Introduction
Deficiency or injury of the endothelium is closely
associated with the initiation of the atherosclerotic process
(1). Endothelial progenitor cells
(EPCs) are mainly involved in vascular regeneration, angiogenesis
and re-endothelialization following vascular damage (2). Thus, it is important to retain the
number and function of EPC at normal levels in patients with
cardiovascular disease.
Ambient particulate matter (PM) has become a major
health threat. Increases in mortality and morbidity due to PM
exposure have been reported (3). PM
is a mixture of different types of debris, the major components of
which are crustal material, metals and bio-aerosols (4). Small PM with a median aerodynamic
diameter of ≤2.5 µm (PM2.5) is the most harmful type and
numerous cardiovascular diseases are associated with exposure to
PM2.5 (5).
PM2.5 exposure causes a reduction in heart rate
variability, as well as vascular dysfunction, vascular
inflammation, an increased coagulation-thrombosis risk and the
acceleration of atherosclerosis (5).
More importantly, in humans and mice, a decreased number and
function of EPC are also associated with PM2.5 exposure
(6,7). A previous study by our group indicated
that exposure of mice to PM of ≤4 µm in size (including
PM2.5) through intranasal instillation for one month
significantly suppressed the number and function of EPCs via
increasing blood intracellular reactive oxygen species (ROS)
production and inflammatory cytokine generation (8). However, the detailed mechanisms by
which the number and function of EPCs decrease after inhalation of
atmospheric PM2.5 have remained to be elucidated.
A notable reduction in atherosclerosis and
restenosis in the coronary artery were observed in subjects treated
with probucol (9,10). This potent drug has anti-oxidant and
anti-inflammatory effects and preserves endothelial function by
reducing the amount of endogenous nitric oxide (NO) synthase
inhibitor, increasing prostacyclin generation, inhibiting the
expression of various adhesion molecules and promoting the
proliferation of endothelial cells, while preventing the apoptosis
of endothelial cells due to oxidative injury (9,10). Of
note, cigarette smoking and oxidized high-density lipoprotein
(Ox-HDL) induced EPC dysfunction may also be reversed by probucol
treatment (11,12). However, the effects of probucol on
EPCs exposed to PM2.5 remain elusive.
The aim of the present study was to determine
whether probucol has any protective effects on EPCs in mice exposed
to PM2.5. It was observed that the diminished EPC levels
in mice under PM2.5 exposure were indeed restored to
normal levels with probucol treatment.
Materials and methods
Animal model of PM2.5
exposure
All animal procedures were performed in accordance
with the Guidelines of the Animal Care Committee of the Shandong
Provincial Hospital affiliated with Shandong University (Jinan,
China). The Animal Care Committee of Shandong University (Jinan,
China) approved the experimental protocols. A total of 40 male
wild-type C57 BL/6 mice (age, 6–8 weeks; weight, 20–25 g) were
purchased from Better Biotechnology Co., Ltd. (Nanjing, China). All
mice were housed at the animal facility for 1 week prior to
exposure. The center of Jinan city (China), a highly polluted area,
was selected for the experiment. The exposure period lasted one
month from December 12, 2016 to January 12, 2017. Ambient PM with a
diameter equal to 2.5 µm was collected using a high flow rate with
an aerosol-into-liquid collector (HRH-PM186; Beijing Huironghe
Technology Co., Ltd., Beijing, China). The mean concentration of PM
in Jinan measured during the experiment was 135.23±42.12
µg/m3. This data was in accordance with previously
published data (13). The
concentration of PM for mice with exposure and for mice with
filtered air (FA; the control mice) was adjusted to 130±65.51 and
2.4±1.1 µg/m3, respectively. A total of 10 mice in each
group were subjected to PM exposure or used as controls who inhaled
FA. All mice were exposed in a chamber system for the experiment as
described (14). The high-efficiency
particulate air filter (Pall Life Sciences; Pall Corporation, Port
Washington, NY, USA) was used for mice with FA exposure. Probucol
was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
To evaluate the protective effect of probucol on EPC from
PM2.5 mice, 5 mg probucol was first dissolved in 100 µl
99% ethanol, then diluted in 99.9 ml PBS to give a final
concentration of 50 µg/ml. The final concentration of ethanol was
adjusted to 1‰ in PBS. Then, the probucol solution was used to
treat mice at a dose of 500 mg/kg/day (10). In a preliminary experiment, probucol
at 100, 250, 500 and 600 mg/kg/day was used to treat the mice
exposed to PM2.5. The maximum anti-oxidant and
anti-inflammatory effects of probucol were observed at the dosage
of 500 mg/kg/day, while no further increases in the protective
effects of probucol were achieved when the dose was 600 mg/kg/day.
Probucol was administered by oral gavage for three days prior to
PM2.5 exposure and for one month during PM2.5
exposure. A total of 40 mice were equally divided into four groups:
The FA group, 10 mice with FA exposure; the PM2.5 group,
10 mice with PM2.5 exposure; the Prob+FA group, 10 mice
with probucol treatment and FA exposure; the Prob+PM2.5
group, 10 mice with probucol treatment and PM2.5
exposure.
Measurement of pro-inflammatory
factors
Mouse serum was collected after one month of
PM2.5 exposure. The pro-inflammatory cytokines tumor
necrosis factor (TNF)-α (cat. no. 430904), interleukin (IL)-1β
(cat. no. 432604) and IL-6 (cat. no. 431304) were measured with an
ELISA kit (BioLegend, San Diego, CA, USA) according to the
manufacturer's protocols.
Assessment of EPC proliferation,
apoptosis and intracellular ROS formation
After PM2.5 or FA exposure, murine blood
was collected, followed by the elimination of red blood cells
(RBCs) with an RBC lysis buffer (cat. no. 420301; BioLegend). The
in vivo EPC number and proliferation rate were measured at
12 h after i.p. injection of 1 mg bromodeoxyuridine (BrdU).
