Introduction
With an aging population in the world, incidence
rate of chronic heart failure (CHF) has been increasing year by
year, resulting in an elevation in mortality rate (1). CHF, as the end-stage of many
cardiovascular diseases, refers to a clinical syndrome caused by
organic or functional variations in the heart with major
manifestations such as anomaly in heart structure, decrease in
cardiac output caused by dysfunction of ventricular filling and/or
ejection, persistent increase in venous pressure and gradual
dysfunction in hemodynamics that can hardly satisfy the requirement
of metabolism, which can result in progressive exacerbation in
heart failure, necrosis in myocardial cells, thereby threatening
the health and life quality of human beings; according to the
degree of failure, CHF can be divided into three types: Left heart
failure, right heart failure and whole heart failure (2). Besides, some younger patients may be
the victims of CHF, and, though CHF can be controlled by treatment,
these patients are more susceptible to the recurrence of CHF for
its irreversible and refractory features (3). Although inhibitor of renin-angiotensin
system (RAS) is dominant in treatment of heart failure with the
ability to stabilize or decrease the pressure and the protective
effect on target organs, it brings about a variety of inevitable
adverse reactions (4). Thus,
searching for a new kind of drug with prominent efficacy and mild
side effect is necessary.
In humans, erythropoietin (EPO), a glycoprotein
secreted by kidneys, can bind to EPO receptor (EPOR) specifically,
thereby activating the proliferation and differentiation of
erythrocytes (5). Enormous number of
studies have indicated that in addition to the function to activate
the hematopoiesis, EPO manifests a protective activity on
myocardial cells (6). EPO, through
binding to EPOR, can activate EPOR to initiate multiple signal
transduction pathways, like signal transducer and activator of
transcription 5 (STAT5), phosphoinositide 3-kinase (PI3-K)/protein
kinase B (Akt, or PKB) signal pathway, or mitogen-activated protein
kinase (MAPK)/extracellular signal-regulated kinase (ERK) signal
pathway, thereby protecting the tissues extensively (7). Not only can EPO protect the heart
through increasing the oxygen supply to tissues by activating the
generation of erythrocytes, but also it can exert its protective
effect on the heart through multiple ways, including
anti-apoptosis, anti-inflammation and pro-angiogenesis effects
(8). Thereupon, medicinal EPO has
been applied in treatment of relevant diseases, but, when applied
as the protective agent, the dose of EPO should be higher than that
in treatment of anemia (9), and such
a high dose of EPO may give rise to dose-dependence, increased
risks in coagulation and thrombosis, or even exacerbation in heart
failure, which may frequently occur particularly in patients at
high risk with thrombus, hypertension, polycythemia or
hyperviscosity syndrome (10). Due
to the adverse reactions above, application of EPO has been largely
limited in clinical treatment of cardiovascular diseases. In 2004,
Fiordaliso et al (11) in an
in vitro experiment obtained carbamylated erythropoietin
(CEPO), a derivative of EPO, which retains anti-inflammation,
anti-apoptosis and tissue-protective effects that are similar to
EPO but without pro-hematopoietic effect. CEPO is much safer than
EPO in treatment of cardiovascular diseases with a much wider
application prospect (11).
PI3-K/Akt (also called PKB) is a major signal
pathway delivering the signal of anti-apoptosis/pro-proliferation,
and PI3-K plays a key role in signal transduction pathway mediated
by the growth factor receptor superfamily (12). Through binding to EPOR, EPO can
activate PI3-K that will further catalyze the transformation of
diphosphoinositide (PIP2) into triphosphoinositide (PIP3), which,
as a second messenger, can activate the following multiple target
proteins, thereby regulating the proliferation, differentiation,
migration and transplantation of cells (13). Akt, also called serine/threonine
protein kinase B (PKB), is one of the downstream target proteins of
PI3-K; once activated, Akt can be phosphorylated into
phosphorylated Akt (pAkt), which can further phosphorylate a series
of apoptotic regulation factors of B-cell lymphoma-2 (Bcl-2)/
B-cell lymphoma-extra-large (Bcl-XL)-associated death promoter
(BAD), caspase and nuclear factor (NF)-κB, so as to affect the
transcription of anti-apoptosis genes, which is conducive to
survival of cells (14). In this
study, we investigated whether CEPO could antagonize CHF in rats
through the PI3-K/Akt signal pathway.
