Introduction
Osteosarcoma (OS) and the Ewing sarcoma family of
tumors (ESFT) are the most common primary bone malignancies
observed in children and adolescents worldwide (1). OS is an osteoid-producing malignancy of
mesenchymal origin that accounts for <0.2% of all cancers
(2). However, the frequency of OS
has been increasing by 0.3% per year over the last 10 years
(2). OS occurs most frequently in
patients between 5 years of age and early adulthood (2). Unfortunately, few improvements have
been made in prognostic and therapeutic methods (3,4). As
such, increasing our understanding of the molecular events
associated with OS is of great importance.
Small ubiquitin-like modifier (SUMO)-ylation is a
dynamic posttranslational modification that occurs on substrate
proteins lysine residues to which a SUMO protein covalently
attached (5,6). SUMO proteins are highly conserved and
serve important roles in a number of cellular processes, including
the pathogenesis of cancer (5,7).
SUMOylation is a reversible process regulated by the SUMO-specific
protease (SENP) family, which exhibits isopeptidase activity to
remove SUMO proteins from substrates (8). A total of six SENP members have been
identified, including SENP1, SENP2, SENP3, SENP5, SENP6 and SENP7,
each of which has various substrate specificities and subcellular
localizations (9,10). SENP5 is reported to be overexpressed
in OS cell lines and tissues (11).
However, it remains unclear whether other SENP family members serve
a role in OS.
SENP2 has a broad de-SUMOylation activity (12). SENP2 null mice exhibit developmental
defects in trophoblast stem cell niches and lineages due to
dysregulation of the Mdm2-p53 signaling pathway in the placenta
(13). SENP2 also acts as a tumor
suppressor in human hepatocellular carcinoma by modulating the
stability of β-catenin (14,15).
The present study demonstrates that SENP2 negatively
regulates the proliferation, migration and invasion of OS cells.
SENP2 was significantly downregulated in OS tissues compared with
adjacent normal samples. SENP2 was associated with and promoted
proteasome-dependent SOX9 ubiquitination and degradation,
exhibiting its tumor suppressor function.
Materials and methods
Samples, cell lines, cell culture and
transfection
A total of 18 paired primary OS tissues and adjacent
non-tumor normal tissues were collected from patients (male, 11 and
female, 7; age range, 9–20 years; mean age, 15.6±4.8 years) with
newly diagnosed with OS who had not received any previous surgical
treatment from June 2014 to May 2016. The present study was
approved by the Institutional Review Board of Jing Zhou Central
Hospital, the Second Clinical Medical College, Yangtze University,
Jing Zhou (China) and written consent was obtained from all
participants or their families. Normal hFOB1.19 cells and OS cell
lines including Saos2, U2OS, Hos and MG63 were obtained from The
Cell Bank of Type Culture Collection of Chinese Academy of Sciences
(Shanghai, China). Cells were cultured in RPMI 1640 (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
fetal bovine serum (HyClone; GE Healthcare Life Sciences), 100
IU/ml penicillin and 100 mg/ml streptomycin and maintained at 37°C
in a humidified atmosphere containing 5% CO2. All
plasmids were purchased from Shanghai GeneChem Co., Ltd. (Shanghai,
China). For transfection, U2OS cells were cultured as described to
70–80% confluence in a 6-well plate. Flag-SENP2 (1 µg) and/or
HA-SOX9 (1 µg) and pcDNA 3.1 control plasmids were co-transfected
using Lipofectamine 2000 (Thermo Fisher Scientific, Inc.) following
the manufacturer's instructions. After 36 h, a total of 20 µM
protease inhibitor MG132 (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) were added for additional 4 h.
Immunoprecipitation (IP) with Flag M2
beads
U2OS cells were cultured to 70–80% confluence in 10
cm dishes and co-transfected with Flag-SENP2 (4 µg) and/or HA-SOX9
(4 µg) and pcDNA 3.1 (4 µg) control plasmids as above described.
Cells were lysed in lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China) for 20 min with gentle rocking at
4°C. Lysates were cleared by centrifugation (12,000 × g for 30 min
at 4°C) and then filtered through 0.45 µm spin filters (Beyotime
Institute of Biotechnology) to further remove cell debris. The
resulting material was subjected to IP with 20 µl anti-FLAG M2
affinity resin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
overnight at 4°C. Resin-containing immune complexes were washed
with ice-cold lysis buffer (2×5 min) followed by TBS washes (2×5
min). Proteins were eluted using 100 µl 150 µg/ml Flag-peptide
(Sigma-Aldrich; Merck KGaA) in TBS for 10 min at room temperature.
Proteins in each fraction were precipitated with 0.5 ml cold
acetone (Sigma-Aldrich; Merck KGaA) overnight at 4°C and collected
by centrifugation (12,000 × g; 30 min; 4°C). Protein samples were
mixed with SDS loading buffer (Beyotime Institute of Biotechnology,
Shanghai, China) prior to denaturation in a boiling water bath for
10 min and subjected to western blot assays.
CRISPR/Cas9 knock out (KO) cell
lines
KO plasmids, including SENP2 CRISPR/Cas9 KO Plasmid
(h): sc-402148 and SENP2 homology directed repair (HDR) Plasmid
(h): sc-402148-HDR, were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Hos cells at 70–80% confluence were
transfected with 2 µg KO and 2 µg HDR plasmids using Lipofectamine
2000 (Thermo Fisher Scientific, Inc.) following the manufacturer's
instructions and cells were selected with 1 µg/ml puromycin over 1
week, as previously described (16).