CD34-Alexa Fluor® 700 (cat. no. 560518; BD Biosciences,
Franklin Lakes, NJ, USA) and CD133-phycoerythrin (cat. no. 141204;
BioLegend) antibodies were used to mark the EPC population.
Specifically, 1 µl CD34 and 1 µl CD133 in 100 µl cell staining
buffer (420201; BioLegend) were added to 1×106 cells.
Then the mixture solution was incubated in ice in a dark room for
30 min. In order to quantify the EPC population, the
CD34+/CD133+ cells were detected in a sample
containing at least 50,000 cells. Anti-BrdU fluorescein
isothiocyanate (FITC) contained in the BrdU Flow Kit (cat. no.
559619; BD Biosciences) was used to measure the cell proliferation.
Blood EPC apoptosis was determined by using the FITC Annexin V
Apoptosis Detection kit (cat. no. 556547; BD Biosciences). Early
[Annexin V FITC-positive and propidium iodide (PI)-negative cells]
and late (Annexin V FITC and PI double-positive cells) apoptotic
cells were measured. Total ROS Assay kit (Thermo Fisher Scientific,
Inc., Waltham, MA, USA), which contained dichlorofluorescein
(DCF)-FITC, was used to measure the intracellular ROS production.
Specifically, following the staining of cells with CD34+
and CD133+ antibodies, cells were washed twice with PBS.
Then, DCF-FITC was added in the mixture solution for 10 min at
37°C. The DCF-FITC-labelled cells were washed twice with PBS and
then suspended in warm PBS (37°C) for analysis using flow
cytometry. The CD34+/CD133+ cells with
DCF-FITC fluorescence-positive cells were quantitatively evaluated
using a BD™ LSR II flow cytometer (BD Biosciences) at
the wavelength of 525 nm, as previously described (8).
Statistical analysis
Values are expressed as the mean ± the standard
deviation. PRISM version 4.0 (GraphPad Software, Inc., La Jolla,
CA, USA) was used for the statistical analyses. The unpaired
Student's t-test (two-sided) was used for comparison between two
groups. One-way analysis of variance followed by a post-hoc
conservative Tukey's test were used for comparison between three or
more groups to minimize type-I errors as appropriate. A two-tailed
P<0.05 was considered to indicate a statistically significant
difference.
Results
PM2.5 treatment reduces
circulating EPCs in association with increased apoptosis and
decreased proliferation
After exposure to PM2.5 for one month,
murine blood cells were collected for EPC analysis. The results
indicated that PM2.5 significantly decreased the
CD34+/CD133+ cell population (0.017±0.007%)
compared with that in the control (0.037±0.012%; Fig. 1A). To identify the possible reasons
for the decrease in the EPC population, the EPC proliferation and
apoptotic rate were assessed. As presented in Fig. 1B, the EPC proliferation rate was
significantly decreased compared with that in the control group
(0.012±0.004% vs. 0.026±0.005%). Furthermore, the early apoptotic
rate (6.74±0.67%) and the late apoptotic rate (14.04±4.38%) of EPCs
were substantially elevated compared with those in the control
group (3.48±0.51 and 6.58±1.77%, respectively; Fig. 1C).
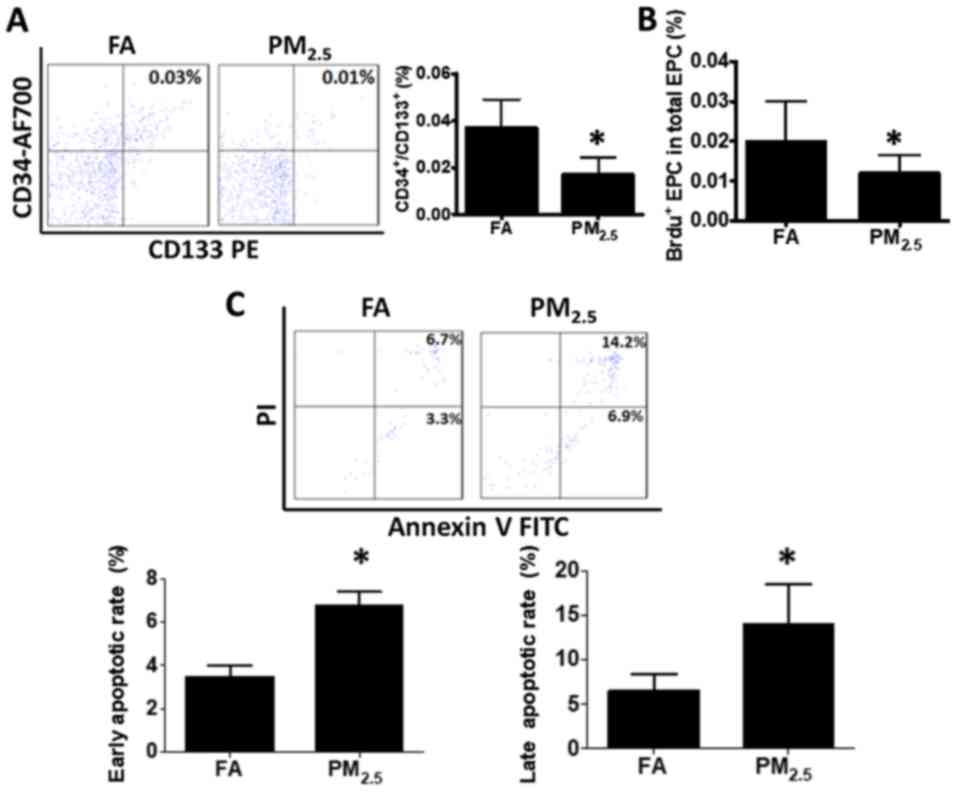 | Figure 1.Exposure to PM2.5
