Introduction
Cerebral infarction is cerebral blood artery
atherosclerosis or thrombosis caused by cerebral insufficiency
(1). Another cause of cerebral
infarction is abnormal blood flow (fluid, gas) into the cerebral
artery caused by blood flow interruption or sudden loss of blood
flow caused by tissue necrosis (2).
Cerebral infarction occurs frequently in the middle-aged and
elderly people. According to the report of Jabbarli et al
(3), there are approximately 600,000
new cerebral infarction patients worldwide in 2015. With the growth
of aging population, incidence of cerebral infarction shows an
increasing trend (4). In addition,
onset age of this disease is becoming increasingly younger
(5). Cerebral infarction is
currently the most common type of cerebrovascular disease with a
high rate of disability and recurrence (6). Fu et al (7) reported that mortality rate of cerebral
infarction patients has reached 28%, and the recurrence rate was as
high as 45%. Due to the high incidence, mortality and recurrence of
cerebral infarction, treatment of this disease is attracting
increasing attention.
Due to insufficient blood circulation and supply,
and reduced immune dysfunction, cerebral infarction patients are
highly susceptible to concurrent disease (8), which in turn increases the difficulties
in the treatment of this disease. Kalra et al (9) reported that approximatly 15% of
patients with cerebral infarction were affected by aspiration
pneumonia. Effective treatment of cerebral infarction complicated
with aspiration pneumonia remains unsatisfactory. Atorvastatin is a
selective and competitive inhibitor of HMG-CoA reductase that can
convert hydroxymethylglutaryl coenzyme A to mevalonate (10). At present, studies (11,12) have
proved that atorvastatin has satisfactory clinical values in the
treatment of patients with cerebral infarction, and no study has
pointed out that atorvastatin can cause adverse reactions to lung.
Therefore, we suppose that atorvastatin can also be used in the
treatment of patients with cerebral infarction and aspiration
pneumonia. Our study provided references for the treatment of
cerebral infarction and aspiration pneumonia.
Materials and methods
Patients
Clinical data of 314 patients with cerebral
infarction complicated with aspiration pneumonia who were admitted
to the emergency department of Beijing Chaoyang Hospital (Beijing,
China) from May 2015 to July 2017 were retrospectively analyzed.
There were 172 males and 142 females, and the age ranged from 45 to
65 years, with an average age of 52.35±9.72 years. Among them, 160
patients who took atorvastatin were treated as the observation
group, and the remaining 154 patients were the control group.
Inclusion and exclusion criteria
Inclusion criteria: Patients aged 45–65 years;
diagnosed with cerebral infarction in Beijing Chaoyang Hospital;
with aspiration pneumonia; received treatments in the hospital;
willing to cooperate with researchers; and patients with complete
clinical data. Exclusion criteria: Patients treated with statins
during the past two months; with other cardiovascular and
cerebrovascular diseases; with family disease history; with a
history of cancer; with other respiratory diseases; drug-sensitive
patients; pregnant patients; transferred to other hospitals during
treatment; with physical disabilities; and patients that accepted
non-hospital treatment. The study was approved by the Ethics
Committee of Beijing Chaoyang Hospital, Capital Medical University
(Beijing, China). Signed informed consents were obtained from the
patients or the guardians.
Methods
Both groups of patients underwent basic treatment
after diagnosis and treatments were conducted in strict accordance
with the guidelines for treatment of cerebral infarction 2013
(13) (Table I). Besides basic treatment, patients
in the observation group also received atorvastatin (oral
medication, 20 mg/day, state approval no. H20120021; Guangdong
Baihe Medical Technology Co., Ltd., Guandong, China). Two groups of
patients were continuously treated for 2 months, and other types of
lipid-lowering drugs and anti-bacterial drugs were not used during
treatment. Venous blood (4 ml) was extracted from two groups of
patients before and after treatment. Beckman Coulter AU5800
automatic biochemical analyzer (Beckman Coulter, Inc., Brea, CA,
USA) was used to detect blood lipids and C-reactive protein (CRP).