Materials and methods
Materials
Experimental animals
A total of 120 healthy clean male Wistar rats
weighing 200–240 g were purchased from Experimental Animal Center
of Academy of Military Medical Sciences (Beijing, China) [approval
no. SCXK-(Military) 2012 0004] and fed with regular food in
separate cages at 20–25°C and 60–70% humidity-controlled
environment, in which rats had free access to water that was
disinfected through ultraviolet. Adjustment in light and
ventilation was performed in accordance with the standards. Padding
was changed twice per week, and regular food was given in 25
g/rat/day. Experiment was carried out following several days of
acclimatization. The study was approved by the Ethics Committee of
The Third Affiliated Hospital of Guangzhou Medical University
(Guangzhou, China).
Reagents
Before administration of CEPO (Amgen, Thousand Oaks,
CA, USA), the dose of CEPO was calculated and weighed in 50 µg/kg
with reference of the total weight of rats in CEPO group. Then CEPO
was dissolved in double distilled water until well mixed, and the
concentration of CEPO was adjusted to 100 µg/ml; isoproterenol
(ISO; 2 ml, 1 mg/bottle, Shanghai Harvest Pharmaceutical Co. Ltd.,
Shanghai, China); LY294002 (LY) (Selleck Chemicals, Houston, TX,
USA); enhanced chemiluminescence (ECL) kit (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA); Rabbit anti-rat pAkt
(Ser473) and Akt monoclonal antibodies (cat. nos. 4058 and 4685
respectively; Cell Signaling Technology, Inc., Danvers, MA, USA);
horseradish peroxidase labeled anti-rabbit IgG polyclonal antibody
(cat. no. 7074; Cell Signaling Technology, Inc.). Bicinchoninic
acid (BCA) protein assay reagent kit (Pierce; Thermo Fisher
Scientific, Inc., Waltham, MA, USA).
Methods
Establishment of CHF models in
rats
After being fed for several days to acclimatize to
the environment, 20 of the rats were selected as the control group,
and the remaining rats as the model group, where rats underwent
intraperitoneal injection of ISO (5 mg/kg) once per day for 3 days
to establish the models. At the same time, rats in the control
group underwent intraperitoneal injection of equivalent normal
saline. During intraperitoneal injection, needle should be inserted
into the abdomen. Pumped back to see whether the blood or
intestinal fluid was drawn, so as to avoid the drugs being
delivered into the vessels, which could lead to the death of rats,
or into the bladder or intestinal tube to lose the activity of
drugs. After 5 weeks of feeding, survived rats were delivered to
evaluate the model establishment. During the feeding period, a
total of 8 rats in the model group died, which might have been
caused by the rapid injection of ISO or delivery of drugs to
vessels due to inappropriate operations, leading to embolism, or
any other unknown factors. In examination of hemodynamics, left
ventricular end-diastolic pressure (LVEDP) ≥15 mmHg suggested that
models were established successfully (15).
Grouping of animals
After the hemodynamics examination for identifying
the successful model establishment, wounds in rats were sutured
followed by administration of gentamycin at a dose of 24,000
U/kg/day for 3 days to prevent infections. A total of 92 rats with
successful model establishment were divided into 4 groups randomly,
i.e. CHF group (n=23), CEPO group (n=23), LY group (n=23) and CEPO
+ LY group (n=23).
CEPO group: After being weighed, rats in CEPO group
underwent intraperitoneal injection of CEPO at a dose of 50 µg/kg
for 4 weeks (16).