decreases circulating EPC levels in mice by suppression of EPC
proliferation and induction of EPC apoptosis. (A) Following
PM2.5 or FA exposure for 1 month, the blood cells from
C57BL/6 mice were collected and stained with CD34-AF700 and
CD133-PE antibody for quantification of EPCs
(CD34+/CD133+). The amount of EPCs was
significantly decreased in C57BL/6 mice subjected to
PM2.5 exposure compared with that in the FA control. (B)
After PM2.5 exposure, mice were injected
intraperitonally with 1 mg BrdU. After 12 h, cells were obtained,
permeabilized and incubated with anti-BrdU FITC after staining with
CD34-AF700 and CD133-PE antibody. The proliferation rate of murine
EPCs was significantly declined with PM2.5 exposure
compared with that in the FA group. (C) Blood cell apoptosis was
measured by staining with annexin V and PI. The early and late
apoptotic rate of murine blood cells was significantly increased
with PM2.5 exposure as compared with that in the FA
group. Values are expressed as the mean ± standard deviation
(n=10). *P<0.05 vs. the FA group. EPCs, bone marrow-derived
circulating endothelial progenitor cells; FA, filtered air;
PM2.5, ambient fine particulate matter of ≤2.5 µm in
diameter; PE, phycoerythrin; PI, propidium iodide; FITC,
fluorescein isothiocyanate; BrdU, bromodeoxyuridine; AF700, Alexa
Fluor® 700. |
PM2.5 increases serum
inflammatory factors
The serum levels of inflammatory factors, including
TNF-α, IL-1β and IL-6, are known to be closely associated with an
increased blood cell apoptosis and decreased circulating EPC
proliferation (8,15). In the present study, the serum levels
of TNF-α, IL-1β and IL-6 were measured after PM2.5
exposure. As presented in Fig. 2,
the TNF-α and IL-1β levels were increased to up to 2-fold of those
in the control group, while IL-6 was 6-fold of that in the control
group.
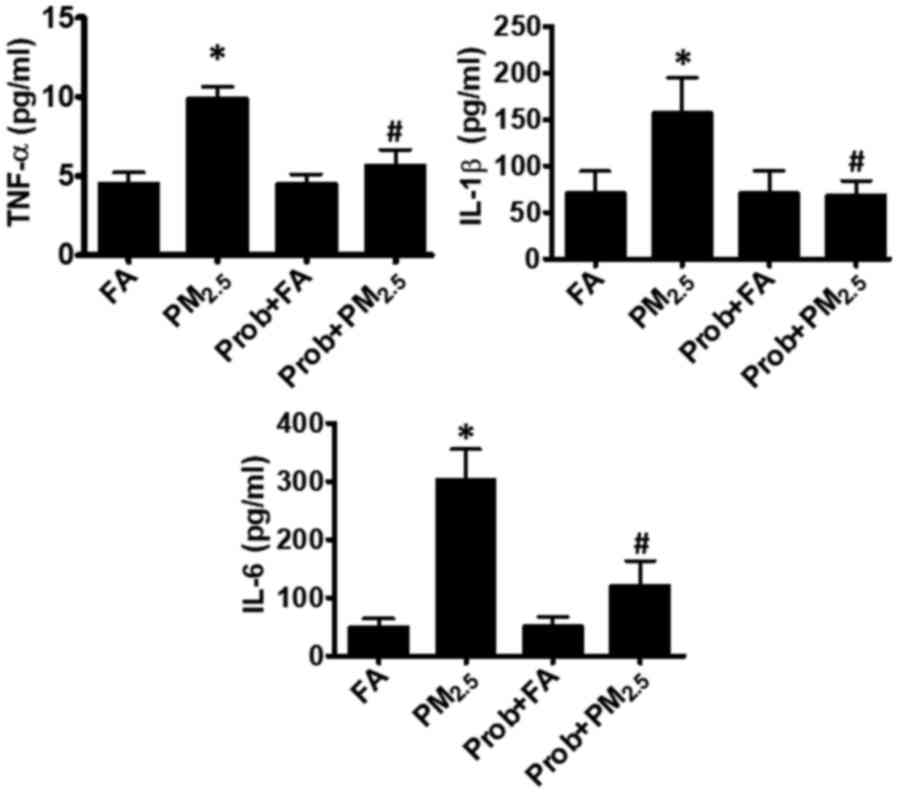 | Figure 2.Prob treatment inhibits increases in
inflammatory cytokines following PM2.5 exposure.
Elevation of the inflammatory cytokines, TNF-α, IL-1β and IL-6,
induced by PM2.5 was effectively prevented by Prob.
Groups: FA, C57BL/6 mice kept in ambient FA; PM2.5,
C57BL/6 mice with PM2.5 exposure; Prob+FA, C57BL/6 mice
with Prob treatment kept in FA; Prob+PM2.5, C57BL/6 mice
with Prob treatment and PM2.5 exposure. Values are
expressed as the mean ± standard deviation (n=10). *P<0.05,
PM2.5 vs. FA or Prob+FA or Prob+PM2.5;
#P<0.05, FA vs. Prob+FA or PM2.5 vs.
Prob+PM2.5. IL, interleukin; TNF, tumor necrosis factor;
FA, filtered air; PM2.5, ambient fine particulate matter
of ≤2.5 µm in diameter; Prob, probucol. |
PM2.5 increases
intracellular ROS levels in blood EPCs
It has been previously reported that intracellular
ROS may cause an elevation of blood cell apoptosis and a decline of
EPC proliferation (8,15). After exposure to PM2.5 for
one month, the ROS levels in the blood EPCs were assessed. As
presented in Fig. 3, the blood
intracellular ROS levels were significantly increased in the mice
with PM2.5 exposure compared to those in the control
group (29.14±2% vs. 18.96±3.14%).