Cells were trypsin-digested and cultured as described in 96-well
for 1–2 weeks. Single clones were selected from individual wells
and cultured as described above in 10 cm dishes for 2 weeks.
Western blotting was used to verify the knockout efficiency.
Retroviral short hairpin (sh)RNA
Retroviral shRNA for SOX9 (Shanghai Genechem Co.,
Ltd.) was cloned into plko.1-puro vector. SOX9 shRNA sequence was
as follows:
5′-CCGGGCGGAGGAAGTCGGTGAAGAACTCGAGTTCTTCACCGACTTCCTCCGCTTTTTG-3′.
For transfection, SENP2 KO hos cells generated by CRISPR/Cas9 as
mentioned were cultured to 70–80% confluence in a 6-well plate.
plko.1 control vector (2 µg) or SOX9 shRNA plko.1 (2 µg) were
transfected using Lipofectamine 2000 (Thermo Fisher Scientific,
Inc.) following the manufacturer's instructions. Following 24 h of
transfection, cells were collected and subjected to migration and
invasion assays.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was harvested from human tissue samples or
cells using the RNA Isolation kit (Ambion; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Then, cDNA was obtained by RT at 37°C for 1 h using a Reverse
Transcription system (Takara Biotechnology Co., Ltd., Dalian,
China). qPCR was performed using a SYBR Green Premix Ex Taq (Takara
Bio, Inc., Otsu, Japan) with the following conditions: Denaturing
DNA at 95°C for 5 min, followed by 45 cycles of amplification with
94°C for 30 sec, 60°C for 60 sec and 72°C for 60 sec. Differences
in gene expression, expressed as fold-changes, were calculated
using the 2−ΔΔCq method (17). Results were normalized to β-actin.
Primer sequences were as follows: Human SENP2, forward,
5′-CTCAGGAACAGGCTGTAACA-3′ and reverse, 5′-CAGGACAGACAGAGTTTCCA-3′;
and human β-actin, forward, 5′-ACGAGACCACCTTCAACTCGATC-3′ and
reverse, 5′-AGGTCCTTCCTGATGTCCACGT-3′.
Western blotting
Tissues and cells were lysed with cold 2X SDS lysis
buffer (Beyotime Institute of Biotechnology) for 1 h at 4°C.
Lysates were centrifuged at 12,000 × g for 15 min at 4°C. The
supernatant was collected and the protein concentration was
determined by a bicinchoninic acid protein concentration
determination kit (Beyotime Institute of Biotechnology, Shanghai,
China). Protein samples (100 µg) were mixed with SDS loading buffer
(Beyotime Institute of Biotechnology) prior to denaturation in a
boiling water bath for 10 min. Samples (10 µg) were separated using
10% SDS-PAGE gels and resolved proteins were transferred to
nitrocellulose membranes on ice and blocked with 50 g/l skimmed
milk at room temperature for 1 h. Then, the membranes were
incubated with the following primary antibodies at 4°C overnight:
Rabbit anti-human SENP2 (1:500; cat. no. sc-130871; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), mouse anti-human
ubiquitin (1:1,000; cat. no. sc-166553; Santa Cruz Biotechnology,
Inc.), mouse anti-SOX9 (1:500; cat. no. sc-166505; Santa Cruz
Biotechnology, Inc.), mouse anti-human GAPDH (1:10,000; cat. no.
sc-47724; Santa Cruz Biotechnology, Inc.), mouse anti-Flag M2
(1:5,000; cat. no. F1804; Sigma-Aldrich; Merck KGaA) and mouse
anti-HA (1:5,000; cat. no. H9658; Sigma-Aldrich; Merck KGaA).
Following three washes with PBS plus Tween-20 (PBST), membranes
were incubated with polyclonal goat anti-mouse horseradish
peroxidase-conjugated secondary antibody (1:20,000; cat. no.
sc-2005; Santa Cruz Biotechnology, Inc.) or polyclonal goat
anti-rabbit horseradish peroxidase-conjugated secondary antibody
(1:20,000; cat. no. sc-2004; Santa Cruz Biotechnology, Inc.) for 1
h at room temperature. Membranes were washed three times with PBST
and developed using an enhanced chemiluminescence detection kit
(Sigma-Aldrich; Merck KGaA) for imaging. Image Lab v3.0 software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to acquire
and analyze imaging signals. GAPDH was used as controls for western
blot assay.
Migration and Matrigel invasion
assays
Migration assay and invasion assay were performed in
24-well plates with Boyden chambers (Corning Incorporated, Corning,
NY, USA) with a filter of 6.5 mm diameter and 8 µm pore size. All
procedures were performed according to the manufacturer's protocol.
Invasion assay was performed using the upper chambers coated with
Matrigel (Collaborative Biomedical Products, Bedford, MA, USA).