CHF group and control group: Rats in CHF group
received intraperitoneal injection of equivalent normal saline at
the same time.
The rats in LY group, after being weighed, received
intraperitoneal injection of LY at a dose of 0.25 µg/100 g twice
per week for 4 weeks.
Rats in CEPO + LY group, after being weighed,
received intraperitoneal injection of LY at a dose of 0.25 µg/100 g
firstly for pretreatment followed by intraperitoneal injection of
CEPO at a dose of 50 µg/kg twice per week for 4 weeks.
After 4 weeks, indicators for hemodynamics were
determined in all survived rats, including heart rate (HR), LVEDP,
left ventricular systolic pressure (LVSP) and maximal increased
rate of left ventricular pressure (LVP)/maximal decreased rate of
LVP (±dp/dtmax). Western blotting assay was utilized to
determine the changes in activity of PI3-K/Akt signal pathway
Examination of hemodynamics
After 12 h of fasting of both food and water, rats
were anesthetized using urethane (25%, 300 mg/kg), and fixed in a
plate in supine position. Following the regular skin preparation
and disinfection, skin of neck was incised to separate the right
common carotid artery (CCA) with a 2-cm segment being freed and
exposed. The distal end of CCA was ligated, and the proximal end
was clamped with a small bulldog clamp. About 1.5 cm in front of
the ligature, polyethylene catheter (containing 0.1% heparin-normal
saline; diameter of 1 mm; micro-pressure sensor deployed at the end
of catheter) of BL-410S bio-function experiment system (Beijing
Temo Technology Co., Ltd., Beijing, China) was inserted through
puncture. Then, the bulldog clamp was open, and catheter was guided
into the left ventricle through ascending aorta with smooth forceps
until the significant flat peak wave in diastolic phase of pressure
signal on the screen, indicating that catheter was delivered into
the left ventricle. After 10 min of stabilization, indicators were
recorded.
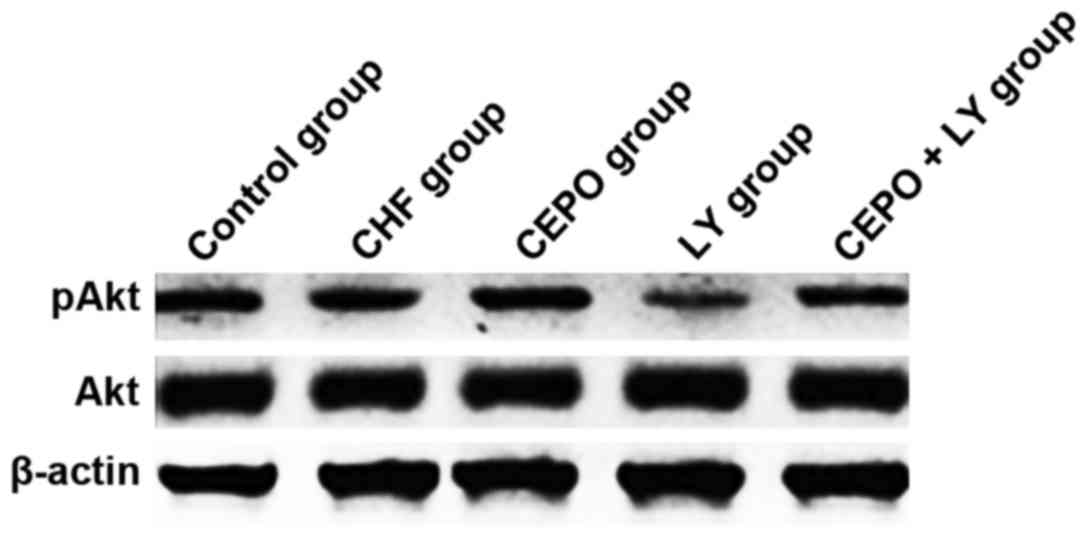
Detection of pAkt and Akt through
western blotting assay
From each group, 4 animals were taken for treatment,
and heart tissues were dissected and shifted into the liquid
N2 within 24 h for preservation and later use. In
accordance with the regular method, total proteins in heart tissues
were extracted for protein quantification with BCA protein
quantification kit. Gel was prepared with 10% separation gel and 3%
spacer gel, and proteins in cytoplasm were extracted in the mixture
of 6X sodium dodecyl sulfate (SDS, 2%) and sample buffer. Five
minutes after the temperature reached 95°C, 10 µl samples were
loaded for electrophoresis until the bromophenol blue gathered at
the bottom of separation gel. Polyvinylidene fluoride membrane was
immersed in membrane-transfer buffer for 15 min, and semi-dry