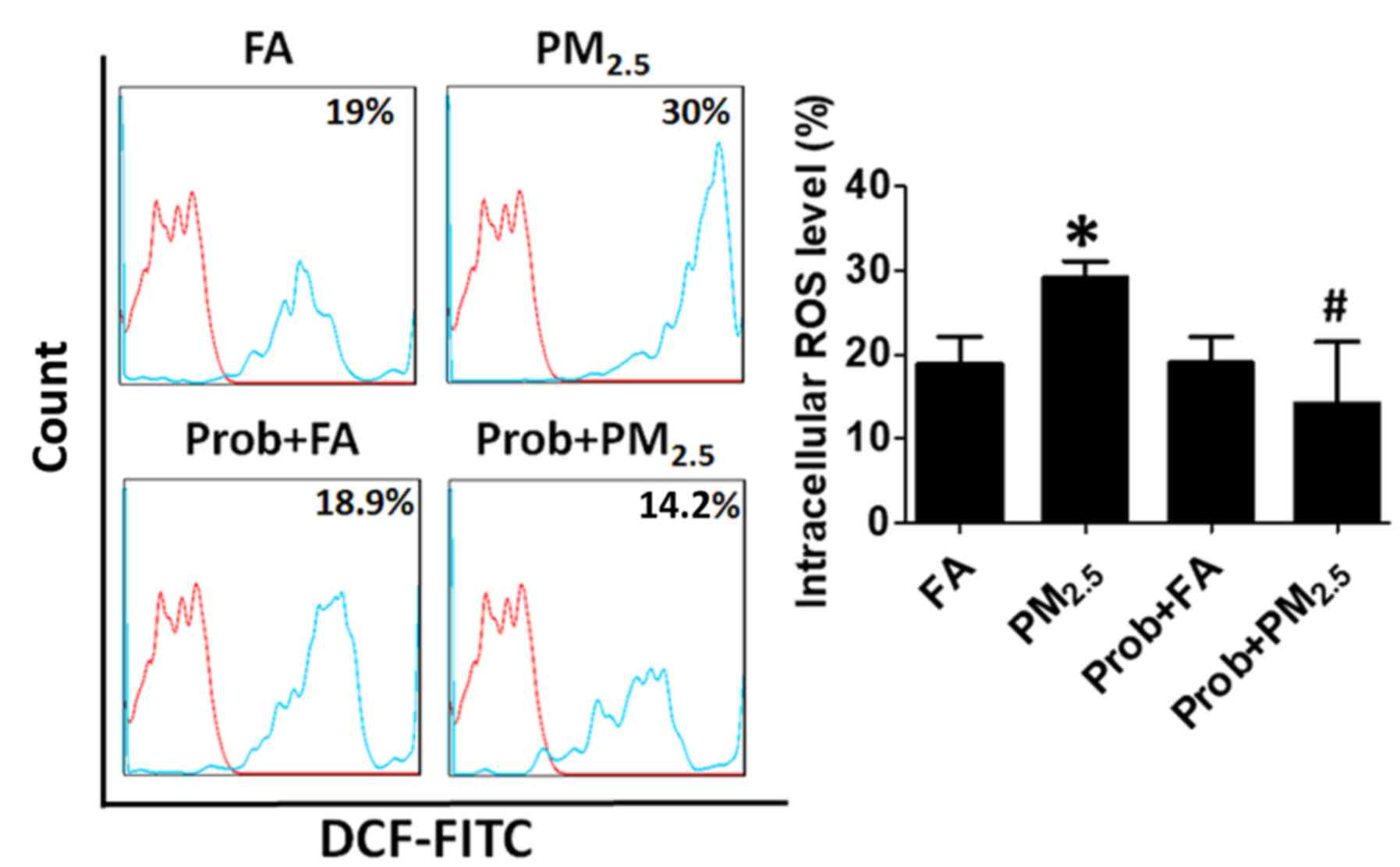 | Figure 3.Prob treatment decreases ROS
production induced by PM2.5 exposure. Increased ROS
production in the blood EPCs of mice exposed to PM2.5
was effectively blocked by Prob treatment. Blood EPCs without DCF
staining was used as baseline in the histograph. Groups: FA,
C57BL/6 mice kept in ambient FA; PM2.5, C57BL/6 mice
with PM2.5 exposure; Prob+FA, C57BL/6 mice with Prob
treatment kept in FA; Prob+PM2.5, C57BL/6 mice with Prob
treatment and PM2.5 exposure. Values are expressed as
the mean ± standard deviation (n=10). *P<0.05, PM2.5
vs. FA or Prob+FA or Prob+PM2.5; #P<0.05,
FA vs. Prob+FA or PM2.5 vs. Prob+PM2.5. FA,
filtered air; PM2.5, ambient fine particulate matter of
≤2.5 µm in diameter; Prob, probucol; ROS, reactive oxygen species;
DCF-FITC, dichlorofluorescein-fluoresceinisothiocyanate. |
Probucol treatment attenuates the
detrimental effects of PM2.5 on EPCs
To evaluate the protective effect of probucol
against the reduction of circulating EPCs due to PM2.5
exposure, mice were pre-treated with probucol prior
PM2.5 exposure for 3 days and treatment was continued
for 1 month with PM2.5 exposure. The intracellular ROS
production was completely blocked by probucol treatment (Fig. 3). In the probucol treatment group,
the PM2.5-associated elevated serum inflammatory factors
(TNF-α, IL-1β and IL-6) were also reduced to the normal level of
the control group (Fig. 2).
