Introduction
Lung cancer is the most common cancer type in men
and women worldwide, causing a large number of cancer-associated
mortalities (1,2). Lung adenocarcinoma constitutes ~50% of
lung cancer cases worldwide (1,2). Despite
advances in the treatment of lung adenocarcinoma in recent decades,
the overall 5-year survival rate of lung adenocarcinoma remains
poor, mainly due to the poor understanding of lung adenocarcinoma
pathogenesis (1). Exploring the
molecular mechanisms underlying lung adenocarcinoma progression may
assist in the development of novel therapeutic strategies.
MicroRNAs (miRs) are a type of small non-coding RNA
containing 22–25 nucleotides and are key regulators of gene
expression by directly interacting with the 3′-untranslated regions
(3′-UTRs) of their target mRNAs, leading to mRNA degradation or
translation inhibition (3,4). A large number of miRs are associated
with the regulation of various biological processes, including cell
proliferation, differentiation, apoptosis and migration, and cell
cycle progression (4). Additionally,
through mediating the expression of oncogenes or tumour
suppressors, numerous miRs have been revealed to serve suppressive
or promoting roles in the initiation and malignant progression of
human cancers including lung adenocarcinoma (5–8).
Exploring the expression and function of miRs in lung
adenocarcinoma may help identify novel biomarkers and therapeutic
targets for this disease.
Recently, miR-148a has been reported to be
aberrantly expressed in different human cancers and serve tumour
suppressive or promoting roles (9–11). For
instance, miR-148a expression was significantly downregulated in
gastric cancer tissues and cell lines, and served as a tumour
suppressor in gastric carcinogenesis by inactivating signal
transducer and activator of transcription (STAT)3 and RAC-α
serine/threonine-protein kinase, thus targeting
gastrin/cholecystokinin type B receptor (CCK-BR) (9). Recently, Li et al (12) reported that the expression of miR-148
was significantly lower in the serum of patients with
non-small-cell lung cancer (NSCLC) compared with that of patients
with benign pulmonary diseases and healthy controls. Additionally,
miR-148a has been reported to serve a suppressive role in NSCLC
(13–15). However, the regulatory mechanism of
miR-148a in lung adenocarcinoma growth remains largely unclear. The
present study aimed to explore the clinical significance of
miR-148a expression in lung adenocarcinoma, and investigate its
function and regulatory mechanisms in lung adenocarcinoma cell
proliferation.
Materials and methods
Tissue samples
The current study was approved by the Ethics
Committee of the Affiliated Hospital of Binzhou Medical College
(Binzhou, China). Lung adenocarcinoma and adjacent non-tumour
tissues were collected from 53 patients with lung adenocarcinoma
between June 2011 and October 2013 at Affiliated Hospital of
Binzhou Medical College. Table I
summarizes the clinical and pathological features of these
patients. Written informed consent was obtained from all patients,
and none of the patients underwent chemotherapy or radiotherapy
prior to surgery. All tissues were quickly snap-frozen in liquid
nitrogen following surgical resection and stored at −80°C prior to
use.
 | Table I.Association between miR-148a
expression and clinicopathological characteristics in lung
adenocarcinoma tissue. |
Table I.
Association between miR-148a
expression and clinicopathological characteristics in lung
adenocarcinoma tissue.
|
|
| miR-148a
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | Cases (n=53) | Low (n=25) | High (n=28) | P-value |
|---|
| Sex |
|
|
| 0.786 |
| Male | 29 | 13 | 16 |
|
|
Female | 24 | 12 | 12 |
|
| Age (years) |
|
|
| 0.785 |
|
<55 | 22 | 11 | 11 |
|
|
≥55 | 31 | 14 | 17 |
|
| Tumor size
(cm) |
|
|
| 0.166 |
|
<3 | 23 | 8 | 15 |
|
| ≥3 | 30 | 17 | 13 |
|
| Smoking history
(years) |
|
|
| 0.777 |
|
<10 | 20 | 11 | 9 |
|
|
≥10 | 33 | 14 | 14 |
|
| Tumor
differentiation |
|
|
| 0.054 |
|
Well-moderate | 25 | 8 | 17 |
|
|
Poor | 28 | 17 | 11 |
|
| TNM stage |
|
|
| 0.006 |
|
I–II | 28 | 8 | 20 |
|
|
III–IV | 25 | 17 | 8 |
|
| Lymph node
metastasis |
|
|
| 0.027 |
|
Negative | 24 | 7 | 17 |
|
|
Positive | 29 | 18 | 11 |
|
Cell culture
Several common lung adenocarcinoma cell lines (H23,
H1975, H2228, and H2085) and a normal bronchial epithelium cell
line (BEAS-2B) were obtained from American Type Culture Collection
(Manassas, VA, USA). These cells were cultured in Dulbecco's
modified Eagle's medium (DMEM) containing 10% foetal bovine serum
(FBS; both Thermo Fisher Scientific, Inc., Waltham, MA, USA). Cells
were all maintained in a humidified incubator containing 5%
CO2 and 95% O2 at 37°C.
