Introduction
Non-small cell lung cancer (NSCLC) is a disease with
high incidence and mortality rates (1). Adenocarcinoma, squamous cell carcinoma
and large cell carcinoma are the three common types of NSCLC
(2,3). In patients with lung cancer, 85–90% of
cases are diagnosed as NSCLC (4).
Compared with small cell carcinoma, the growth of NSCLC is slower
(2). The clinical symptoms of NSCLC
include fever, chest pain, shortness of breath, coughing and bloody
sputum (4,5). Clinically, NSCLC is typically treated
with surgery, chemotherapy, radiotherapy, immunotherapy and
combination therapy (6).
Radiotherapy is frequently used in combination with chemotherapy
for the treatment of NSCLCs if surgery alone is not possible
(6,7). Radiation can be used to induce NSCLC
cell death and can relieve the symptoms caused by NSCLC, including
breathing problems and swelling (8).
Additionally, radiation therapy can be used to kill lung cancer
cells that have metastasized to the other part of the body, such as
the bones (7).
Although radiotherapy is an important cancer
treatment, it can cause side effects, including neurotoxicity,
nephrotoxicity, myelosuppression, nausea and vomiting (9). Radiation kills tumor cells and normal
cells alike, which leads to limitations in its clinical application
(10). Therefore, the combination of
certain auxiliary anticancer drugs and radiotherapy has become an
area of interest for cancer research. The optimal
radiotherapy-anticancer drug combination should enhance the
antitumor effects and reduce radiation-induced side effects.
Oridonin is an active kaurene diterpene that is
derived from the traditional Chinese herb Rabdosia
rubescens, which possesses antitumor activity and exhibits
little toxicity (11). It has been
reported that oridonin has antiproliferative and apoptosis-inducing
effects on cells from various types of cancer, including colon
cancer (12), gastric cancer
(13), prostate cancer (14), laryngeal cancer (15), lung cancer (16), breast cancer (17) and gallbladder cancer (18). Murayama et al (19) demonstrated that oridonin induced
radiosensitization in Chinese hamster V79 cells, alone or in
combination with misonidazole (19).
The radiosensitivity enhancement ratios of oridonin and
misonidazole for hypoxic cells were 1.16 and 1.59, respectively
(19). In addition, the concurrent
administration of Asian botanicals, including vincristine, and
radiotherapy may increase the anticancer effects of therapies
(20). However, the role of oridonin
on the radiosensitivity of lung cancer cells remains unclear. The
present study investigated whether oridonin could enhance the
radiosensitivity of lung cancer cells and simultaneously to reduce
the side effects of radiation, which would provide a novel method
for the radiotherapy of human lung cancer.
Materials and methods
Cell lines and reagents
Human lung adenocarcinoma HCC827
(CRL-2868™) cells were obtained from the American Type
Culture Collection (Manassas, VA, USA). The human lung
adenocarcinoma cell line SPC-A-1 (cat. no. YB-ATCC-4637) was
purchased from Shybio Corporation (Shanghai, China) and was
maintained in the laboratory of the Department of Medical Oncology,
Daqing Oilfield General Hospital (Daqing, China). The HCC827 and
SPC-A-1 cells were cultured in Dulbecco's Modified Eagles Medium
supplemented with 10% fetal bovine serum (both HyClone; GE
Healthcare Life Sciences, Logan, Utah, USA) at 37°C with 5%
CO2. Oridonin (cat. no. O9639) was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
MTT assay
The inhibitory effect of oridonin on HCC827 and
SPC-A-1 cells was measured using the MTT assay. Briefly, the cells
were seeded into 96-well plates at a density of 2×103
cells/well. The cells were pretreated with series of increasing
oridonin concentrations (0–80 µm) for different amounts of time
(24, 48 or 72 h). Cells treated with 0.1% dimethyl sulfoxide (DMSO)
were used as the negative control group. The cells were cultured
for the indicated length and for further 4 h following MTT
treatment (5.0 mg/l, 20 µl) prior to testing. The crystals that had
formed were dissolved with DMSO. Subsequently, the plates were read
at a test wavelength of 490 nm and a reference wavelength of 570
nm.
Clonogenic assay
Cells in the logarithmic phase of growth were
irradiated with 6 MeV X-rays, which were generated by a linear
accelerator (Varian 2100C; Varian Medical Systems, Inc., Palo Alto,
CA, USA). Briefly, the lung cancer cells were plated into 6 cm
plates at a density of 5,000 cells/plate and irradiated at a dose
of 0, 2, 4, 6, 8 or 10 Gy. The cultured medium was replaced every
other day and the cells were cultured for 22 days. The cells were
then fixed with paraformaldehyde (40 g/l) for 15 min at room
temperature and stained with 1 g/l crystal violet for 20 min at
room temperature. Colonies of >50 cells were counted under a
light microscope. The surviving fraction (%) was calculated as
follows: Colony forming efficiency in the experimental group/colony
forming efficiency in the control group × 100; with colony forming
efficiency = number of colonies formed/number of cells planted
×100%. A single-hit multitarget model was used to fit the survival
curves and radiobiological parameters, including D0 and N, which
were calculated using GraphPad Prism software (version 5.0;
GraphPad Software, Inc., La Jolla, CA, USA). D0 is the
radiosensitivity parameter describing the mean lethal dose. It was
determined as the reciprocal slope in the semi-logarithmic survival
curve. The N-value is the extrapolation value and was determined at
the intersection with the Y-axis.