To determine whether probucol treatment of mice
exposed to PM2.5 is able to restore the circulating EPC
proliferation and blood EPCs apoptotic rate to the normal level,
the EPC proliferation and blood cell apoptotic rate were measured
after probucol treatment. As presented in Fig. 4B, the EPC proliferation rate was
recovered with probucol treatment, while the early and late
apoptotic rate of blood cells were also restored to the normal
level in the mice with probucol treatment (Fig. 4C).
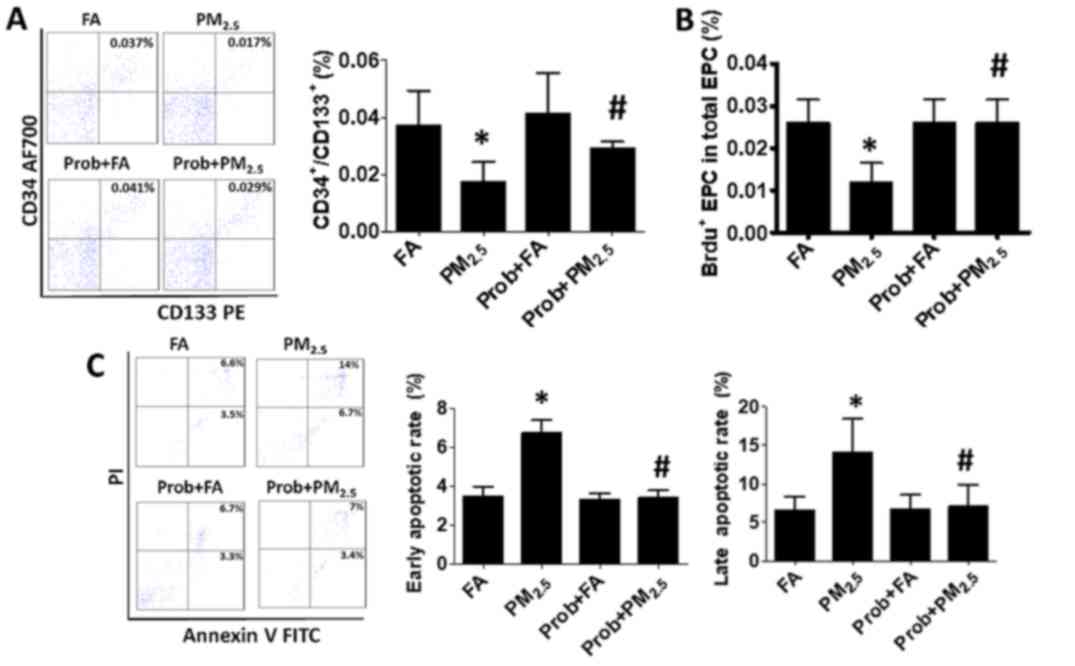 | Figure 4.Prob treatment recovers the EPC
population in mice exposed to PM2.5 by restoring EPC
proliferation and preventing EPC apoptosis. (A and B) The decreased
(A) EPC population and (B) EPC proliferation due to
PM2.5 exposure were effectively recovered with Prob
treatment. (C) The early and late apoptotic rate were restored to
the normal level with Prob treatment. Groups: FA, C57BL/6 mice kept
in ambient FA; PM2.5, C57BL/6 mice with PM2.5
exposure; Prob+FA, C57BL/6 mice with Prob treatment kept in FA;
Prob+PM2.5, C57BL/6 mice with Prob treatment and
PM2.5 exposure. Values are expressed as the mean ±
standard deviation (n=10). *P<0.05, PM2.5 vs. FA or
Prob+FA or Prob+PM2.5; #P<0.05, FA vs.
Prob+FA or PM2.5 vs. Prob+PM2.5. EPCs, bone
marrow-derived circulating endothelial progenitor cells; FA,
filtered air; PM2.5, ambient fine particulate matter of
≤2.5 µm in diameter; PE, phycoerythrin; PI, propidium iodide; FITC,
fluorescein isothiocyanate; BrdU, bromodeoxyuridine; AF700, Alexa
Fluor® 700; Prob, probucol. |
Finally, it was assessed whether probucol was able
to prevent the reduction in EPCs after PM2.5 exposure.
As presented in Fig. 4A, the
decrease in CD34+/CD133+ cells (0.017±0.007%)
associated with PM2.5 exposure was inhibited by probucol
treatment, resulting in near normal levels (0.029±0.002%).
These data indicated that probucol effectively
inhibited the effects of PM2.5 on murine circulating
EPCs via the inhibiton of blood cell apoptosis and increase in EPCs
proliferation through the blocking of intracellular ROS production
and inflammatory cytokine secretion (Fig. 5).