Serum levels of inflammatory cytokines were measured by ELISA.
Neurological score of the patients was evaluated according to 2013
Neurological Function Scale (14).
Length of hospital stay and the recovery of aspiration pneumonia in
both groups were recorded. Patients were followed up for a period
of six months and the mortality rate was recorded.
 | Table I.Basic treatment. |
Table I.
Basic treatment.
| Treatment
content | Drug | Way of
administration | Dosage (mg) | Times per day |
|---|
| Intracranial pressure
dehydration | Mannitol | Intravenous
injection | 125 | 3 |
| Hypoglycemic
treatment | Acarbose tablets | Oral treatment | 50 | 3 |
| Antihypertensive
treatment | Nifedipine controlled
release tablets | Oral treatment | 30 | 1 |
| Anti-platelet
aggregation | Aspirin
enteric-coated tablets | Oral treatment | 100 | 1 |
Observation indicators and evaluation
criteria
Observations indicators: Clinical data of the two
groups of patients; CRP; inflammatory factors: IL-6, IL-8 and
TNF-α; hospitalization; prognosis survival rate.
Cerebral infarction rehabilitation evaluation
criteria: Decline of neurological deficit score of 91–100% was
excellent, decline of 46–90% was good, decline of 18–45% as fair,
and decline <17% was considered as poor. Total efficiency =
excellent rate + good rate. Evaluation of pneumonia rehabilitation
was performed according to the guidelines for the treatment of
inflammatory diseases 2013 (15).
Levels of inflammatory factors lower than normal were
rehabilitated.
Statistical methods
Data were analyzed using SPSS 22.0 statistical
software (IBM Corp., Armonk, NY, USA). Measurement data were
expressed as mean ± standard deviation (SD), and comparisons
between two groups were performed by t-test. Intragroup comparisons
were performed w paired t-test. Enumeration data are expressed as
rates, and Chi-square tests were used for comparisons among groups.
Kaplan-Meier was used for survival analysis, and log-rank test was
used for testing. P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparison clinical data
There was no significant difference in age, weight,
onset time, sex composition and smoking habits between two groups
of patients (P>0.05) (Table
II).
 | Table II.Comparison of clinical data between
two groups of patients (n, %). |
Table II.
Comparison of clinical data between
two groups of patients (n, %).
| Variable | Observation
(n=160) | Control (n=154) | t/χ2 | P-value |
|---|
| Age (years) | 54.83±8.69 | 55.94±9.09 | 1.11 | 0.27 |
| Weight (kg) | 82.35±7.42 | 81.62±8.04 | 0.84 | 0.40 |
| Onset time (h) | 16.24±2.54 | 16.05±3.06 | 0.60 | 0.55 |
| Sex |
|
| 0.09 | 0.76 |
| Male | 150 (60.00) | 154 (63.64) |
|
|
|
Female | 100 (40.00) | 88 (36.36) |
|
|
| Smoking |
|
| 0.29 | 0.59 |
| Yes | 138 (55.20) | 129 (53.31) |
|
|
| No | 112 (44.80) | 113 (46.69) |
|
|
Comparison of CRP before and after
treatment
Before treatment, CRP were found between two groups
of patients (P=092). After treatment, indicators of both groups
were improved significantly (P<0.05). After treatment, levels of
CRP in the observation group were significantly lower than those in
the control group (P<0.01) (Table
III).
 | Table III.Comparison of CRP before and after
treatment (mg/ml). |
Table III.
Comparison of CRP before and after
treatment (mg/ml).
| Treatment | Observation
(n=160) | Control (n=154) | t | P-value |
|---|
| Before | 6.15±0.82 | 6.19±0.90 | 0.52 | 0.61 |
| After |
3.65±0.68a |
4.82±0.75a | 18.21 | <0.01 |
Comparison of levels of inflammatory
cytokines before and after treatment
Before treatment, no significant differences in
levels of IL-6, IL-8 and TNF-α were found between the two groups of
patients (P>0.05). After treatment, levels of inflammatory
cytokines were significantly improved in both groups (P<0.05).