Western blotting and antibodies
The lung cancer cells were washed with ice-cold PBS
and cell lysates were prepared using radioimmunoprecipitation assay
buffer (cat. no. P0013B; Beyotime Institute of Biotechnology,
Nanjing, China). Proteins were separated by SDS-PAGE, as previously
described (21–23). Briefly, 20 µg protein was loaded per
lane, separated using 10% SDS-PAGE gels and transferred to
polyvinylidene fluoride membranes. The primary antibodies used
included anti-apoptosis regulator BAX (Bax; 1:1,000; cat. no.
AP1302a-ev; Abgent, Inc., San Diego, CA, USA), anti-apoptosis
regulator Bcl-2 (Bcl-2; 1:1,000; cat. no. AP1303a-ev; Abgent, Inc.)
and anti-β-actin (1:1,000; cat. no. ab8227; Abcam, Cambridge, UK).
Incubation with primary antibodies was overnight at 4°C.
Horseradish peroxidase-conjugated goat anti-mouse secondary
antibodies (1:5,000; cat. co. sc-2031) were purchased from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). Incubation with the
secondary antibody was 1 h at room temperature. Bands were
visualized using an enhanced chemiluminescence detection kit
(Amersham; GE Healthcare, Chicago, IL, USA) and analyzed with
ImageJ software (V1.8.0; National Institutes of Health, Bethesda,
MD, USA) for quantification.
Statistical analysis
The data was analyzed using SPSS (version 20.0; IBM
Corp., Armonk, NY, USA) and GraphPad Prism software 5.0 (GraphPad
Software, Inc.). The results are presented as the mean ± standard
deviation. Experiments were repeated twice with each sample run in
triplicate. Comparisons between multiple groups were analyzed using
one-way analysis of variance followed by a post hoc Tukey's range
test. The survival fraction analysis was performed using an
independent samples t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Oridonin suppresses the proliferation
of lung cancer cells in a time- and dose-dependent manner
Oridonin is a naturally derived substance with low
toxicity that possesses antitumor activity against various types of
cancer (12,18,24). To
explore the antitumor activities of oridonin on lung cancer cells,
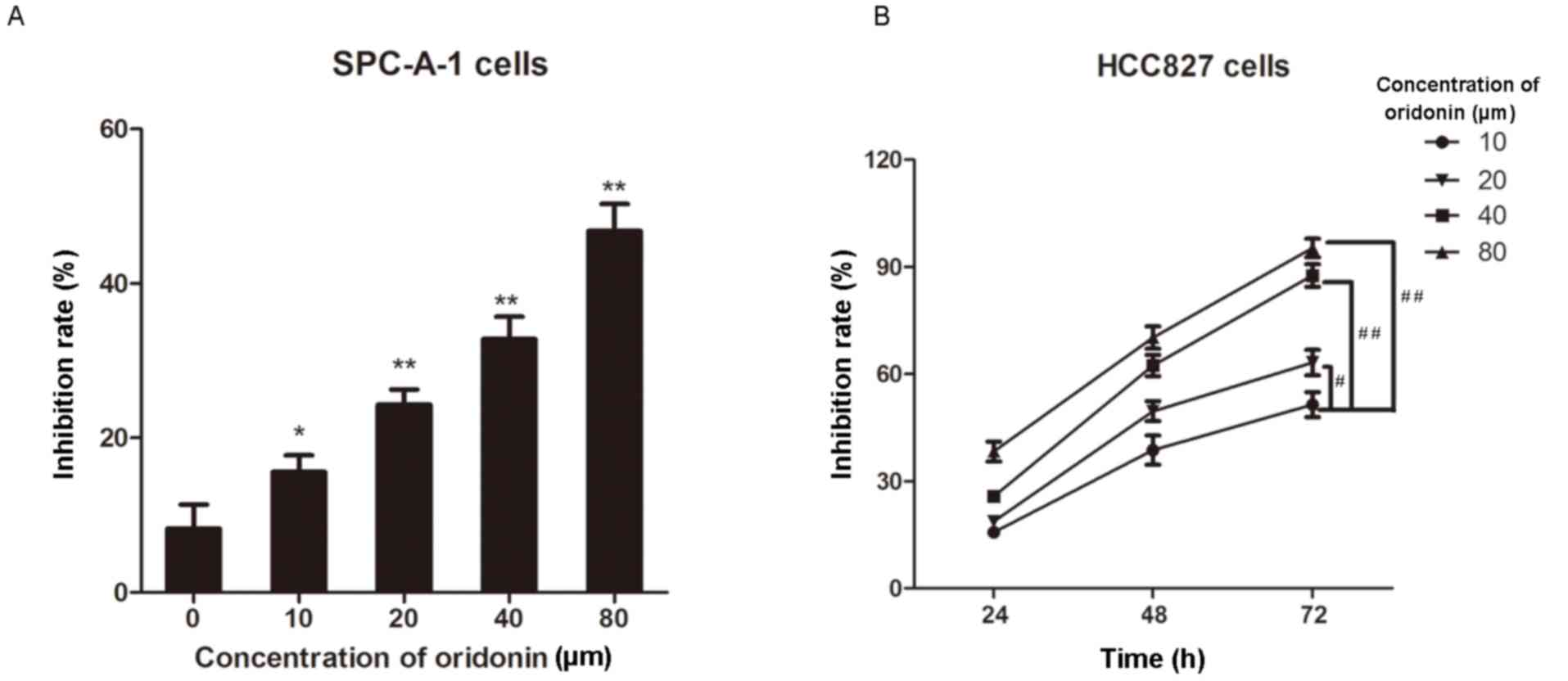
human lung cancer SPC-A-1 cells were treated with increasing
concentrations of oridonin (0–80 µm) for 24 h. Cell viability was
then determined by the MTT assay and the results demonstrated that
the inhibition rate of oridonin on the cells gradually increased in
a dose-dependent manner (all P<0.05 vs. the 0 µm oridonin group;
Fig. 1A). In addition, the HCC827
cells were treated with oridonin (0–80 µm) for 24, 48 or 72 h and
the MTT assay was performed to determine cell proliferation.