Matrigel was thawed at 4°C overnight and diluted with RPMI-1640
medium. The mixture (20 µl) was then added into the upper chamber
(BD Biosciences) and incubated at 37°C for 1 h. Cells
(4×104) were seeded into the upper chamber containing
0.2 ml serum-free RPMI-1640 medium. In addition, 0.5 ml RPMI-1640
medium supplemented with 10% fetal bovine serum was added into the
lower chamber. Following 48 h at 37°C, the chamber was removed and
cells in the upper chamber were wiped off. Following fixing with 4%
formaldehyde for 10 min at room temperature, samples were stained
at room temperature for 2 h using 0.2% crystal violet in 10%
methanol. Migration and invasion activities were evaluated by
counting the number of migrated cells on the lower surface of the
filters using a light microscope (magnification, ×100; EclipseE200;
Nikon Corporation, Tokyo, Japan).
Cycloheximide inhibition test
Control and SENP2 KO cells (5×105) were
cultured to 70–80% confluence in a 6-well plate and treated with 20
µg/ml cycloheximide (CHX; Sigma-Aldrich; Merck KGaA) for 0, 1, 2, 4
or 8 h at 37°C. SOX9 and SENP2 protein expression was measured by
western blot as described, using GAPDH as loading control.
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. Statistical significance was
evaluated using one-way analysis of variance with the Newman-Keuls'
post hoc test. Data were analyzed using GraphPad PRISM 6 (GraphPad
Software, Inc., La Jolla, CA, USA) and P<0.05 was considered to
indicate a statistically significant difference.
Results
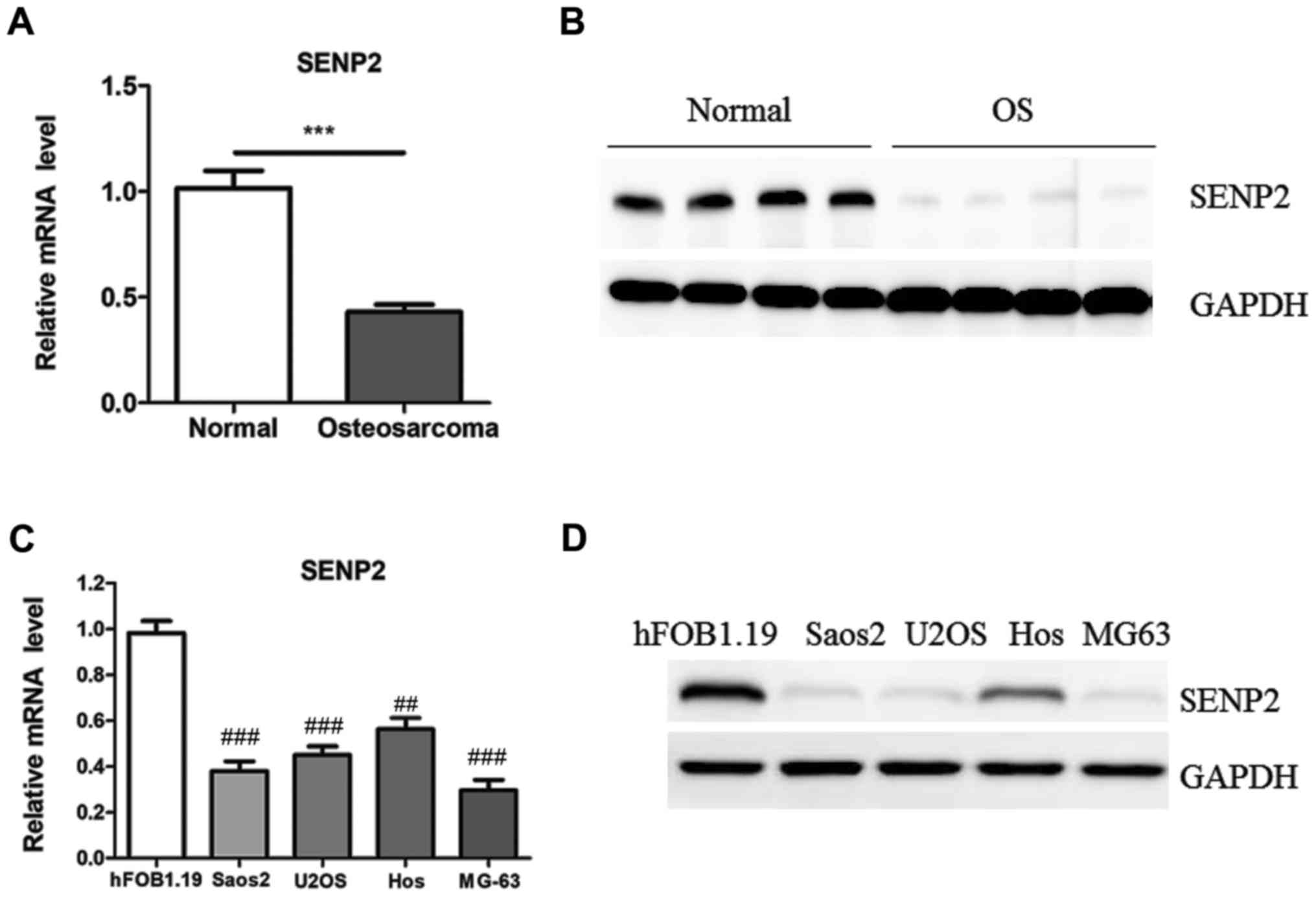
SENP2 was downregulated in OS samples
and cell lines
To investigate the expression of SENP2 in clinic
samples, SENP2 mRNA was measured in 18 paired primary OS samples
and adjacent normal tissues using RT-qPCR. SENP2 expression was
significantly decreased in OS samples compared with adjacent normal
tissues (Fig. 1A). SENP2 protein
expression was compared between 4 paired OS samples using western
blotting. The results demonstrated that SENP2 protein was also
downregulated in OS samples compared with adjacent normal tissues
(Fig. 1B). SENP2 mRNA and protein
expression was also significantly downregulated in Saos2, U2OS, Hos
and MG63 OS cell lines compared with normal hFOB1.19 cells
(Fig. 1C and D). Taken together,
these data demonstrate that SENP2 is downregulated in OS.