After treatment, levels of IL-6, IL-8 and TNF-α in the observation
group were significantly lower than those in the control group
(P<0.01) (Table IV).
 | Table IV.Comparison of levels of inflammatory
cytokines before and after treatment (pg/ml). |
Table IV.
Comparison of levels of inflammatory
cytokines before and after treatment (pg/ml).
| Variables | Observation
(n=160) | Control (n=154) | t | P-value |
|---|
| Before |
| IL-6 | 69.58±11.63 | 71.34±12.88 | 1.27 | 0.20 |
| IL-8 | 86.34±10.59 | 85.92±11.31 | 0.34 | 0.73 |
|
TNF-α | 169.45±19.82 | 172.62±22.13 | 1.34 | 0.18 |
| After |
| IL-6 |
32.53±9.82a |
48.16±8.34a | 15.17 | <0.01 |
| IL-8 |
31.35±6.72a |
51.34±8.36a | 23.40 | <0.01 |
|
TNF-α |
61.45±12.67a |
89.85±11.09a | 21.10 | <0.01 |
Comparison of therapeutic efficacy
between two groups
Hospital stay in the observation group was
21.85±8.62 days, which was significantly shorter than that in the
control group 32.37±11.26 days (P<0.01). Excellent, good, fair
and poor rate of the observation group were 37.50, 49.38, 12.50 and
0.63%, respectively, while in the control group were 22.73, 52.60,
22.08 and 2.60%, respectively. Compared with the control group
(75.33%), total efficacy was significantly higher in the
observation group (86.88%) (P=0.01). In the observation group
(86.25%, 138 cases) of patients with pneumonia recovered
significantly, which was significantly better than that of the
control group (75.32%, 116 cases) (P<0.05) (Table V).
 | Table V.Comparison of therapeutic efficacy
between two groups (n, %). |
Table V.
Comparison of therapeutic efficacy
between two groups (n, %).
| Variables | Observation
(n=160) | Control
(n=154) |
t/χ2 | P-value |
|---|
| Hospital stay
(days) | 21.85±8.62 | 32.37±11.26 | 9.32 | <0.01 |
|
Excellent | 60 (37.50) | 35 (22.73) |
|
|
|
Good | 79 (49.38) | 81 (52.60) |
|
|
|
Fair | 20 (12.50) | 34 (22.08) |
|
|
|
Poor | 1 (0.63) | 4 (2.60) |
|
|
| Total efficacy rate
(%) | 86.88 | 75.33 | 6.86 | 0.01 |
| Pneumonia | 138 (86.25) | 116 (75.32) | 6.06 | 0.01 |
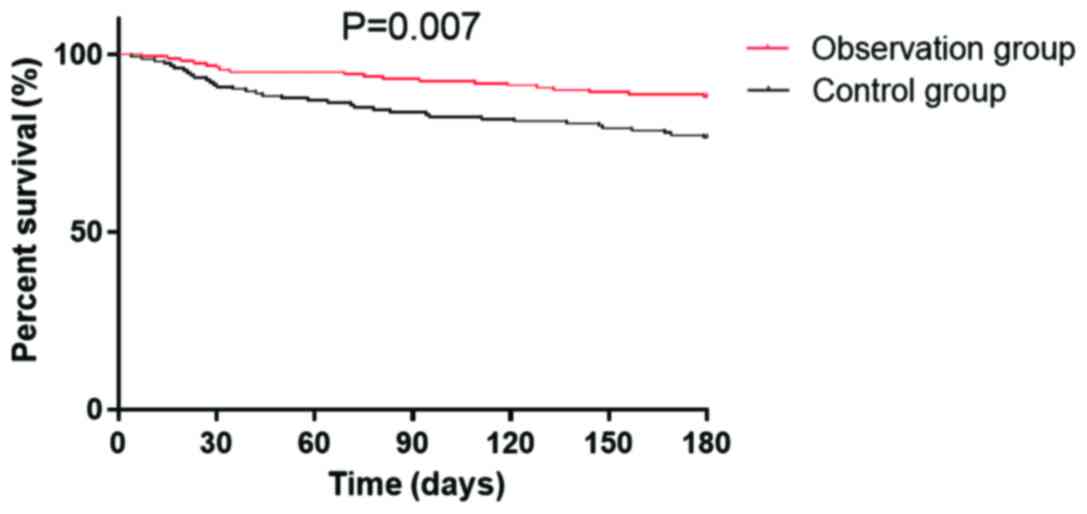
Prognosis survival rate
The patients were followed up for a period of six
months, and 312 patients completed the follow-up, and follow-up
success rate was 99.36%. Six patients were lost during follow-up,
including 2 in the observation group and 4 in the control group.