membrane transfer was then initiated with gel in contact with the
negative electrode of semi-dry transfer cell membrane-transfer
apparatus (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and PVDF
with the positive electrode under a constant voltage of 15 V for
2–3 h. Thereafter, membrane was blocked in 4°C blocking buffer [5%
skimmed milk powder and 1Χ Tris-buffered saline-Tween-20 (TBS-T)]
overnight. Membrane was then incubated with primary antibody (Akt,
1:400; pAkt, 1:400) overnight at 4°C, followed by washing. After
that, horseradish peroxidase labeled anti-rabbit IgG (1:2,000) was
added on the membrane which was later prepared for color
development reaction using chemiluminescent method with ECL kit.
The X-ray images were prepared for color development. Scanning and
identification of images: In this study, the quantitative analysis
of grey value was performed with MHImage 1.63 image analysis
system.
Statistical analysis. With Statistical Product and
Service Solutions (SPSS 21.0; IBM Corp., Armonk, NY, USA), t-test
and one-way analysis of variance (one-way ANOVA) were performed and
SNK test was the post hoc test. Measurement data are presented as
mean ± standard deviation (SD). P<0.05 was set as the critical
value.
Results
Observation of general
manifestations
During the whole test, manifestations in the CHF
group such as dry and shaggy hair without gloss, lack of energy,
drowsiness and hypoactivity, and decline in food were observed
sequentially, and in some severe cases, manifestations including
massive loss of hair, disturbance in respiration, cyanosis and
persistent poor sense on balance. Symptoms of heart failure in
varying degrees were also observed in rats of the CEPO group before
injection of CEPO, but were ameliorated significantly after
medication. However, in the CEPO + LY group, after medication,
symptoms of heart failure in varying degrees that were observed
before medication had no significant improvement. In addition,
symptoms of heart failure of rats in the LY group were more severe
than those in the CHF group.
Hemodynamics indicators
As shown in Table I,
LVSP and ±dp/dtmax in the CHF, CEPO, CEPO + LY and LY
groups were significantly lower than those in the control group
(P<0.05), while LVEDP and HR in these groups were increased
obviously when compared with the control group (P<0.05); in
comparison with the CHF, LY and CEPO + LY groups, LVSP and
±dp/dtmax were elevated significantly in the CEPO group
(P<0.05) with significant decrease in LVEDP and HR (P<0.05);
when compared with the CHF group, LVSP and ±dp/dtmax in
the LY group were significantly decreased (P<0.05) with
remarkable elevations in LVEDP and HR (P<0.05).
 | Table I.Comparison of the hemodynamics
indicators among groups (mean ± SD). |
Table I.
Comparison of the hemodynamics
indicators among groups (mean ± SD).