Consistently, the inhibition rate increased as the concentration of
oridonin increased (Fig. 1B).
Notably, oridonin exhibited a marked inhibitory effect on HCC827
cell proliferation in a time-dependent manner (Fig. 1B). These results indicate that
oridonin inhibits the proliferation of lung cancer cells in a time-
and dose-dependent manner. As cell growth was increased and more
consistent using SPC-A1 cells compared with HCC827, SPC-A1 cells
were chosen for further experiments.
Irradiation inhibits cell
proliferation of SPC-A-1 cells
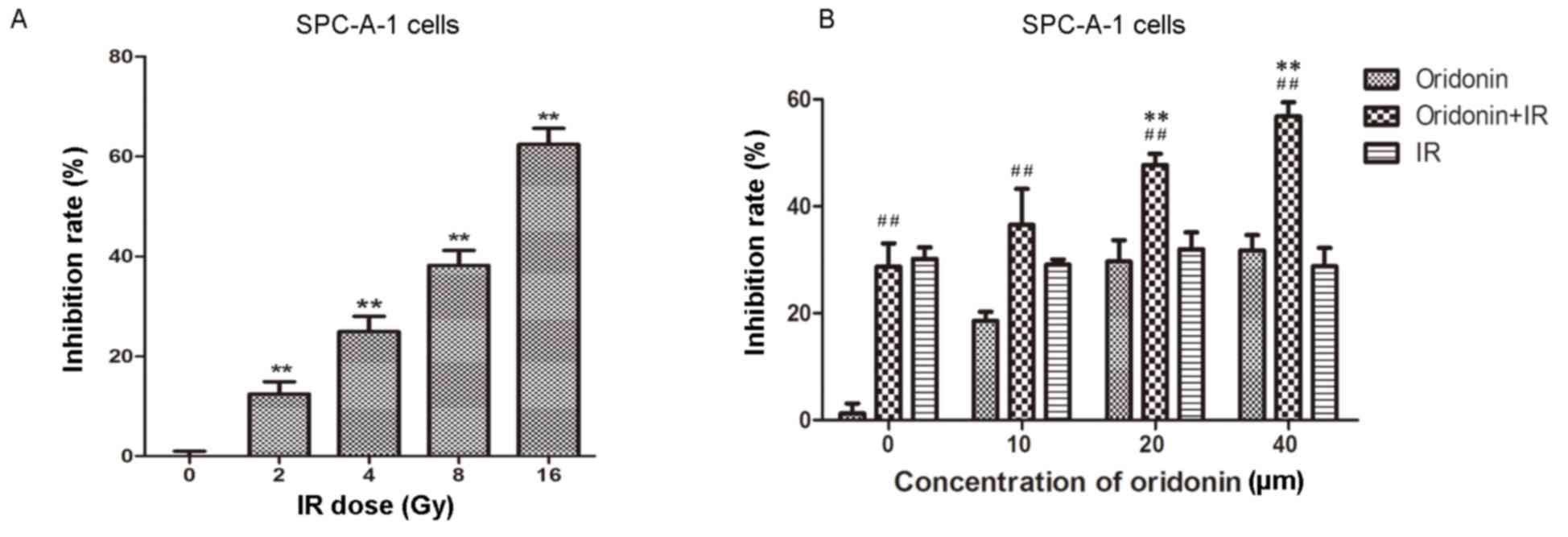
In order to identify an appropriate irradiation dose
for SPC-A-1 cells, a series of irradiation doses were used to treat
the cells, which were then cultured for 24 h. Cell viability was
then determined using the MTT assay. The results demonstrated that
the proliferation of SPC-A-1 cells was significantly inhibited with
increasing irradiation doses (all P<0.01 vs. the 0 Gy group;
Fig. 2A). Cells were irradiated with
2, 4, 8 and 16 Gy; the inhibition rate was 62.4% at 16 Gy. In the
following experiments, a 4 Gy irradiation dose (~30% inhibition
rate) was used to treat the lung cancer cells.
