lesion: A diagnostic meta-analysis. Med
Ultrason. 1:43–49. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chamming's F, Latorre-Ossa H, Le
Frere-Belda MA, Fitoussi VT, Quibel T, Assayag F, Marangoni E,
Autret G, Balvay D, Pidial L, et al: Shear wave elastography of
tumour growth in a human breast cancer model with pathological
correlation. Eur Radiol. 23:2079–2086. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lakhani SR, Ellis IO, Schnitt SJ, Tan PH
and van de Vijver MJ: WHO Classification of Tumours of the Breast
(4th). 2012.
|
|
7
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer I. The value of histological
grade in breast cancer: Experience from a large study with
long-term follow-up. Histopathology. 41:154–161. 2002.PubMed/NCBI
|
|
8
|
Li T, Mello-Thoms C and Brennan PC:
Descriptive epidemiology of breast cancer in China: Incidence,
mortality, survival and prevalence. Breast Cancer Res Treat.
159:395–406. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang J, Clark GM, Allred DC, Mohsin S,
Chamness G and Elledge RM: Survival of patients with metastatic
breast carcinoma: Importance of prognostic markers of the primary
tumor. Cancer. 97:545–553. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weidner N, Cady B and Goodson WH III:
Pathologic prognostic factors for patients with breast carcinoma:
Which factors are important. Surg Oncol Clin N Am. 6:415–462. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Irshad A, Ackerman SJ, Pope TL, Moses CK,
Rumboldt T and Panzegrau B: Rare breast lesions: Correlation of
imaging and histologic features with who classification.
Radiographics. 28:1399–1414. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang WS, Hardesty L, Borgstede J,
Takahashi J and Sams S: Breast cancers found with digital breast
tomosynthesis: A comparison of pathology and histologic grade.
Breast J. 22:651–656. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim TH, Kang DK, Kim JY, Han S and Jung Y:
Histologic grade and decrease in tumor dimensions affect axillary
lymph node status after neoadjuvant chemotherapy in breast cancer
patients. J Breast Cancer. 18:394–399. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blaichman J, Marcus JC, Alsaadi T,
El-Khoury M, Meterissian S and Mesurolle B: Sonographic appearance
of invasive ductal carcinoma of the breast according to histologic
grade. Am J Roentgenol. 199:402–408. 2012. View Article : Google Scholar
|
|
15
|
Rotstein AH and Neerhut PK: Ultrasound
characteristics of histologically proven grade 3 invasive ductal
breast carcinoma. Australas Radiol. 49:476–479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gozzi G, Cressa C, Bazzocchi M, Stanta G
and Vidali C: Causes of attenuation of the sound waves in neoplasms
of the breast: Histologic and echographic correlation study (In
Italian). Radiol Med. 72:195–198. 1986.PubMed/NCBI
|
|
17
|
Zhang FJ, Han RL and Zhao XM: The value of
virtual touch tissue image (VTI) and virtual touch tissue
quantification (VTQ) in the differential diagnosis of thyroid
nodules. Eur J Radiol. 83:2033–2040. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao Y, Xi J, Zhao B, Xiong W, Jiang D,
Yang L, Cai Z, Liu T, Jiang H, Rong S and Jin X: Preliminary
evaluation of virtual touch tissue imaging quantification for
differential diagnosis of metastatic and nonmetastatic cervical
lymph nodes. J Ultrasound Med. 36:557–563. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Onoyama T, Koda M, Fujise Y, Takata T,
Kawata S, Okamoto T, Miyoshi K, Matono T, Sugihara T, Matsumoto K,
et al: Utility of virtual touch quantification in the diagnosis of
pancreatic ductal adenocarcinoma. Clin Imaging. 42:64–67. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin ZQ, Li XR, Zhou HL, Chen JX, Huang X,
Dai HX, Li JW, Chen XD and Xu XH: Acoustic radiation force impulse
elastography of breast imaging reporting and data system category 4
breast lesions. Clin Breast Cancer. 12:420–427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou BG, Wang D, Ren WW, Li XL, He YP, Liu
BJ, Wang Q, Chen SG, Alizad A and Xu HX: Value of shear wave
arrival time contour display in shear wave elastography for breast
masses diagnosis. Sci Rep. 7:70362017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tozaki M, Isobe S and Fukuma E:
Preliminary study of ultrasonographic tissue quantification of the
breast using the acoustic radiation force impulse (ARFI)
technology. Eur J Radiol. 80:e182–e187. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Golatta M, Schweitzer-Martin M, Harcos A,
Schott S, Gomez C, Stieber A, Rauch G, Domschke C, Rom J, Schütz F,
et al: Evaluation of virtual touch tissue imaging quantification, a
new shear wave velocity imaging method, for breast lesion
assessment by ultrasound. Biomed Res Int. 2014:9602622014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Evans A, Whelehan P, Thomson K, McLean D,
Brauer K, Purdie C, Baker L, Jordan L, Rauchhaus P and Thompson A:
Invasive breast cancer: Relationship between shear-wave
elastographic findings and histologic prognostic factors.
Radiology. 263:673–677. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Youk JH, Gweon HM, Son EJ, Kim JA and
Jeong J: Shear-wave elastography of invasive breast cancer:
Correlation between quantitative mean elasticity value and
immunohistochemical profile. Breast Cancer Res Treat. 138:119–126.
2013. View Article : Google Scholar : PubMed/NCBI
|