|
| Control group | CHF group | CEPO group | CEPO+LY group | LY group |
|---|
| n | 20 | 23 | 23 | 23 | 23 |
| LVSP (mmHg) | 134.71±6.31 |
108.40±8.24a |
119.72±7.84ab |
112.48±8.01ac |
97.2±6.76a–c |
| +dp/dtmax
(mmHg/sec) | 4444.54±230.56 |
2965.74±235.41a |
3721.50±200.07ab |
3328.64±210.43ac |
2794.47±198.52a–c |
| -dp/dtmax
(mmHg/sec) | 3926.60±208.78 |
2879.95±219.93a |
3343.28±207.41ab |
3014.7±209.87ac |
2689.98±207.34a–c |
| LEVDP (mmHg) | 5.36±0.78 |
19.46±1.17a |
11.70±1.14ab |
14.43±1.07ac |
22.62±1.21a–c |
| HR (beat/min) | 340.43±11.61 |
416.11±13.63a |
388.84±11.85ab |
427.84±12.43ac |
470.46±11.93a–c |
Comparison of results in western
blotting assay
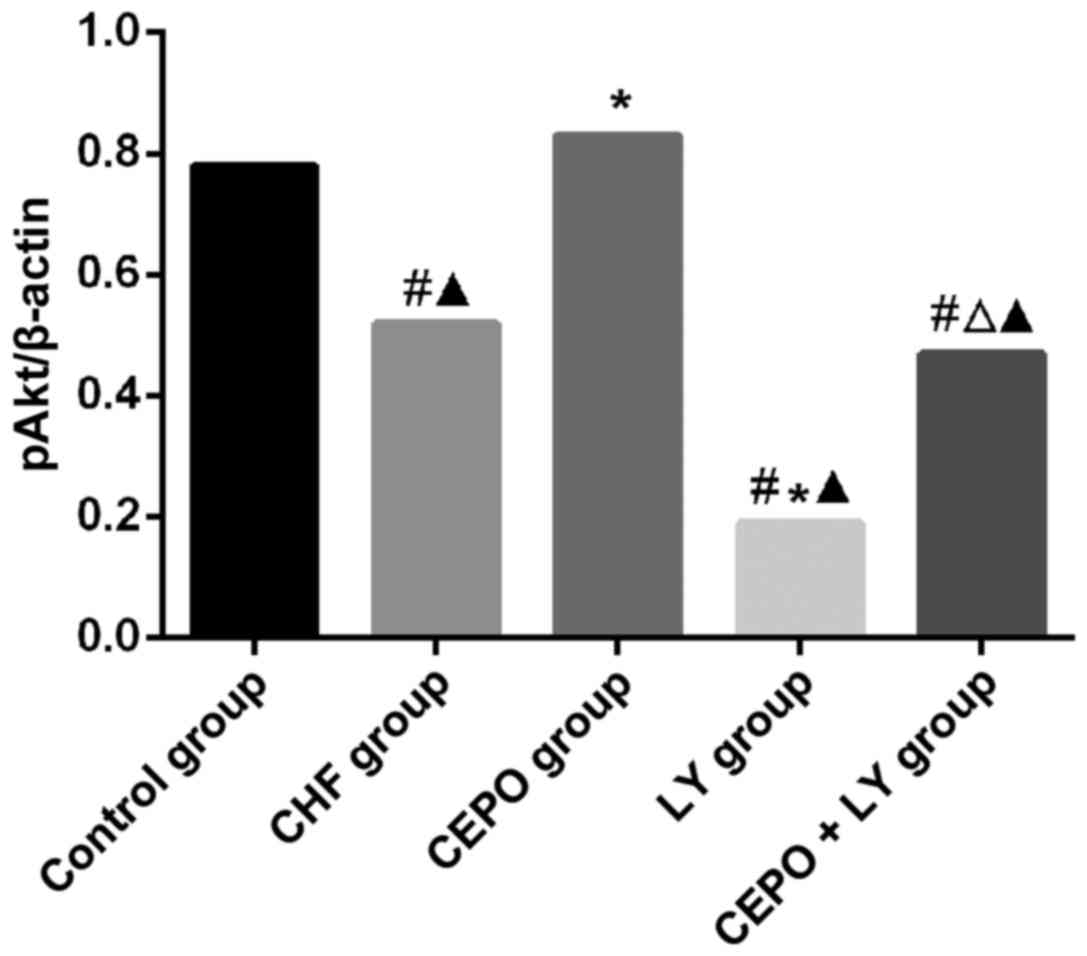
In comparison with the control group, pAkt levels in
CHF, CEPO + LY and LY groups were significantly declined
(P<0.05), while that in the CEPO group was significantly higher
(P<0.05); in the LY group, pAkt level was significantly lower
than that in the CHF group (P<0.05), suggesting that CEPO
increased the phosphorylation level of Akt, which was inhibited by
LY. Comparisons of Akt levels among groups showed that differences
had no statistical significance (Figs.