Oridonin increases the
radiosensitivity of SPC-A-1 cells
An increasing concentration of oridonin (0–40 µm)
was used to pretreat the human lung cancer SPC-A-1 cells for 24 h
prior to a 4 Gy irradiation dose. Subsequently, cell viability was
determined using the MTT assay. As shown in Fig. 2B, the inhibition rate was
significantly increased in the 20 and 40 µM oridonin pretreatment
groups compared with the group treated with irradiation alone
(P<0.01).
SPC-A-1 cell survival is enhanced by
oridonin treatment prior to irradiation
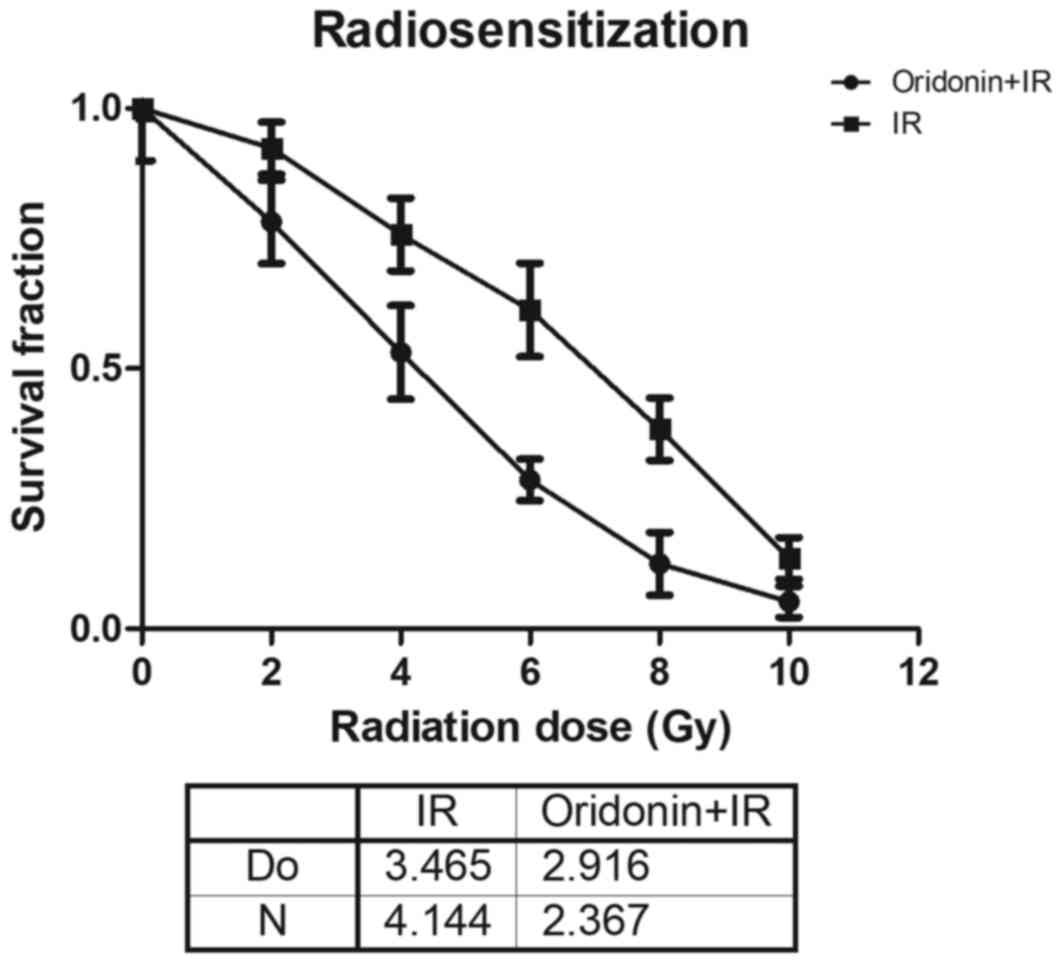
In order to further determine the role of oridonin
on the radiosensitivity of lung cancer cells, SPC-A-1 cells were
pretreated with 40 µm oridonin for 24 h and then exposed to various
irradiation doses (2, 4, 6, 8 and 10 Gy). The results demonstrated
that the survival fraction in the oridonin and irradiation treated
group was lower compared with that in the irradiation alone group
at all doses (Fig. 3). In the
single-hit multitarget model, for its associated parameters the D0
value was 2.916±0.063 in the oridonin pretreatment group, which was
significantly decreased compared with that of 3.465±0.239 in the
group without oridonin pretreatment (P<0.05; Fig. 3). The N value was 2.367±0.334 in
oridonin-pretreated cells compared with 4.144±1.519 in the cells
without oridonin pretreatment.