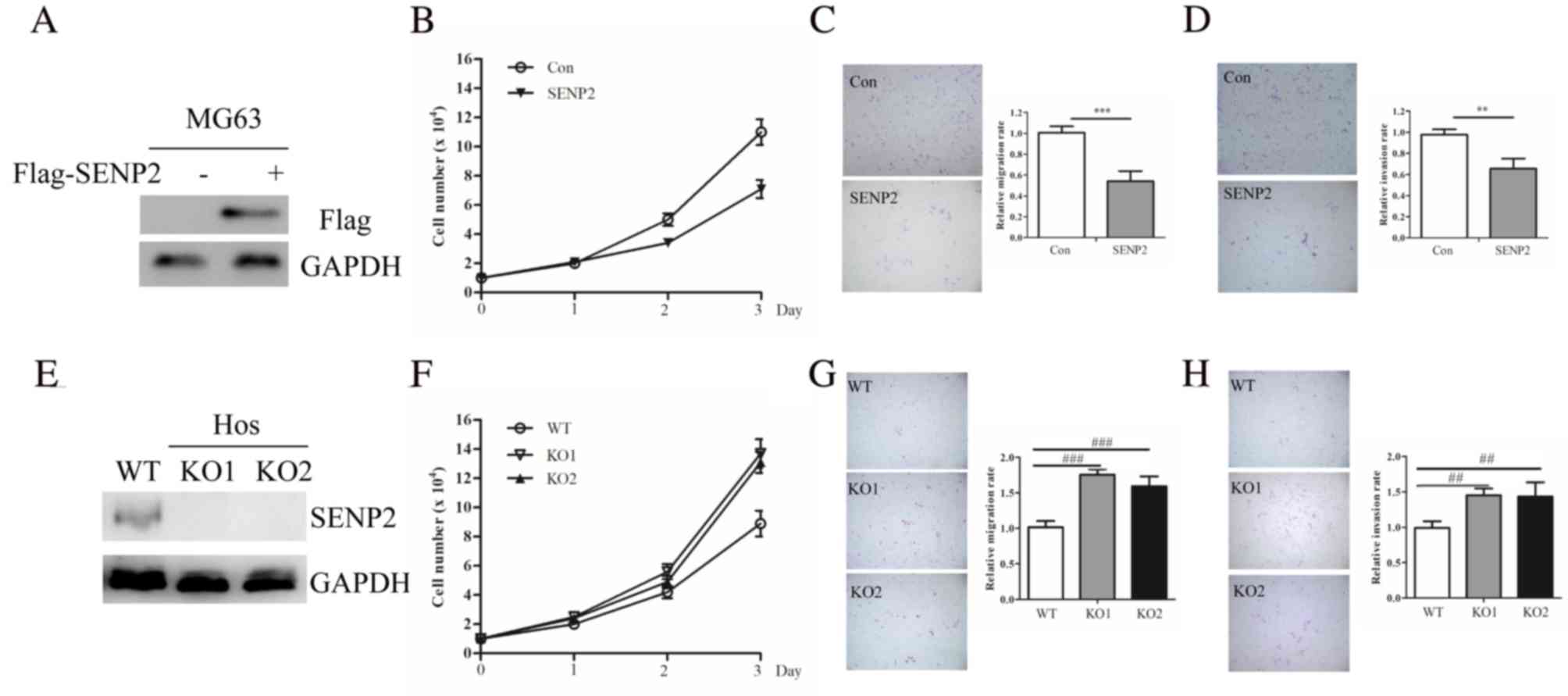
SENP2 regulates proliferation,
migration and invasion in OS cells
SENP2 is downregulated in OS cells and it was
observed that MG63 cells had the lowest expression; as such, MG63
cells were selected for use in following experiments (Fig. 2A). SENP2 overexpression significantly
inhibited the proliferation, migration and invasion of MG63 cells
(Fig. 2B-D). SENP2 knockdown was
achieved in Hos cells, which had the highest SENP2 expression of OS
cells assessed in the present study, using a CRISPR/Cas9 system.
Two clones were selected and SENP2 silencing was confirmed using
western blotting (Fig. 2E). KO
clones exhibited increased cell proliferation compared with control
cells (Fig. 2F). Consistent with
this, SENP2 depletion was demonstrated to enhance OS cell migration
and invasion (Fig. 2G-H).