1 and 2).
Discussion
CHF is one of the major causes of death contributed
by cardiovascular diseases. Once the cardiac output is decreased to
a degree that can hardly satisfy the requirement of the metabolism,
a series of organic or functional variations take place, which can
threaten the safety and quality of life of humans (17). CEPO, as a carbamylated derivative of
EPO, has protective effect on myocardial cells but no
pro-hematopoietic effect (18).
Millet et al (19) reported
that in the nerve system, EPO and CEPO can inhibit the opening of
mitochondrial permeability transition pore (mPTP) to sustain the
membrane potential of mitochondria and calcium homeostasis, thereby
reducing the death of cells induced by ischemia. Similarly, other
researchers (20,21) also confirmed that EPO and CEPO have
nourishing and protective effect on nerves.
LVSP mainly reflects the systolic function of
myocardium, and decrease of LVSP suggests a decline in systolic
function of myocardium; +dp/dtmax can also indicate the
performance of the myocardium in systolic phase almost regardless
of the effect of load, and its decrease means that the systolic
capability of myocardium is decreased; -dp/dtmax serves
as an indicator of diastolic capability of myocardium, and its
decrease reflects that the diastolic capability of myocardium is
curbed; LVEDP is used to evaluate the preload of left ventricle and
reflect the diastolic capability of myocardium, and the increase of
LVEDP shows that the diastolic capability of myocardium is weakened
(22). In this study, diastolic and
systolic capabilities of rats in CHF group were decreased, which,
however, were ameliorated after medication of CEPO. In addition,
pretreatment of LY blocked the ability of CEPO to ameliorate the
heart function. Results of western blotting assay showed that CEPO
could elevate the level of pAkt, which was inhibited by treatment
of LY, suggesting that CEPO can ameliorate the heart function of
CHF rats through PI3-K/Akt signal pathway. Results in this study
agreed with those of He et al (23).
In addition to the prophylactic effect on apoptosis
of myocardial cells and protective effect on myocardial cells,
normal activation of PI3-K/Akt signal pathway is also indispensable
to other life activities (2,24–28).
However, excessive activation of this signal pathway may promote
the development of cancer (29). In
this study, LVSP and ±dp/dtmax were decreased and LVEDP
and HR increased in the LY group compared with in the CHF group,
which might be caused by the blocking effect of LY on PI3-K/Akt
signal pathway; this further affected the life activities
associated with this signal pathway, and the descended life quality
could hardly be sustained, which exacerbated the CHF in rats,
further weakening the diastolic and systolic capabilities of the
heart.
In conclusion, CEPO can generate the antagonist
effect on CHF in rats through activation of PI3-K/Akt signal
pathway.
Acknowledgements
Not applicable.
Funding
This study was funded by The Department of Science
and Technology of Guangdong Province (project no.
2013B022000103).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZH contributed significantly to writing the
manuscript and establishment of CHF models. WX and JW helped with
animal grouping and treatment. SC conducted examination of
hemodynamics. XC performed western blot analysis. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Third Affiliated Hospital of Guangzhou Medical University
(Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ismailov RM: Global heart failure rates
and erythropoietin. Iran J Med Hypotheses Ideas. 6:70–74. 2012.
View Article : Google Scholar
|
|
2
|
Zhang XJ, Liu J, Wan L, Sun Y, Wang F, Qi
YJ and Huang CB: Relations of synovial angiogenesis and
PTEN/PI3K/AKT signaling pathway in rats with adjuvant arthritis.