Oridonin promotes the expression of
Bax and decreases the expression of Bcl-2 in SPC-A-1 cells
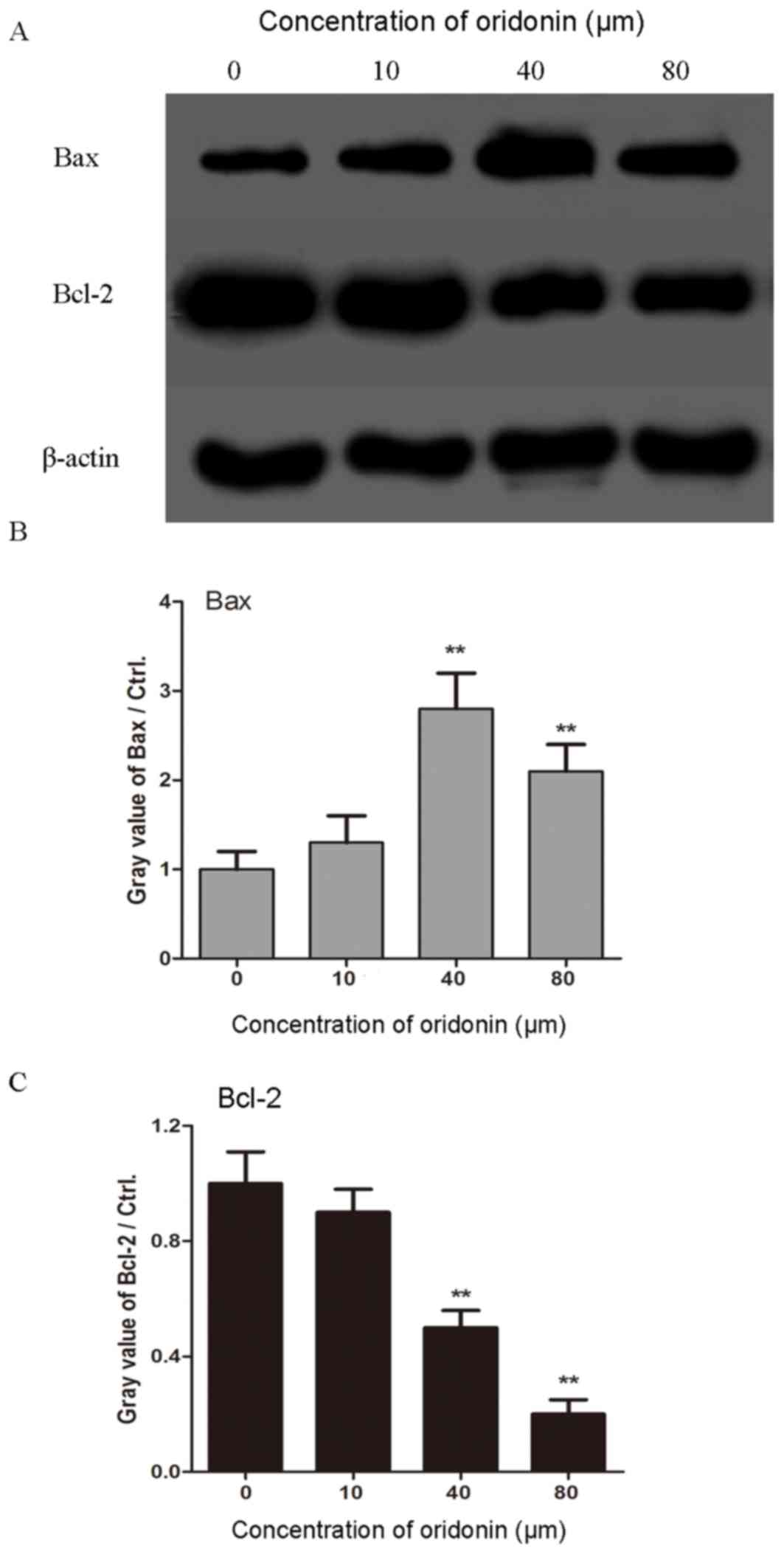
Furthermore, the underlying molecular mechanism of
the effect of oridonin on lung cancer cells was investigated by
western blotting analysis. The SPC-A-1 cells were treated with
different doses of oridonin (10, 40 and 80 µm) for 24 h prior to
analysis. As shown in Fig. 4, the
level of Bax gradually increased as the concentration of oridonin
increased, up until a dose of 40 µm, and conversely, the level of
Bcl-2 decreased with an increasing concentration of oridonin. These
results indicate that oridonin pretreatment promotes the apoptosis
of lung cancer cells.