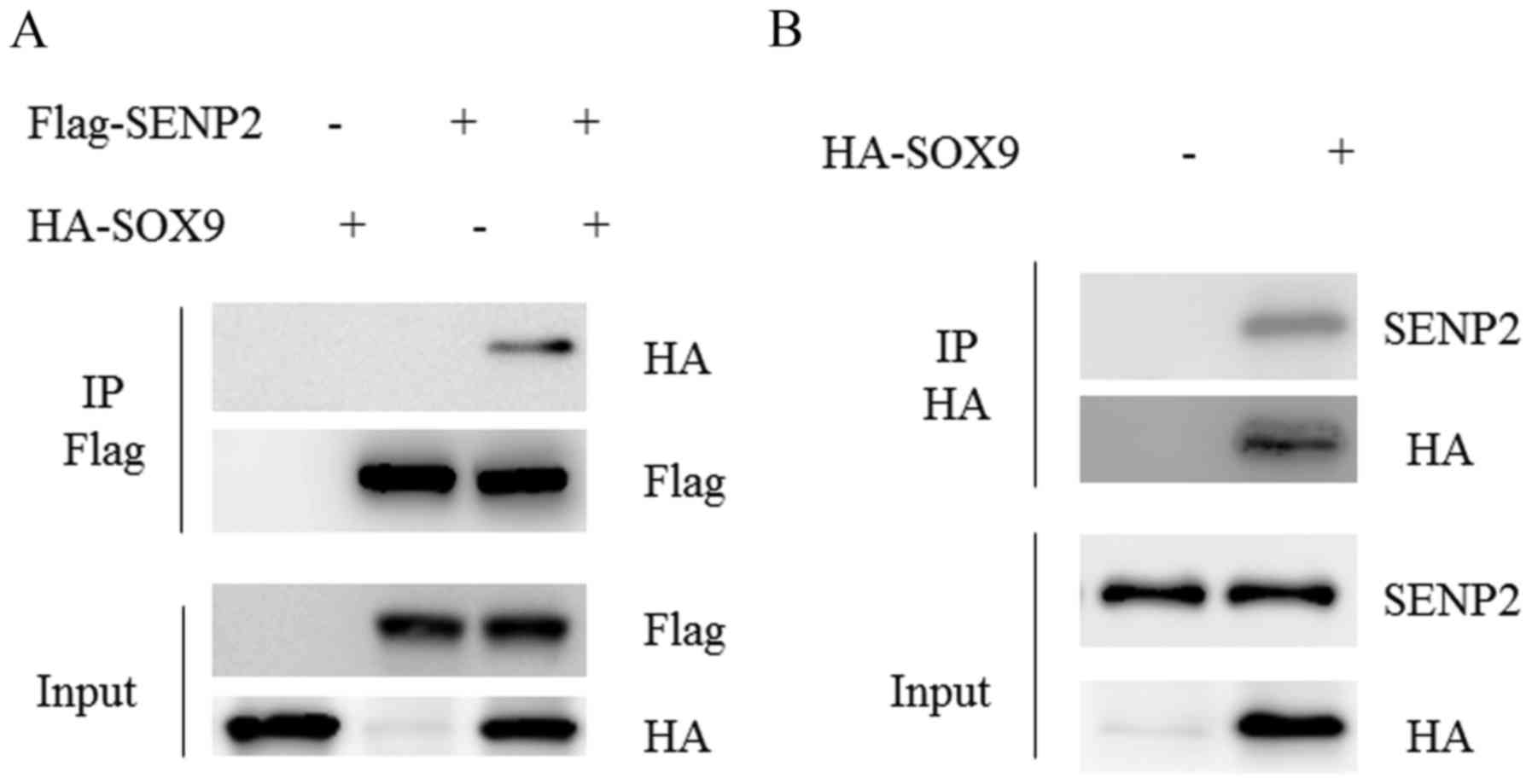
SENP2 is associated with SOX9
SOX9 is a transcription factor that serves crucial
roles in tissue development and tumorigenesis (18). In a previous study, SOX9 was
overexpressed in 120 out of 166 (72.3%) OS specimens and was
demonstrated to be associated with advanced clinical stage,
metastasis and poor response to chemotherapy (19). Given that SENP2 serves a tumor
suppressing role in OS cells and SOX9 is a SUMOylated protein
(20), the potential association
between SENP2 and SOX9 was investigated. To this end, U2OS cells
were co-transfected with Flag-SENP2 and HA-SOX9 for 48 h, cells
were then harvested and subjected to immunoprecipitation with Flag
M2 beads. The results revealed that HA-SOX9 was readily detected in
Flag-SENP2 immunoprecipitate (Fig.
3A). Furthermore, endogenous SENP2 was detected in HA-SOX9
immunoprecipitate (Fig. 3B). These
results suggest that SENP2 interacts with SOX9 in OS.
SENP2 promotes SOX9 ubiquitination and
degradation
In the presence of SENP2, the expression of
exogenous SOX9 was decreased (Fig.
4A); however, this effect was reversed by treatment with the
proteasome inhibitor MG132 (Fig.
4A), suggesting that SENP2 may regulate the protein stability
of SOX9. SOX9 protein expression was upregulated in SENP2 KO cells
(Fig. 4B). The effects of SENP2 on
SOX9 protein stability were assessed using a cycloheximide (CHX)
assay. CHX inhibits new protein synthesis and it was revealed that
the half-life of SOX9 was significantly extended in OS cells in the
absence of SENP2 (Fig. 4C).
Furthermore, SENP2 WT was demonstrated to significantly promote the
ubiquitination of SOX9. SENP2 Mut, a deSUMOylation catalytic
inactive form of SENP2, was unable to induce SOX9 ubiquitination
(Fig. 4D). Together, these data
suggest that SENP2 promotes SOX9 ubiquitination and degradation
dependent on its deSUMOylation activity.