Cell transfection
Cells transfection was conducted using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.)
following the manufacturer's protocol. 100 nM miR-148a mimics (cat.
no. HmiR0102-MR04), negative control (NC) miR mimics (miR-NC; cat.
no. CmiR0001-MR04), miR-148a inhibitor (cat. no.
HmiR-AN0204-SN-10), NC inhibitor (cat. no. CmiR-AN0001-SN; all
Guangzhou Fulengen Co., Ltd., Guangzhou, China), or 1 mg
pcDNA3.1-E2F3 expression plasmid or blank pcDNA3.1 plasmid (both
Hunan Nanhua Aishi Pulin Biotechnology; NanHua Bio-medicine Co.,
Ltd., Changsha, China) were transfected into H23 and H1975 cells
(5×106 cells/well). Following transfection for 48 h,
subsequent experiments were conducted.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues and cell lines
using TRIzol Reagent (Thermo Fisher Scientific, Inc.) and then
reverse transcribed into cDNA using SuperScript III Reverse
Transcriptase (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. qPCR was conducted using the SYBR-Green
Real-Time Master mix (Toyobo Life Science, Osaka, Japan) according
to the manufacturer's protocol. The reaction was performed under
the following conditions: 95°C for 5 min, and 40 cycles at 95°C for
15 sec and 60°C for 15 sec. GAPDH and U6 were used as internal
controls to normalise the expression of E2F3 and miR-148a,
respectively. The relative expression was calculated by the
2−ΔΔCq method (16). The
primer sequences were as follows: E2F3 forward,
5′-AGAAAGCGGTCATCAGTACCT-3′ and reverse,
5′-TGGACTTCGTAGTGCAGCTCT-3′; GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′; U6 forward,
5′-CTCGCTTCGGCAGCACATATACT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGT-3′. In addition, the primers for miR-148a
were purchased from Guangzhou Fulengen Co., Ltd. (cat. no.
HmiRQP0204) and the sequences were not supplied by the
manufacturer. The experiments were repeated three times.
Cell proliferation analysis
Following transfection, the H23 and H1975 cells were
seeded onto 96-well plates at a density of 2×103
cells/well and cultured in DMEM supplemented with 10% FBS at 37°C
for 0, 24, 48 and 72 h. Cell proliferation was determined using
Cell Counting kit-8 (Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. Absorbance was detected at an optical
density of 450 nm by a spectrophotometer. The experiments were
repeated three times.
Cell cycle analysis
Transfected H23 and H1975 cells were washed twice
with ice-cold PBS and fixed with 70% ethanol at −20°C overnight.
Cells were incubated with 50 mg/ml propidium iodide (Thermo Fisher
Scientific, Inc.) and 1 mg/ml RNase for 30 min in the dark at room
temperature. The distribution of the cell cycle was analysed using
a flow cytometer and BD Accuri™ C6 software (version
1.0; BD Biosciences, Franklin Lakes, NJ, USA). The experiments were
repeated three times.
Colony formation assay
Transfected H23 and H1975 cell suspensions (300
cells/well) were seeded in a 6-well plate and cultured in a
humidified incubator containing 5% CO2 at 37°C for 2
weeks. Then, the cells were fixed with 4% polyformaldehyde at room
temperature for 30 min and stained with 0.1% crystal violet at room
temperature for 10 min. The number of colonies was counted under a
light microscope (magnification, ×10). The experiments were
repeated three times.