Survival rates in the observation group were 96.35, 93.13 and
88.13% in the first, third and sixth month, and 90.91, 83.77 and
76.62% in the control group, respectively (Table VI). The survival curve is shown is
Fig. 1.
 | Table VI.Prognosis survival rate (n, %). |
Table VI.
Prognosis survival rate (n, %).
| Variables | Observation
(n=160) | Control
(n=154) | χ2 | P-value |
|---|
| Survival rate in
the 1st month | 154 (96.35) | 140 (90.91) | 3.753 | 0.053 |
| Survival rate in
the 3rd month | 149 (93.13) | 129 (83.77) | 6.771 | 0.009 |
| Survival rate in
the 6th month | 141 (88.13) | 118 (76.62) | 7.185 | 0.007 |
Discussion
Cerebral infarction is extremely harmful and has
great negative impacts on health. In severe cases, cerebral
infarction may lead to disability in self-care and even death
(16). With the development and
progress of medical technology, prognosis of patients with cerebral
infarction has been improved. However, incidence and mortality of
cerebral infarction are still on the rise due to accelerated
population aging and differences in regional medical conditions
(17). Patients with cerebral
infarction complicated with aspiration pneumonia are more difficult
to treat because of concurrent infection diseases, and their
prognosis is even worse. Universal standard for the clinical
treatment of those patients remains unknown, and cerebral
infarction and aspiration pneumonia are usually separately treated.
However, different drugs may cause different reactions, leading to
poor treatment outcomes (18). In
this study, we analyzed the efficacy of the use of atorvastatin
alone in the treatment of patients with cerebral infarction
complicated with aspiration pneumonia. The aim of this study is to
provide references for the treatment of this disease.
Results of this study showed that the CRP levels in
the observation group treated with atorvastatin combined with basic
treatment were significantly lower than those in patients of the
control group who were only treated with basic therapy (P<0.01).
However, after treatment, HDL-C in the observation group was
significantly higher than that in the control group (P<0.01).
After treatment, levels of inflammatory cytokines IL-6, IL-8 and
TNF-α in the observation group were significantly lower than those
in the control group (P<0.01). Hospital stay and treatment
efficiency of the observation group were also better than those of
the control group (P<0.05). The six-month follow-up of all
patients showed that the half-year total mortality (11.88%) in the
observation group was significantly lower than that in the control
group (23.38%, P=0.01).
These data indicate that atorvastatin treatment can
effectively reduce the levels of blood lipids and inflammatory
factors in patients with cerebral infarction and aspiration
pneumonia and can improve prognosis. Du et al (19) also showed that the application of
atorvastatin in the treatment of cerebral infarction can reduce the
levels of inflammatory cytokines. Elevated levels of TC and TG is
the main cause of the formation of cerebrovascular endocardial
atherosclerotic plaque in patients with cerebral infarction, and
high TC and TG levels can also lead to increased plasma viscosity
and abnormal platelet aggregation (20). Therefore, lipid-lowering is important
for the treatment of patients with cerebral infarction.
Atorvastatin has a strong regulatory effect on platelets (21), which can intervene abnormal blood
flow and reduce levels of blood lipids and cholesterol in the body.