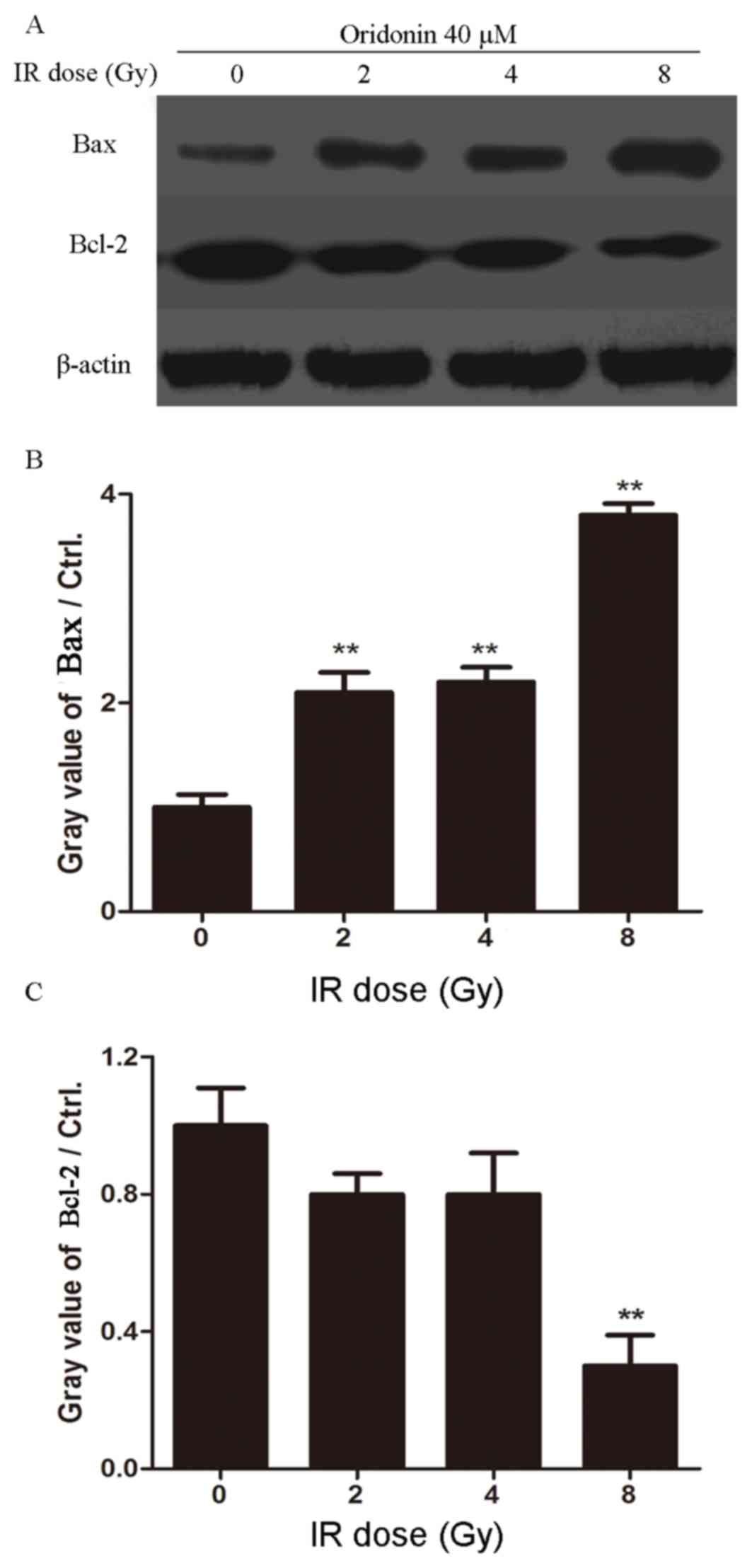
Oridonin in combination with
irradiation increases the level of Bax and decreases the level of
Bcl-2 in SPC-A-1 cells
To investigate whether pretreatment with oridonin
promoted the apoptosis of lung cancer cells in response to
irradiation, western blotting was performed to detect the
expression levels of Bax and Bcl-2 after oridonin and irradiation
treatment. SPC-A-1 cells were pretreated with oridonin (40 µM) for
24 h and then subjected to a 0, 2, 4 or 8 Gy irradiation dose. As
shown in Fig. 5, the expression of
Bax was increased and the expression of Bcl-2 was decreased with
increasing irradiation doses. These results suggest that oridonin
increases the radiosensitivity of human lung cancer cells through
inducing apoptosis.
Discussion
In recent years, great progress has been made in the
diagnosis of and clinical therapy for lung cancer. However, the
mortality rate of patients with lung cancer remains high, with a
5-year survival rate of only 15% (25–27).
Oridonin is a compound that is extracted from the traditional
Chinese herb R. rubescens, which possesses anticancer
properties through inhibiting proliferation and promoting apoptosis
(24,28,29). The
present study investigated the role of oridonin in the
radiosensitivity of lung cancer cells.
Two human lung cancer cell lines, HCC827 and
SPC-A-1, were used as cell models of lung cancer. The human lung
cancer cells were treated with different concentrations of oridonin
for 24, 48 or 72 h and cell viability was determined using the MTT
assay. The results demonstrated that oridonin inhibited lung cancer
cell proliferation in a time- and dose-dependent manner. Next, the
lung cancer cells were exposed to different doses of radiation,
which revealed that irradiation inhibited the proliferation of
SPC-A-1 cells. Furthermore, different concentrations of oridonin
were used to pretreat the SPC-A-1 cells for 24 h prior to a 4 Gy
irradiation dose. The results demonstrated that oridonin increased
the radiosensitivity of SPC-A-1 cells.
The underlying molecular mechanism of the effect of
oridonin and irradiation on lung cancer cells was explored.
Notably, the results demonstrated that oridonin in combination with
irradiation promoted the apoptosis of SPC-A-1 cells, as the
Bax/Bcl-2 ratio was increased in the cells treated with combination
therapy compared with each therapy alone. Bcl-2 is an antiapoptotic
protein that is primarily located on the nuclear membrane,
endoplasmic reticulum and the outer mitochondrial membrane, and
inhibits the progression of X-ray-induced apoptosis (30,31).