Zhongguo Gu Shang. 28:71–74. 2015.(In Chinese). PubMed/NCBI
|
|
3
|
Polat N, Oz F, Baykız D, Cizgici AY, Altun
I, Buğra Z, Umman B, Tufan F and Oflaz H: Predictors of functional
capacity in younger and elderly chronic heart failure patients: An
observational study. Anadolu Kardiyol Derg. 13:778–783.
2013.PubMed/NCBI
|
|
4
|
Iwanami J, Mogi M, Iwai M and Horiuchi M:
Inhibition of the renin-angiotensin system and target organ
protection. Hypertens Res. 32:229–237. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suzuki N, Mukai HY and Yamamoto M: In vivo
regulation of erythropoiesis by chemically inducible dimerization
of the erythropoietin receptor intracellular domain. PLoS One.
10:e01194422015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wan LJ and Wu XH: Effects of
erythropoietin on cardiomyocyte apoptosis and endoplasmic reticulum
stress-related proteins in neonatal rats with asphyxia. Zhongguo
Dang Dai Er Ke Za Zhi. 15:890–895. 2013.(In Chinese). PubMed/NCBI
|
|
7
|
Chang ZY, Yeh MK, Chiang CH, Chen YH and
Lu DW: Erythropoietin protects adult retinal ganglion cells against
NMDA-, trophic factor withdrawal-, and TNF-α-induced damage. PLoS
One. 8:e552912013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Doue T, Ohtsuki K, Ogawa K, Ueda M, Azuma
A, Saji H, Strauss HW and Matsubara H: Cardioprotective effects of
erythropoietin in rats subjected to ischemia-reperfusion injury:
Assessment of infarct size with 99mTc-annexin V. J Nucl Med.
49:1694–1700. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wen Y, Xu J, Ma X and Gao Q: High-dose
erythropoietin in acute ST-segment elevation myocardial infarction:
A meta-analysis of randomized controlled trials. Am J Cardiovasc
Drugs. 13:435–442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Minamino T, Toba K, Higo S, Nakatani D,
Hikoso S, Umegaki M, Yamamoto K, Sawa Y, Aizawa Y and Komuro I;
EPO-AMI-II study investigators, . Design and rationale of low-dose
erythropoietin in patients with ST-segment elevation myocardial
infarction (EPO-AMI-II study): A randomized controlled clinical
trial. Cardiovasc Drugs Ther. 26:409–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fiordaliso F, Chimenti S, Staszewsky L,
Bai A, Carlo E, Cuccovillo I, Doni M, Mengozzi M, Tonelli R, Ghezzi
P, et al: A nonerythropoietic derivative of erythropoietin protects
the myocardium from ischemia-reperfusion injury. Proc Natl Acad Sci
USA. 102:2046–2051. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kretz A, Happold CJ, Marticke JK and
Isenmann S: Erythropoietin promotes regeneration of adult CNS
neurons via Jak2/Stat3 and PI3K/AKT pathway activation. Mol Cell
Neurosci. 29:569–579. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu X, Lv S, Mi Y, Wang L and Wang G:
Neuroprotective effect of miR-665 against sevoflurane
anesthesia-induced cognitive dysfunction in rats through PI3K/Akt
signaling pathway by targeting insulin-like growth factor 2. Am J
Transl Res. 9:1344–1356. 2017.PubMed/NCBI
|
|
14
|
Gasparotto EPL, Tognon R, Ferreira AF,
Oliveira GLV, Palma PVB, Zanichelli MA, Souto EX, Velano CEE,
Carrara RCV, Kashima S, et al: Deregulated expression of A1,
Bcl-2, Bcl-xL, and Mcl-1 antiapoptotic proteins and Bid,
Bad, and Bax proapoptotic genes in polycythemia vera patients. Braz
J Pharm Sci. 47:873–886. 2011. View Article : Google Scholar
|
|
15
|
Sattarzadeh R, Tavoosi A and Tajik P:
Echocardiographic estimation of left ventricular filling pressures
in patients with mitral valve stenosis. Cardiovasc J Afr. 25:34–39.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Takemura G, Okada H, Miyata S,
Maruyama R, Li L, Higuchi M, Minatoguchi S, Fujiwara T and Fujiwara
H: Reduction of inflammatory cytokine expression and oxidative
damage by erythropoietin in chronic heart failure. Cardiovasc Res.