Bioinformatics analysis and
dual-luciferase reporter gene assay
TargetScan 7.1 software (http://www.targetscan.org/) was used to predict the
putative targets of miR-148a. The wild type (WT) and mutant type
(MT) sequences in the 3′-UTR of E2F3 mRNA, which contained the
putative binding sites of miR-148a, were cloned into the
pMIR-REPORT luciferase reporter plasmids (Promega Corporation,
Madison, WI, USA). Transfected H23 and H1975 cells were then
transfected with miR-148a mimics or miR-NC with WT E2F3 or MT E2F3
plasmids, respectively, using Lipofectamine 2000. After the cells
were transfected for 48 h, the relative luciferase activity was
determined using the Dual-Luciferase® Reporter Assay
system (Promega Corporation) according to the manufacturer's
protocol. The activity of firefly luciferase was normalized to the
activity of Renilla luciferase. The experiments were
repeated three times.
Western blot analysis
Total protein was isolated from H23 and H1975 cells
using a radioimmunoprecipitation assay lysis buffer (Thermo Fisher
Scientific, Inc.). The protein concentration was determined using a
BCA Protein Assay kit (Thermo Fisher Scientific, Inc.). Protein (50
µg/lane) was separated by 12% SDS-PAGE, which was then transferred
onto a polyvinylidene fluoride membrane (Thermo Fisher Scientific,
Inc.). The membrane was blocked with 5% non-fat milk in TBST
overnight at 4°C. Following three washes with TBST, the membrane
was incubated with rabbit anti-human E2F3 (cat. no. ab50917) and
GAPDH (cat. no. ab9485; both 1:200; Abcam, Cambridge, MA, USA)
primary antibodies at room temperature for 3 h. Following three
washes with TBST, the membrane was incubated with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (cat. no.
ab6721; 1:5,000; Abcam) at room temperature for 1 h. The protein
signal was visualized using an enhanced chemiluminescence detection
system (GE Healthcare, Chicago, IL, USA) and analysed with Quantity
One® software (version 4.62; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). GAPDH was used as an internal control. The
experiments were repeated three times.
Statistical analysis
Data are expressed as mean ± standard deviation.
SPSS software (version 19.0; IBM Corp., Armonk, NY, USA) was used
to perform statistical analysis. The experiments were repeated