Another apoptotic effector, Bax, also belongs to Bcl-2 family. Bax
can bind to Bcl-2 to form heterodimers, which promote apoptosis
(32,33). In the present study, treatment with
oridonin prior to irradiation notably increased the ratio of
Bax/Bcl-2, suggesting that apoptosis was promoted by the
combination of oridonin and irradiation compared with each
treatment alone. Active Bax is recruited to the mitochondria and
forms pores leading to the release of cytochrome c (33). Thus, the present study hypothesized
that combination treatment with oridonin and irradiation activated
the mitochondrial-mediated apoptosis signaling pathway. However,
the present study only detected the levels of Bax and Bcl-2.
Further studies are required to measure the apoptotic rate of lung
cancer cells after oridonin treatment and irradiation. In
conclusion, the results of the present study indicate that oridonin
has a potential application as a radiosensitizing agent for the
treatment of human NSCLC.
Acknowledgements
The authors would like to thank Professor Guanghui
Cheng (Department of Radiation, China-Japan Union Hospital of Jilin
University, Changchun, China) for guidance with the experiments and
the publication of the work and Professor Dan Shi (Department of
Radiation, China-Japan Union Hospital of Jilin University,
Changchun, China) for scientific discussions. Furthermore the
support of Liangyu Zhang and Fang Yang is gratefully
acknowledged.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL and GC contributed to the conception and design
of the study. SL was involved with the clinical studies. DS and FY
performed the experiments and analysed the data. LZ performed the
statistical analysis and prepared the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen J, Chen J, Wu X, Shi T and Kang M:
Efficacy of targeted agents in the treatment of elderly patients
with advanced non-small-cell lung cancer: A systematic review and
meta-analysis. OncoTargets Ther. 9:4797–4803. 2016. View Article : Google Scholar
|
|
2
|
Yano M, Yoshida J, Koike T, Kameyama K,
Shimamoto A, Nishio W, Yoshimoto K, Utsumi T, Shiina T, Watanabe A,
et al: The outcomes of a limited resection for non-small cell lung
cancer based on differences in pathology. World J Surg.
40:2688–2697. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Giopanou I, Lilis I, Papaleonidopoulos V,
Marazioti A, Spella M, Vreka M, Papadaki H and Stathopoulos GT:
Comprehensive evaluation of nuclear factor-κ β expression patterns
in non-small cell lung cancer. PloS One. 10:e01325272015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arrieta O, Carmona A, Ramirez-Tirado LA,
Flores-Estrada D, Macedo-Pérez EO, Martínez-Hernández JN,
Corona-Cruz JF, Cardona AF and de la Garza J: Survival of patients
with advanced non-small cell lung cancer enrolled in clinical
trials. Oncology. 91:185–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fernandez-Lopez C, Exposito-Hernandez J,
Arrebola-Moreno JP, Calleja-Hernández MÁ, Expósito-Ruíz M,
Guerrero-Tejada R, Linares I and Cabeza-Barrera J: Trends in phase
III randomized controlled clinical trials on the treatment of
advanced non-small-cell lung cancer. Cancer Med. 5:2190–2197. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paleiron N, Bylicki O, Andre M, Rivière E,
Grassin F, Robinet G and Chouaïd C: Targeted therapy for localized
non-small-cell lung cancer: A review. OncoTargets Ther.
9:4099–4104. 2016. View Article : Google Scholar
|
|
7
|
Aridgides P and Bogart J: Stereotactic
body radiation therapy for stage i non-small cell lung cancer.
Thoracic Surg Clin. 26:261–269. 2016. View Article : Google Scholar
|
|
8
|
Jiang J, Liang X, Zhou X, Huang R and Chu
Z: A meta-analysis of randomized controlled trials comparing
carboplatin-based to cisplatin-based chemotherapy in advanced
non-small cell lung cancer. Lung Cancer. 57:348–358. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
D'Addario G, Pintilie M, Leighl NB, Feld
R, Cerny T and Shepherd FA: Platinum-based versus
non-platinum-based chemotherapy in advanced non-small-cell lung
cancer: A meta-analysis of the published literature. J Clin Oncol.
23:2926–2936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Forsythe B and Faulkner K: Overview of the
tolerability of gefitinib (IRESSA) monotherapy: Clinical experience
in non-small-cell lung cancer. Drug Saf. 27:1081–1092. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wong AM, Zhang Y, Kesler K, Deng M,
Burhenn L, Wang D, Moro A, Li Z and Heber D: Genomic and in vivo
evidence of synergy of a herbal extract compared to its most active
ingredient: Rabdosia rubescens vs. oridonin. Exp Ther Med.
1:1013–1017. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu QX, Yuan SX, Ren CM, Yu Y, Sun WJ, He
BC and Wu K: Oridonin upregulates PTEN through activating p38 MAPK
and inhibits proliferation in human colon cancer cells. Oncol Rep.
35:3341–3348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi M, Lu XJ, Zhang J, Diao H, Li G, Xu L,
Wang T, Wei J, Meng W, Ma JL, et al: Oridonin, a novel lysine
acetyltransferases inhibitor, inhibits proliferation and induces
apoptosis in gastric cancer cells through p53- and
caspase-3-mediated mechanisms. Oncotarget. 7:22623–22631.