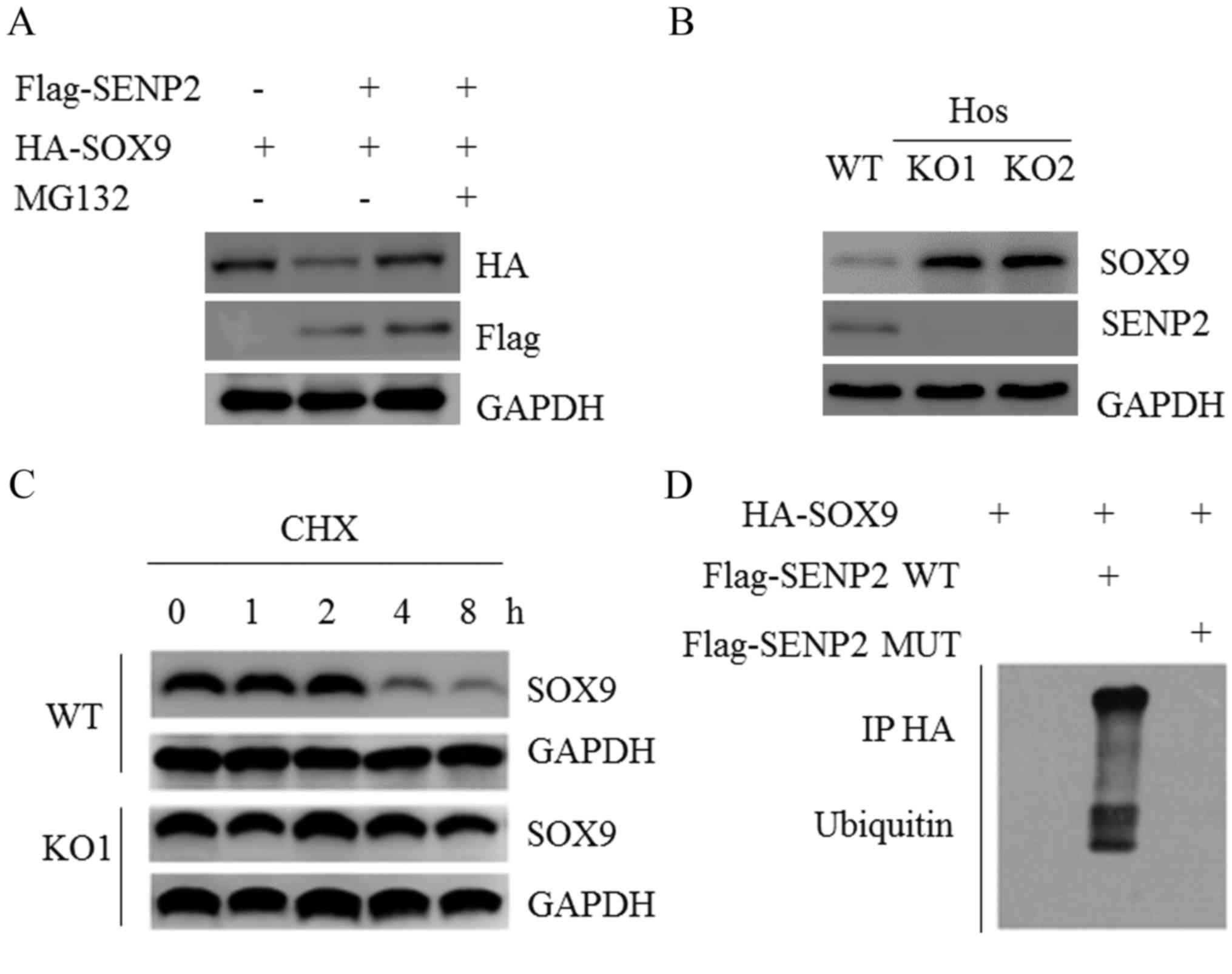 | Figure 4.SENP2 promotes SOX9 ubiquitination and
degradation. (A) U2OS cells were transfected with the indicated
plasmids for 36 h and subsequently treated with 20 µM MG132 for 4
h, following which western blotting was performed. (B) SOX9 protein
expression was measured in control and SENP2 KO cells. (C) Control
and SENP2 KO cells were treated with 20 µg/ml CHX for 0, 1, 2, 4 or
8 h and the protein expression of SOX9 was measured. (D) U2OS cells
were transfected with the indicated plasmids and subjected to
immunoprecipitation with HA-antibody and western blotting. SENP2,
small ubiquitin-like modifier-specific protease 2; SOX9, Sry-box-9;
KO, knockout; MUT, mutant; WT, wild type; HA, hemagglutinin. |
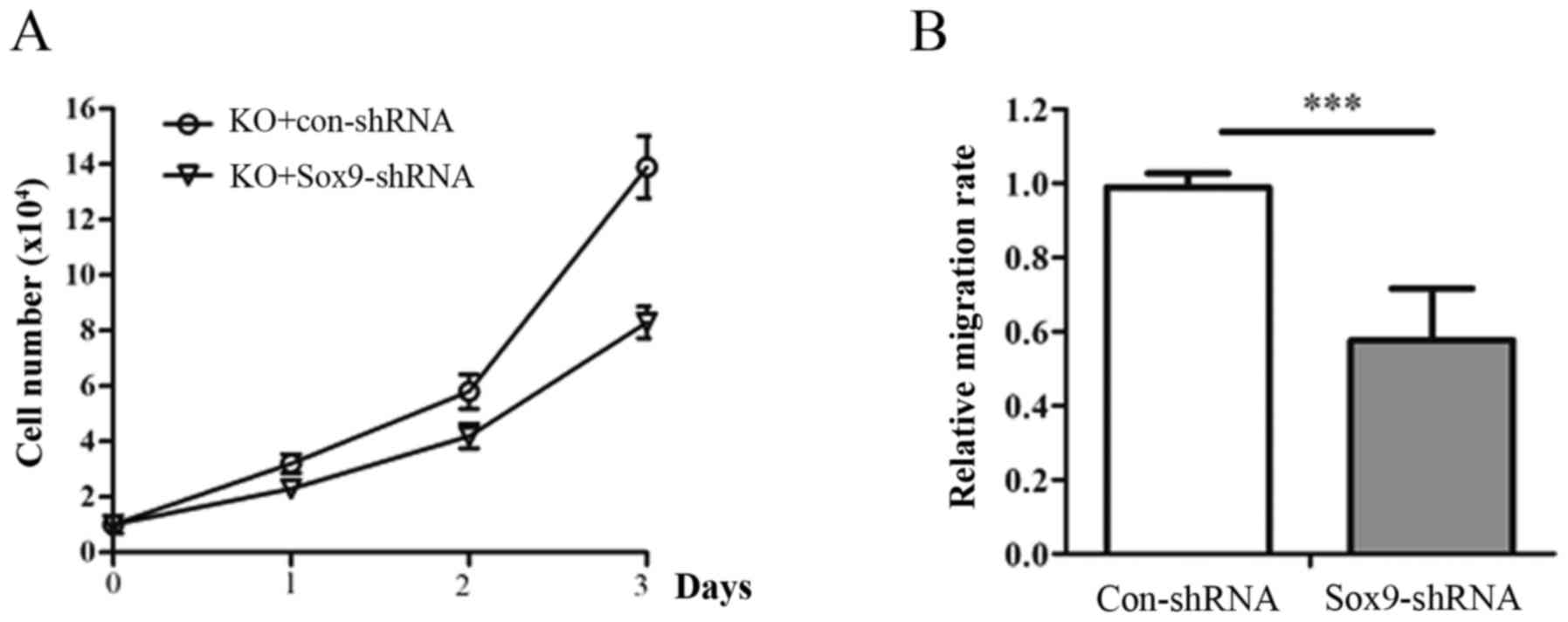
SENP2-SOX9 axis regulates OS cell
proliferation and invasion
To determine whether SOX9 is required for the
biological function of SENP2 in OS, SENP2 KO cells were further
transfected with shRNAs against SOX9. It was revealed that SOX9
knockdown largely inhibited the proliferation and invasion of SENP2
KO cells (Fig. 5A-B), suggesting
that SOX9 is a downstream target of SENP2 in OS cells.
Discussion
The transcription factor SOX9 is associated with the
development of a variety of malignant tumors and increased
aggressiveneness in OS. SOX9 knockdown reduced the proliferation of
OS cells by decreasing the expression of Wnt1 and its receptor Fzd1
(19). SOX9 protein levels are
regulated by SUMOylation and the ubiquitin-proteasome pathway
(21). SUMO1 and SUMOylation are
able to suppress the transactivation of SOX9 (22). SOX9 has a HMG box and its
transactivation serves critical roles in a number of developmental