Increased HDL-C level can protect patient's arterial wall to
maintain smooth blood flow, which in turn avoid the formation of
thrombosis on the blood vessel wall caused by the accumulation of
LDL (22). In addition, atorvastatin
can reduce the production of inflammatory cytokines in the blood by
regulating blood lipids (23). In
this study, levels of IL-6, IL-8, TNF-α were significantly reduced
in the observation group patients compared with the control group,
suggesting that atorvastatin not only can regulate the level of
blood lipids, but also can inhibit inflammatory responses in those
patients. Atorvastatin can inhibit the secretion of inflammatory
cytokines in patients to inhibit the formation of macrophage tissue
factor-induced plaque, so as to relieve carotid atherosclerosis and
prevent the recurrence of cerebral infarction, thereby increasing
the survival rate of patients (24).
This study is also limited by some shortcomings.
Patients from different regions with different ethics backgrounds
were not included and the follow-up is relatively short. We will
solve these problems in our future studies.
In conclusion, atorvastatin is effective in the
treatment of cerebral infarction patients complicated with
aspiration pneumonia. Atorvastatin has satisfactory inhibitory
effect on inflammation and can improve the prognosis of patients.
So, it should be popularized in clinical practice.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
BW was responsible for the conception and design of
the study, and drafted the manuscript. BW and YL collected,
analyzed and interpreted the patient data, and revised the
manuscript for important intellectual content. Both authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Beijing Chaoyang Hospital, Capital Medical University (Beijing,
China). Signed informed consents were obtained from the patients or
the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tan XL, Xue YQ, Ma T, Wang X, Li JJ, Lan
L, Malik KU, McDonald MP, Dopico AM and Liao FF: Partial eNOS
deficiency causes spontaneous thrombotic cerebral infarction,
amyloid angiopathy and cognitive impairment. Mol Neurodegener.
10:242015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zeng Q, Lin K, Yao M and Wei L:
Significant correlation between cystatin C, cerebral infarction,
and potential biomarker for increased risk of stroke. Curr
Neurovasc Res. 12:40–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jabbarli R, Reinhard M, Roelz R, Shah M,
Niesen WD, Kaier K, Taschner C, Weyerbrock A and Van Velthoven V:
Early identification of individuals at high risk for cerebral
infarction after aneurysmal subarachnoid hemorrhage: The BEHAVIOR
score. J Cereb Blood Flow Metab. 35:1587–1592. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ye H, Wang L, Yang XK, Fan LP, Wang YG and
Guo L: Serum S100B levels may be associated with cerebral
infarction: A meta-analysis. J Neurol Sci. 348:81–88. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ye L, Cai R, Yang M, Qian J and Hong Z:
Reduction of the systemic inflammatory induced by acute cerebral
infarction through ultra-early thrombolytic therapy. Exp Ther Med.
10:1493–1498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jabbarli R, Reinhard M, Niesen WD, Roelz
R, Shah M, Kaier K, Hippchen B, Taschner C and Van Velthoven V:
Predictors and impact of early cerebral infarction after aneurysmal
subarachnoid hemorrhage. Eur J Neurol. 22:941–947. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fu HJ, Zhao LB, Xue JJ, Wu ZX, Huang YP,
Liu W and Gao Z: Elevated serum homocysteine (Hcy) levels may
contribute to the pathogenesis of cerebral infarction. J Mol
Neurosci. 56:553–561. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu W, Guan Y, Xu K, Fu XJ, Lei XF, Lei LJ,
Zhang ZQ, Cheng Y and Li YQ: Plasma homocysteine levels predict the
risk of acute cerebral infarction in patients with carotid artery
lesions. Mol Neurobiol. 53:2510–2517. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalra L, Irshad S, Hodsoll J, Simpson M,
Gulliford M, Smithard D, Patel A and Rebollo-Mesa I; STROKE-INF
Investigators, . Prophylactic antibiotics after acute stroke for
reducing pneumonia in patients with dysphagia (STROKE-INF): A
prospective, cluster-randomised, open-label, masked endpoint,
controlled clinical trial. Lancet. 386:1835–1844. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang JW and Hu ZP: Neuroprotective effects
of atorvastatin against cerebral ischemia/reperfusion injury
through the inhibition of endoplasmic reticulum stress. Neural
Regen Res. 10:1239–1244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang N, Lin M, Wang BG, Zeng WY, He YF,
Peng HY, Zeng J, Wu ZY and Zhong Y: Low level of low-density
lipoprotein cholesterol is related with increased hemorrhagic
transformation after acute ischemic cerebral infarction. Eur Rev
Med Pharmacol Sci. 20:673–678. 2016.PubMed/NCBI
|
|
12
|
Fang X, Tao D, Shen J, Wang Y, Dong X and
Ji X: Neuroprotective effects and dynamic expressions of MMP9 and
TIMP1 associated with atorvastatin pretreatment in
ischemia-reperfusion rats. Neurosci Lett. 603:60–65. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumar A, Brown R, Dhar R, Sampson T,
Derdeyn CP, Moran CJ and Diringer MN: Early vs. delayed cerebral
infarction after aneurysm repair after subarachnoid hemorrhage.