71:684–694. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martins QC, Aliti G and Rabelo ER:
Decreased cardiac output: Clinical validation in patients with
decompensated heart failure. Int J Nurs Terminol Classif.
21:156–165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu W, Shen Y, Plane JM, Pleasure DE and
Deng W: Neuroprotective potential of erythropoietin and its
derivative carbamylated erythropoietin in periventricular
leukomalacia. Exp Neurol. 230:227–239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Millet A, Bouzat P, Trouve-Buisson T,
Batandier C, Pernet-Gallay K, Gaide-Chevronnay L, Barbier EL,
Debillon T, Fontaine E and Payen JF: Erythropoietin and its
derivates modulate mitochondrial dysfunction after diffuse
traumatic brain injury. J Neurotrauma. 33:1625–1633. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li X, Bai ZY, Zhang FY and Xu XY:
Neuroprotection of herbs promoting EPO on cerebral ischemia.
Zhongguo Zhong Yao Za Zhi. 40:2265–2271. 2015.(In Chinese).
PubMed/NCBI
|
|
21
|
Gu YX, Du J, Si MS, Mo JJ, Qiao SC and Lai
HC: The roles of PI3K/Akt signaling pathway in regulating MC3T3-E1
preosteoblast proliferation and differentiation on SLA and SLActive
titanium surfaces. J Biomed Mater Res A. 101:748–754. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stewart JT, Grbic M and Sigwart U: Left
atrial and left ventricular diastolic function during acute
myocardial ischaemia. Br Heart J. 68:377–381. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He H, Qiao X and Wu S: Carbamylated
erythropoietin attenuates cardiomyopathy via PI3K/Akt activation in
rats with diabetic cardiomyopathy. Exp Ther Med. 6:567–573. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ueba H, Brines M, Yamin M, Umemoto T, Ako
J, Momomura S, Cerami A and Kawakami M: Cardioprotection by a
nonerythropoietic, tissue-protective peptide mimicking the 3D
structure of erythropoietin. Proc Natl Acad Sci USA.
107:14357–14362. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Q, Xu T, Li D, Pan D, Wu P, Luo Y, Ma
Y and Liu Y: JNK/PI3K/Akt signaling pathway is involved in
myocardial ischemia/reperfusion injury in diabetic rats: effects of
salvianolic acid A intervention. Am J Transl Res. 8:2534–2548.
2016.PubMed/NCBI
|
|
26
|
de Oliveira MR, Ferreira GC, Schuck PF and
Dal Bosco SM: Role for the PI3K/Akt/Nrf2 signaling pathway in the
protective effects of carnosic acid against methylglyoxal-induced
neurotoxicity in SH-SY5Y neuroblastoma cells. Chem Biol Interact.
242:396–406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang N, Li T and Han P: The effect of
tianmai xiaoke pian on insulin resistance through PI3-K/AKT
signal pathway. J Diabetes Res. 2016:92612592016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cen L, Hsieh FC, Lin HJ, Chen CS, Qualman
SJ and Lin J: PDK-1/AKT pathway as a novel therapeutic target in
rhabdomyosarcoma cells using OSU-03012 compound. Br J Cancer.
97:785–791. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wei X, Wang G, Li W, Hu X, Huang Q, Xu K,
Lou W, Wu J, Liang C, Lou Q, et al: Activation of the JAK-STAT3
pathway is associated with the growth of colorectal carcinoma
cells. Oncol Rep. 31:335–341. 2014. View Article : Google Scholar : PubMed/NCBI
|