processes, including sex determination and chondrogenesis (23). However, it is not yet known whether
SOX9 transactivation is required for the development of OS.
Unlike other SENP family members, SENP2 is regarded
as a tumor suppressor in a number of malignant tumors (24–26). In
the present study it was demonstrated that SENP2 exerted anti-tumor
effects in OS cells. Firstly, SENP2 expression is downregulated in
OS tissue samples and cultured cell lines. Secondly, SENP2
overexpression decreased the proliferation and invasion of OS
cells, whereas SENP2 knockdown had the opposite effect. Thirdly,
SOX9 was demonstrated to be a post-transcriptional target of SENP2
in OS cells. The results of the present study demonstrate that
SENP2 is associated with SOX9 and promotes its proteasome-dependent
degradation in a SUMOylation-dependent manner. Furthermore, SOX9
silencing was reveal to counteract the effects of SENP2
depletion.
In summary, the in vitro gain- and
loss-of-function studies performed in the present study demonstrate
that the SENP2-SOX9 regulatory axis serves a role in the regulation
of OS cell proliferation and invasion. These results suggest that
SENP2 dysregulation is an important feature of OS. As such,
restoring the expression or activity of SENP2 may be a promising
therapeutic treatment for patients with OS. However, the present
study is limited by the relative small number of clinical samples
utilized. Future studies may include a larger number of clinical
samples and animal experiments may further elucidate the biological
function of SENP2-SOX9 regulatory axis in the development and
treatment of OS.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets and materials used in the current study
are available from the corresponding author upon reasonable
request.