2016.PubMed/NCBI
|
|
14
|
Ming M, Sun FY, Zhang WT and Liu JK:
Therapeutic effect of oridonin on mice with prostate cancer. Asian
Pac J Trop Med. 9:184–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang N, Cao SJ, Zhou Y, He H, Tashiro S,
Onodera S, Qiu F and Ikejima T: Inhibition of caspase-9 by
oridonin, a diterpenoid isolated from Rabdosia rubescens, augments
apoptosis in human laryngeal cancer cells. Int J Oncol.
47:2045–2056. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang YY, Lv YF, Lu L and Cai L: Oridonin
inhibits mTOR signaling and the growth of lung cancer tumors.
AntiCancer Drugs. 25:1192–1200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang S, Zhong Z, Wan J, Tan W, Wu G, Chen
M and Wang Y: Oridonin induces apoptosis, inhibits migration and
invasion on highly-metastatic human breast cancer cells. Am J Chin
Med. 41:177–196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bao R, Shu Y, Wu X, Weng H, Ding Q, Cao Y,
Li M, Mu J, Wu W, Ding Q, et al: Oridonin induces apoptosis and
cell cycle arrest of gallbladder cancer cells via the mitochondrial
pathway. BMC Cancer. 14:2172014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Murayama C, Nagao Y, Sano S, Ochiai M,
Fuji K, Fujita E and Mori T: Effect of oridonin, a Rabdosia
diterpenoid, on radiosensitization with misonidazole. Experientia.
43:1221–1223. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sagar SM: Can the therapeutic gain of
radiotherapy be increased by concurrent administration of Asian
botanicals? Integr Cancer Ther. 9:5–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nishitani H, Sugimoto N, Roukos V,
Nakanishi Y, Saijo M, Obuse C, Tsurimoto T, Nakayama KI, Nakayama
K, Fujita M, et al: Two E3 ubiquitin ligases, SCF-Skp2 and
DDB1-Cul4, target human Cdt1 for proteolysis. EMBO J. 25:1126–1136.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng L, Xu Z, Zhou Y, Yang T, Liang ZQ and
Zhang M: Effect of rosiglitazone on cells cycle, apoptosis and
expression of Skp2 and p27Kip1 in hepatocellular carcinoma cell
line. Zhonghua Gan Zang Bing Za Zhi. 18:148–149. 2010.(In Chinese).
PubMed/NCBI
|
|
23
|
Schulman BA, Carrano AC, Jeffrey PD, Bowen
Z, Kinnucan ER, Finnin MS, Elledge SJ, Harper JW, Pagano M and
Pavletich NP: Insights into SCF ubiquitin ligases from the
structure of the Skp1-Skp2 complex. Nature. 408:381–386. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Wang Y, Wang S, Gao Y, Zhang X and
Lu C: Oridonin phosphate-induced autophagy effectively enhances
cell apoptosis of human breast cancer cells. Med Oncol. 32:3652015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen H, Cao Y, Li X, Tan Y, Chen J, Yang
Z, Kong Y and Yuan Y: Surgical intervention improves survival for
metastatic non-small cell lung cancer patients. Medicine
(Baltimore). 95:e38002016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Santarpia M, Rolfo C, Peters GJ, Leon LG
and Giovannetti E: On the pharmacogenetics of non-small cell lung
cancer treatment. Expert Opin Drug Metab Toxicol. 12:307–317. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ohtaki Y, Shimizu K, Kaira K, Nagashima T,
Obayashi K, Nakazawa S, Kakegawa S, Igai H, Kamiyoshihara M,
Nishiyama M and Takeyoshi I: Risk factors associated with
recurrence of surgically resected node-positive non-small cell lung
cancer. Surg Today. 46:1196–1208. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang XH, Liu YX, Jia M, Han JS, Zhao M,
Ji SP and Li AM: Oridonin inhibits tumor growth in glioma by
inducing cell cycle arrest and apoptosis. Cell Mol Biol
(Noisy-le-grand). 60:29–36. 2014.PubMed/NCBI
|
|
29
|
Xu B, Shen W, Liu X, Zhang T, Ren J, Fan Y
and Xu J: Oridonin inhibits BxPC-3 cell growth through cell
apoptosis. Acta Biochim Biophys Sin (Shanghai). 47:164–173. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dong Y, Zheng Y, Xiao J, Zhu C and Zhao M:
Regulatory effect of Bcl-2 in ultraviolet radiation-induced
apoptosis of the mouse crystalline lens. Exp Ther Med. 11:973–977.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhen L, Li J, Zhang M and Yang K: MiR-10b
decreases sensitivity of glioblastoma cells to radiation by
targeting AKT. J Biol Res (Thessalon). 23:142016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Q, Si T, Xu X, Liang F, Wang L and Pan
S: Electromagnetic radiation at 900 MHz induces sperm apoptosis
through bcl-2, bax and caspase-3 signaling pathways in rats. Reprod
Health. 12:652015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mendes F, Sales T, Domingues C, Schugk S,
Abrantes AM, Gonçalves AC, Teixo R, Silva R, Casalta-Lopes J, Rocha
C, et al: Effects of X-radiation on lung cancer cells: The
interplay between oxidative stress and P53 levels. Med Oncol.
32:2662015. View Article : Google Scholar : PubMed/NCBI
|