Neurosurgery. 73:617–623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen B, Grothe C and Schaller K:
Validation of a new neurological score (FOUR Score) in the
assessment of neurosurgical patients with severely impaired
consciousness. Acta Neurochir (Wien). 155:2133–2139. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Whyte J, Nordenbo AM, Kalmar K, Merges B,
Bagiella E, Chang H, Yablon S, Cho S, Hammond F, Khademi A, et al:
Medical complications during inpatient rehabilitation among
patients with traumatic disorders of consciousness. Arch Phys Med
Rehabil. 94:1877–1883. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li RY, Cao ZG, Li Y and Wang RT: Increased
whole blood viscosity is associated with silent cerebral
infarction. Clin Hemorheol Microcirc. 59:301–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koh SH and Lo EH: The role of the PI3K
pathway in the regeneration of the damaged brain by neural stem
cells after cerebral infarction. J Clin Neurol. 11:297–304. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Honeybul S, Ho KM and Gillett G: Outcome
following decompressive hemicraniectomy for malignant cerebral
infarction: Ethical considerations. Stroke. 46:2695–2698. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Du R, Teng JF, Wang Y, Zhao XY and Shi ZB:
Clinical study of Butylphthalide combined with Xue Shuan Tong on
serum inflammatory factors and prognosis effect of patients with
cerebral infarction. Pak J Pharm Sci. 28 Suppl:1823–1827.
2015.PubMed/NCBI
|
|
20
|
Yang N, Lin M, Wang BG, Zeng WY, He YF,
Peng HY, Zeng J, Wu ZY and Zhong Y: Low level of low-density
lipoprotein cholesterol is related with increased hemorrhagic
transformation after acute ischemic cerebral infarction. Eur Rev
Med Pharmacol Sci. 20:673–678. 2016.PubMed/NCBI
|
|
21
|
Kucera M, Balaz D, Kruzliak P, Ciccocioppo
R, Oravec S, Rodrigo L, Zulli A, Hirnerova E, Sabaka P, Komornikova
A, et al: The effects of atorvastatin treatment on the mean
platelet volume and red cell distribution width in patients with
dyslipoproteinemia and comparison with plasma atherogenicity
indicators - A pilot study. Clin Biochem. 48:557–561. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu W, Guan Y, Xu K, Fu XJ, Lei XF, Lei LJ,
Zhang ZQ, Cheng Y and Li YQ: Plasma homocysteine levels predict the
risk of acute cerebral infarction in patients with carotid artery
lesions. Mol Neurobiol. 53:2510–2517. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang J, Mu X, Breker DA, Li Y, Gao Z and
Huang Y: Atorvastatin treatment is associated with increased BDNF
level and improved functional recovery after atherothrombotic
stroke. Int J Neurosci. 127:92–97. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pantan R, Tocharus J, Suksamrarn A and
Tocharus C: Synergistic effect of atorvastatin and
Cyanidin-3-glucoside on angiotensin II-induced inflammation in
vascular smooth muscle cells. Exp Cell Res. 342:104–112. 2016.
View Article : Google Scholar : PubMed/NCBI
|