Authors' contributions
HP and ZZ collaborated to design the study. HP, LC,
QL and KW were responsible for experiments. HP, SC and ZL analyzed
the data. HP and ZZ wrote the paper. All authors collaborated to
interpret results and develop the manuscript. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Jing Zhou Central Hospital, the Second Clinical
Medical College, Yangtze University, Jing Zhou (China) and written
consent was obtained from all participants or their families.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jackson TM, Bittman M and Granowetter L:
Pediatric malignant bone tumors: A review and update on current
challenges, and emerging drug targets. Curr Probl Pediatr Adolesc
Health Care. 46:213–228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ballatori SE and Hinds PW: Osteosarcoma:
Prognosis plateau warrants retinoblastoma pathway targeted therapy.
Signal Transduct Target Ther. 1:160012016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Botter SM, Neri D and Fuchs B: Recent
advances in osteosarcoma. Curr Opin Pharmacol. 16:15–23. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hattinger CM, Fanelli M, Tavanti E, Vella
S, Ferrari S, Picci P and Serra M: Advances in emerging drugs for
osteosarcoma. Expert Opin Emerg Drugs. 20:495–514. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seeler JS and Dejean A: SUMO and the
robustness of cancer. Nat Rev Cancer. 17:184–197. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
David R: Sumoylation: Targeting SUMO. Nat
Rev Mol Cell Biol. 11:3872010. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li XC, Zeng Y, Sun RR, Liu M, Chen S and
Zhang PY: SUMOylation in cardiac disorders-a review. Eur Rev Med
Pharmacol Sci. 21:1583–1587. 2017.PubMed/NCBI
|
|
8
|
Guo C and Henley JM: Wrestling with
stress: Roles of protein SUMOylation and deSUMOylation in cell
stress response. IUBMB Life. 66:71–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bawa-Khalfe T and Yeh ET: SUMO losing
balance: SUMO proteases disrupt SUMO homeostasis to facilitate
cancer development and progression. Genes Cancer. 1:748–752. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JH and Baek SH: Emerging roles of
desumoylating enzymes. Biochim Biophys Acta. 1792:155–162. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang K and Zhang XC: Inhibition of SENP5
suppresses cell growth and promotes apoptosis in osteosarcoma
cells. Exp Ther Med. 7:1691–1695. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mikolajczyk J, Drag M, Békés M, Cao JT,
Ronai Z and Salvesen GS: Small ubiquitin-related modifier
(SUMO)-specific proteases: Profiling the specificities and
activities of human SENPs. J Biol Chem. 282:26217–26224. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chiu SY, Asai N, Costantini F and Hsu W:
SUMO-specific protease 2 is essential for modulating p53-Mdm2 in
development of trophoblast stem cell niches and lineages. PLoS
Biol. 6:e3102008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen HJ, Zhu HY, Yang C and Ji F: SENP2
regulates hepatocellular carcinoma cell growth by modulating the
stability of β-catenin. Asian Pac J Cancer Prev. 13:3583–3587.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang QF, Tian YW, Shen Q, Xue HZ and Li
K: SENP2 regulated the stability of β-catenin through WWOX in
hepatocellular carcinoma cell. Tumour Biol. 35:9677–9682. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen L, Pei H, Lu SJ, Liu ZJ, Yan L, Zhao
XM, Hu B and Lu HG: SPOP suppresses osteosarcoma invasion via
PI3K/AKT/NF-κB signaling pathway. Eur Rev Med Pharmacol Sci.
22:609–615. 2018.PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jo A, Denduluri S, Zhang B, Wang Z, Yin L,
Yan Z, Kang R, Shi LL, Mok J, Lee MJ and Haydon RC: The versatile
functions of Sox9 in development, stem cells, and human diseases.
Genes Dis. 1:149–161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu H, Chen Y, Zhou F, Jie L, Pu L, Ju J,
Li F, Dai Z, Wang X and Zhou S: Sox9 regulates hyperexpression of
Wnt1 and Fzd1 in human osteosarcoma tissues and cells. Int J Clin
Exp Pathol. 7:4795–4805. 2014.PubMed/NCBI
|
|
20
|
Gill G: SUMO changes Sox for developmental
diversity. Mol Cell. 20:495–496. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hattori T, Kishino T, Stephen S,
Eberspaecher H, Maki S, Takigawa M, de Crombrugghe B and Yasuda H:
E6-AP/UBE3A protein acts as a ubiquitin ligase toward SOX9 protein.
J Biol Chem. 288:35138–35148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oh HJ, Kido T and Lau YF: PIAS1 interacts
with and represses SOX9 transactivation activity. Mol Reprod Dev.
74:1446–1455. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lefebvre V and Dvir-Ginzberg M: SOX9 and
the many facets of its regulation in the chondrocyte lineage.
Connect Tissue Res. 58:2–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tan MY, Mu XY, Liu B, Wang Y, Bao ED, Qiu
JX and Fan Y: SUMO-specific protease 2 suppresses cell migration
and invasion through inhibiting the expression of MMP13 in bladder
cancer cells. Cell Physiol Biochem. 32:542–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu XY, Liu Z, Zhang KL, Feng J, Liu XF,
Wang LY and Wang ZW: SUMO-specific protease 2-mediated
deSUMOylation is required for NDRG2 stabilization in gastric cancer
cells. Cancer Biomark. 21:195–201. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tan M, Zhang D, Zhang E, Xu D, Liu Z, Qiu
J, Fan Y and Shen B: SENP2 suppresses epithelial-mesenchymal
transition of bladder cancer cells through deSUMOylation of
TGF-βRI. Mol Carcinog. 56:2332–2341. 2017. View Article : Google Scholar : PubMed/NCBI
|