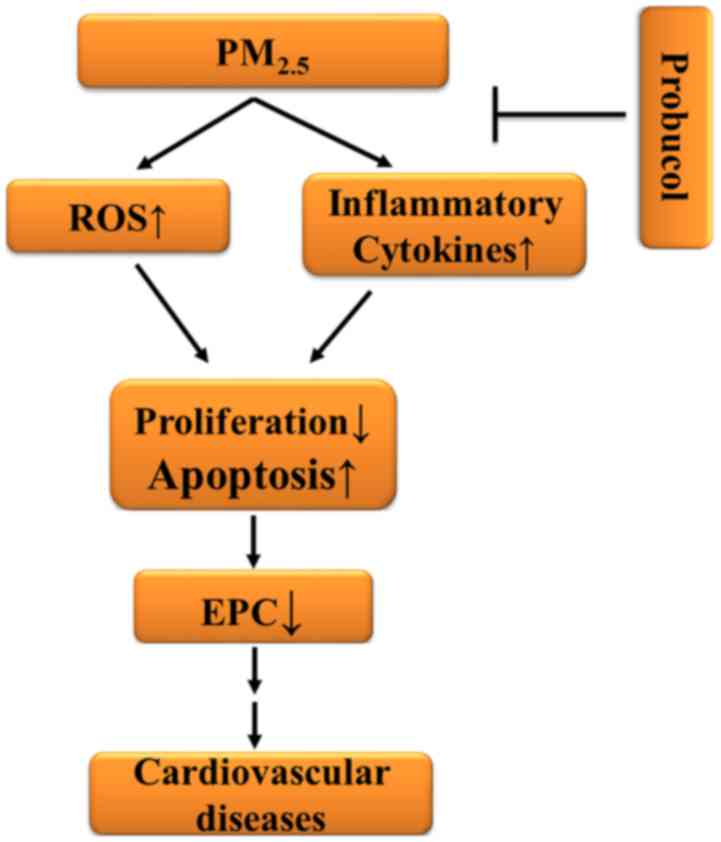 | Figure 5.Schematic illustrating the possible
mechanisms for the decreased number of circulating EPCs in the mice
subjected to PM2.5 exposure. Blood intracellular ROS
production and inflammatory cytokine levels were elevated following
PM2.5 exposure. The proliferation rate of the EPCs was
notably decreased, while the apoptotic rate of blood EPCs was
significantly increased. As a result, the number of circulating
EPCs was extensively decreased, which is associated with the
development of cardiovascular diseases. Prob efficiently inhibited
the PM2.5-induced ROS generation, increase in
inflammatory cytokines and apoptotic rate of blood cells, and
decrease in EPC proliferation. The number of circulating EPCs was
significantly increased with Prob treatment after PM2.5
exposure. ROS, reactive oxygen species; EPC, endothelial progenitor
cell; ↑, increase; ↓, decrease; Prob, probucol; PM2.5,
ambient fine particulate matter of ≤2.5 µm in diameter. |
Discussion
In the present study, it was demonstrated that
exposure to PM2.5 induced apoptosis of blood cells and
suppressed EPC proliferation to reduce the number of EPCs via
increasing the blood intracellular ROS levels and serum
inflammatory cytokine levels. However, probucol prevented the blood
intracellular ROS generation, increases in serum inflammatory
cytokines and EPC apoptosis. The proliferation rate and the
percentage of circulating EPCs were also recovered with probucol
treatment.
It has been widely reported that PM2.5
exposure is associated with various systemic diseases, including
cardiovascular (5), neuronal
(16) and hepatic disease (17), as well as diabetes (18). The mechanisms mainly involve
PM2.5 triggering systemic oxidative stress and
inflammation (19). Of note, the
increases in ROS production and the levels of inflammatory factors
following PM exposure are associated with endothelial injury as
well as a decreased number of EPCs (20,21). The
results of the present study also suggested that intracellular ROS
production as well as serum inflammatory factors were increased
after PM2.5 exposure. These contributed to the decrease
in the number of circulating EPCs, which is associated with an
increased risk of cardiovascular disease.
It is well documented that after endothelial injury,
EPCs contribute to angiogenesis and vascular regeneration, as well
as maintaining a normal endothelial function (2). Of note, increases in cardiovascular
disease are associated with a decreased number and function of EPCs
after PM exposure (5). It has been
reported that after PM2.5 or nickel exposure, the number
of murine bone marrow and circulating EPCs
(CD34+/CD31+/CD45+/CD133+)
was decreased (21), and the
function of EPCs (CD34+/vascular endothelial growth
factor receptor-2+/CD11b−), including tube
formation and chemotaxis, were also significantly suppressed
(6). A study on a Chinese cohort
also reported that circulating EPCs [CD34+/kinase insert
domain receptor (KDR)+,
CD34+/KDR+/CD45− or
CD34+/KDR+/CD133+] were notably
reduced following PM2.5 exposure (22). Furthermore, in accordance with a
previously published study (8), the
present results indicated that PM exposure significantly suppressed
the circulating EPC population in mice through promoting apoptosis
of EPCs (CD34+/CD133+) in association with an
elevated ROS generation as well as serum TNF-α and IL-1β levels
in vivo. Furthermore, the present study confirmed that
inhalation of ambient PM2.5 suppressed the proliferation
of EPCs and promoted their apoptosis via increasing the levels of
ROS in blood cells and of TNF-α, IL-1β and IL-6 in the serum.
However, certain studies reported that circulating EPCs increased
following short-term PM2.5–10 exposure (7,23). The
mechanisms were described to mainly involve sympathetic nervous
system activation and decreased mobilization of bone marrow EPCs
into the circulation through a systemic reaction to an acute
‘endothelial injury’ following PM exposure.
Reagents and methods for the protection of EPCs have
been reported in numerous studies and included the use of microRNA,
triterine, granulocyte-macrophage colony-stimulating factor,
urinary trypsin and inhibition of CD40 (24–27). Of
note, probucol, as a cholesterol modulator, significantly inhibits
the initiation and progression of atherosclerosis. The mechanisms
mainly include the suppression of ROS formation (10), promotion of endothelial recovery,
inhibition of monocyte activation and adhesion (28), attenuation of vascular smooth muscle
cell (VSMC) growth and migration (29), an influence on VSMC and macrophage
proliferation and apoptosis, as well as a decrease of cytokine
secretion by macrophages (30,31). Of
note, the cigarette smoke-induced impairment and ischemia-triggered
neovascularization was rescued by probucol through its protective
effects on EPCs (11). The
deleterious effects of Ox-HDL on EPCs were also reversed by
probucol (12). In addition,
probucol prevents ROS-induced inactivation of endothelium-derived
NO, decreases endogenous NO synthase inhibitor formation and
increases the level and function of NO to further benefit EPCs
(9,10,32). In
the present study, after the treatment of mice with probucol at 500
mg/kg/day for one month, it was observed the population and the
proliferation of circulating EPCs were effectively restored, EPC
apoptosis was inhibited, ROS formation blocked and serum
inflammatory cytokines were reduced in the mice with
PM2.5 exposure.
Although the benefits of probucol on EPCs have been
reported in numerous studies, the detailed mechanisms of its
protective effects on EPCs following PM2.5 exposure have
remained elusive. Further questions, including what specific type
of ROS is generated following PM2.5 exposure, whether
any other mechanisms are involved in the protection of EPCs by
probucol, and whether ROS and inflammatory cytokines that are
induced by PM2.5 exposure are the two major factors that
impair EPCs remain to be addressed in further studies. Future
projects, including an experiment to discriminate between different
types of ROS generated in EPCs and the application of vitamin C
after PM2.5 exposure, are currently in planning.
In conclusion, the present study indicated that
probucol effectively prevented the effects of PM2.5 on
murine circulating EPCs via inhibition of blood cell apoptosis and
recovery of EPC proliferation through blocking of blood
intracellular ROS generation and inflammatory cytokine secretion.
Thus, probucol may be an effective medicine for the prevention and
treatment of PM2.5-induced cardiovascular diseases.
Acknowledgements
The manuscript was revised by Dr. Jiangbing Li
(Department of Cardiology, Shandong Provincial Hospital affiliated
to Shandong University, Jinan, China).
Funding
The current work was supported by grants from The
National Nature Science Foundation of China to YC (grant no.
81600222), the Shandong Provincial Nature Science Foundation of
China to LC (grant no. ZR2016HM22), and the Clinical Medical
Science and Technology Innovation Development Plan Project of Jinan
in China to LC (grant no. 201704106).
Availability of data and materials
All data generated or analyzed during the current
study are included in this published article.
Authors' contributions
YC, LC, QS and ZL designed the experiments, and YC,
KH, LY, HX, PZ and HS performed them. ZS, HL, LC and HB collected
and analyzed the data. YC wrote the manuscript.
Ethical approval and consent to
participate
The Animal Care Committee of Shandong University
(Jinan, China) approved the experimental protocols.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Natarelli L and Schober A: MicroRNAs and
the response to injury in atherosclerosis. Hamostaseologie.
35:142–150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laurenzana A, Fibbi G, Margheri F,
Biagioni A, Luciani C, Del Rosso M and Chillà A: Endothelial
progenitor cells in sprouting angiogenesis: Proteases pave the way.
Curr Mol Med. 15:606–620. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crouse DL, Peters PA, Hystad P, Brook JR,
van Donkelaar A, Martin RV, Villeneuve PJ, Jerrett M, Goldberg MS,
Pope CA III, et al: Ambient PM2.5, O3 and NO2
Exposures and associations with mortality over 16 years of
follow-up in the Canadian census health and environment cohort
(CanCHEC). Environ Health Perspect. 123:1180–1186. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Robertson S and Miller MR: Ambient air
pollution and thrombosis. Part Fibre Toxicol. 15:12018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cui Y, Sun Q and Liu Z: Ambient
particulate matter exposure and cardiovascular diseases: A focus on
progenitor and stem cells. J Cell Mol Med. 20:782–793. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liberda EN, Cuevas AK, Gillespie PA,
Grunig G, Qu Q and Chen LC: Exposure to inhaled nickel
nanoparticles causes a reduction in number and function of bone
marrow endothelial progenitor cells. Inhal Toxicol. 22 Suppl
2:S95–S99. 2010. View Article : Google Scholar
|
|
7
|
Brook RD, Bard RL, Kaplan MJ, Yalavarthi
S, Morishita M, Dvonch JT, Wang L, Yang HY, Spino C, Mukherjee B,
et al: The effect of acute exposure to coarse particulate matter
air pollution in a rural location on circulating endothelial
progenitor cells: Results from a randomized controlled study. Inhal
Toxicol. 25:587–592. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cui Y, Xie X, Jia F, He J, Li Z, Fu M, Hao
H, Liu Y, Liu JZ, Cowan PJ, et al: Ambient fine particulate matter
induces apoptosis of endothelial progenitor cells through reactive
oxygen species formation. Cell Physiol Biochem. 35:353–363. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang M, Hou Y, Shen Y, Guo X, Shang D and
Zhang D: Probucol reverses homocysteine induced inflammatory
monocytes differentiation and oxidative stress. Eur J Pharmacol.
818:67–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Q, Chen L, Si Z, Bu H, Narasimhulu
CA, Song X, Cui MY, Liu H, Lu T, He G, et al: Probucol protects
endothelial progenitor cells against oxidized low-density
lipoprotein via suppression of reactive oxygen species formation in
vivo. Cell Physiol Biochem. 39:89–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Turgeon J, Dussault S, Haddad P, Groleau
J, Ménard C, Michaud SE, Maingrette F and Rivard A: Probucol and
antioxidant vitamins rescue ischemia-induced neovascularization in
mice exposed to cigarette smoke: Potential role of endothelial
progenitor cells. Atherosclerosis. 208:342–349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu J, He Z, Gao X, Wu F, Ding R, Ren Y,
Jiang Q, Fan M, Liang C and Wu Z: Oxidized high-density lipoprotein
impairs endothelial progenitor cells' function by activation of
CD36-MAPK-TSP-1 pathways. Antioxid Redox Signal. 22:308–324. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Zhou X, Wang Z, Yang L, Wang J
and Wang W: Trace elements in PM2.5 in Shandong
Province: Source identification and health risk assessment. Sci
Total Environ. 621:558–577. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ying Z, Xie X, Bai Y, Chen M, Wang X,
Zhang X, Morishita M, Sun Q and Rajagopalan S: Exposure to
concentrated ambient particulate matter induces reversible increase
of heart weight in spontaneously hypertensive rats. Parti Fibre
Toxicol. 12:152015. View Article : Google Scholar
|
|
15
|
Cui Y, Jia F, He J, Xie X, Li Z, Fu M, Hao
H, Liu Y, Liu DZ, Cowan PJ, et al: Ambient fine particulate matter
suppresses in vivo proliferation of bone marrow stem cells through
reactive oxygen species formation. PLoS One. 10:e01273092015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Calderon-Garciduenas L, Gonzalez-Gonzalez
LO, Kulesza RJ, Fech TM, Pérez-Guillé G, Luna MAJ, Soriano-Rosales
RE, Solorio E, Miramontes-Higuera JJ, Chew Gómez-Maqueo A, et al:
Exposures to fine particulate matter (PM2.5) and ozone
above USA standards are associated with auditory brainstem
dysmorphology and abnormal auditory brainstem evoked potentials in
healthy young dogs. Environ Res. 158:324–332. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng Z, Zhang X, Wang J, Dandekar A, Kim
H, Qiu Y, Xu X, Cui Y, Wang A, Chen LC, et al: Exposure to fine
airborne particulate matters induces hepatic fibrosis in murine
models. J Hepatol. 63:1397–1404. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun Q, Zhang G, Chen R, Li R, Wang H,
Jiang A, Li Z, Kong L, Fonken LK, Rajagopalan S, et al: Central
IKK2 inhibition ameliorates air pollution mediated hepatic glucose
and lipid metabolism dysfunction in mice with type II diabetes.
Toxicol Sci. 164:240–249. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
An Z, Jin Y, Li J, Li W and Wu W: Impact
of particulate air pollution on cardiovascular health. Curr Allergy
Asthma Rep. 18:152018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pope CA III, Bhatnagar A, McCracken JP,
Abplanalp W, Conklin DJ and O'Toole T: Exposure to fine particulate
air pollution is associated with endothelial injury and systemic
inflammation. Circ Res. 119:1204–1214. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
O'Toole TE, Hellmann J, Wheat L,
Haberzettl P, Lee J, Conklin DJ, Bhatnagar A and Pope CA III:
Episodic exposure to fine particulate air pollution decreases
circulating levels of endothelial progenitor cells. Circ Res.
107:200–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Niu J, Liberda EN, Qu S, Guo X, Li X,
Zhang J, Meng J, Yan B, Li N, Zhong M, et al: The role of metal
components in the cardiovascular effects of PM2.5. PLoS One.
8:e837822013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haberzettl P, Lee J, Duggineni D,
McCracken J, Bolanowski D, O'Toole TE, Bhatnagar A and Conklin DJ:
Exposure to ambient air fine particulate matter prevents
VEGF-induced mobilization of endothelial progenitor cells from the
bone marrow. Environ Health Perspect. 120:848–856. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu C, Yu X, Zuo K, Zhang X, Cao C, Xu J,
Wang S, Tang T, Ye M, Pei E, et al: Tripterine treatment improves
endothelial progenitor cell function via integrin-linked kinase.
Cell Physiol Biochem. 37:1089–1103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo W, Li Z, Xie X, Qin T, Wu Y, Li Z,
Chai J, Yi F, Tan T, Zhu H and Wang S: Urinary trypsin inhibitor
attenuates acute lung injury by improving endothelial progenitor
cells functions. Cell Physiol Biochem. 36:1059–1068. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ye M, Li D, Yang J, Xie J, Yu F, Ma Y, Zhu
X, Zhao J and Lv Z: MicroRNA-130a targets MAP3K12 to modulate
diabetic endothelial progenitor cell function. Cell Physiol
Biochem. 36:712–726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
YanYun P, Wang S, Yang J, Chen B, Sun Z,
Ye L, Zhu J and Wang X: Interruption of CD40 pathway improves
efficacy of transplanted endothelial progenitor cells in
monocrotaline induced pulmonary arterial hypertension. Cell Physiol
Biochem. 36:683–696. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang KQ, Chen D, Sun DQ, Zhang H, Li B
and Fu Q: Probucol improves erectile function by restoring
endothelial function and preventing cavernous fibrosis in
Streptozotocin-induced diabetic rats. Urology. 91:241.e9–241.e16.
2016. View Article : Google Scholar
|
|
29
|
Li JF, Chen S, Feng JD, Zhang MY and Liu
XX: Probucol via inhibition of NHE1 attenuates LPS-accelerated
atherosclerosis and promotes plaque stability in vivo. Exp Mol
Pathol. 96:250–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ku G, Doherty NS, Wolos JA and Jackson RL:
Inhibition by probucol of interleukin 1 secretion and its
implication in atherosclerosis. Am J Cardiol. 62:77B–81B. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zucoloto AZ, Manchope MF,
Staurengo-Ferrari L, Pinho-Ribeiro FA, Zarpelon AC, Saraiva ALL,
Cecílio NT, Alves-Filho JC, Cunha TM, Menezes GB, et al: Probucol
attenuates lipopolysaccharide-induced leukocyte recruitment and
inflammatory hyperalgesia: Effect on NF-кB activation and cytokine
production. Eur J Pharmacol. 809:52–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu BB, Wang H, Chi YF, Wang YM, Yao XM,
Liu S, Qiu H, Fang J, Yin PH, Zhang XM and Peng W: Protective
effects of probucol on Ox-LDL-induced epithelial-mesenchymal
transition in human renal proximal tubular epithelial cells via
LOX1/ROS/MAPK signaling. Mol Med Rep. 17:1289–1296. 2018.PubMed/NCBI
|



