three times. Differences between the groups were analysed using an
unpaired Student's t-test or a paired Student's t-test. Differences
between more than two groups were analysed using one-way analysis
of variance with a Turkey's post hoc test. The Kaplan-Meier method
and log-rank test was used for the survival analysis of patients
grouped by high and low miR-148a expression. The association
between miR-148a and the clinicopathological features in lung
adenocarcinoma was analysed using a χ2 test. Pearson's
correlation coefficient was used to analyse the correlation between
the miR-148a and E2F3 expression levels in the lung adenocarcinoma
tissues. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-148a is downregulated in lung
adenocarcinoma
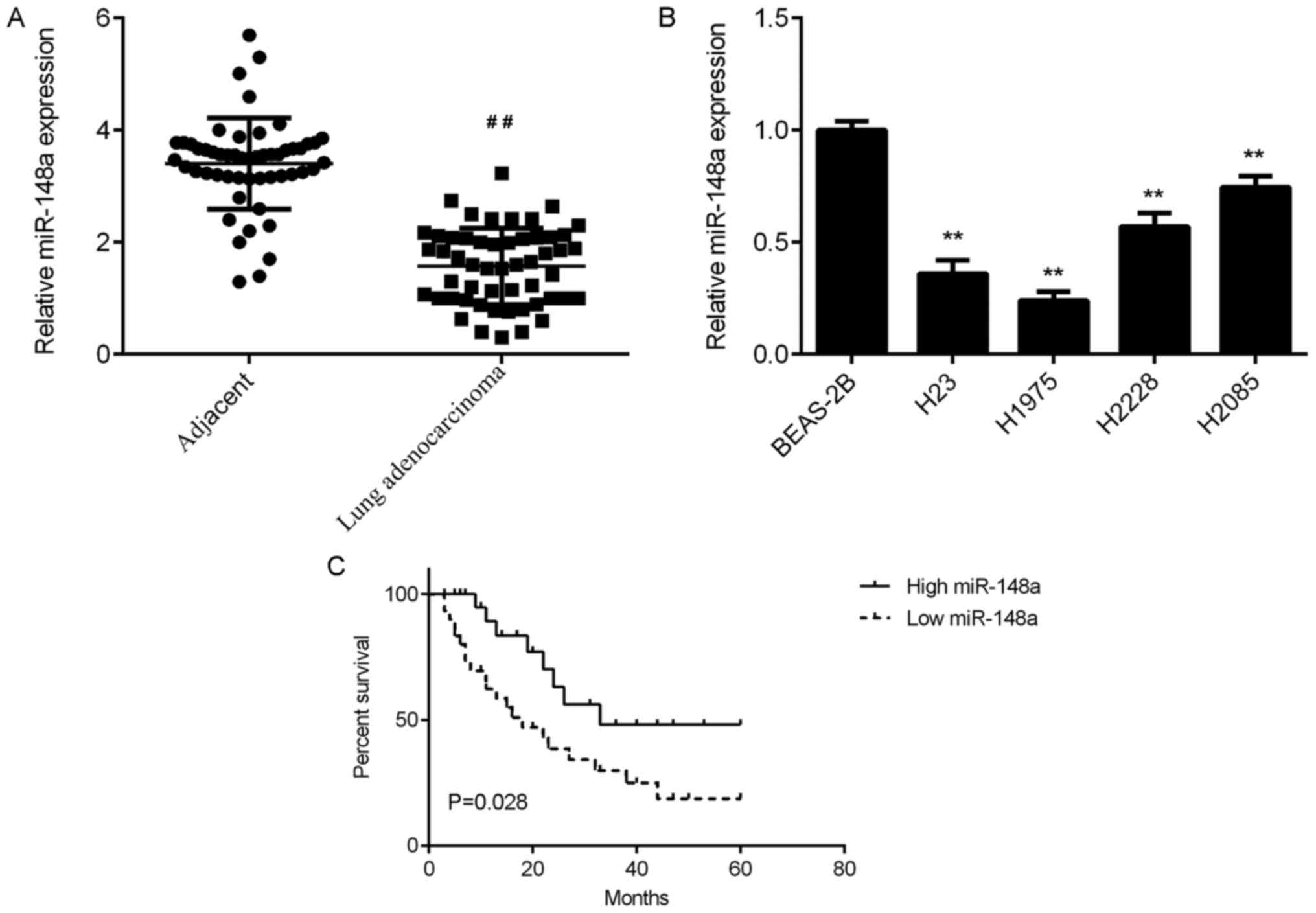
To examine the expression of miR-148a in lung
adenocarcinoma, RT-qPCR was conducted. The data demonstrated that
the expression of miR-148a was significantly reduced in lung
adenocarcinoma tissues compared with that in adjacent non-tumour
lung tissues (Fig. 1A). The
association between miR-148a expression and clinicopathological
characteristics were then studied in lung adenocarcinoma patients.
Based on the mean expression value of miR-148a (1.57) in lung
adenocarcinoma tissues, these lung adenocarcinoma patients were
divided into the low expression and high expression groups. It was
revealed that low miR-148a expression was significantly associated
with advanced Tumor, Node, Metastasis (TNM) stages and lymph node
metastasis of lung adenocarcinoma (Table
I).
Then, the miR-148a expression was examined in
several common lung adenocarcinoma cell lines compared with normal
bronchial epithelium BEAS-2B cells. The data indicated that
miR-148a was also downregulated in these lung adenocarcinoma cell
lines compared with BEAS-2B cells (Fig.
1B). Therefore, miR-148a is downregulated in lung
adenocarcinoma cell lines, which may contribute to the malignant
progression of this disease.
In addition, patients with low miR-148a expression
had a shorter survival time compared with those with high miR-148a
expression (Fig. 1C). These findings
suggest that low miR-148a expression may predict poor prognosis for
patients with lung adenocarcinoma.
miR-148a overexpression inhibits cell
proliferation, colony formation and cell cycle progression of lung
adenocarcinoma cells
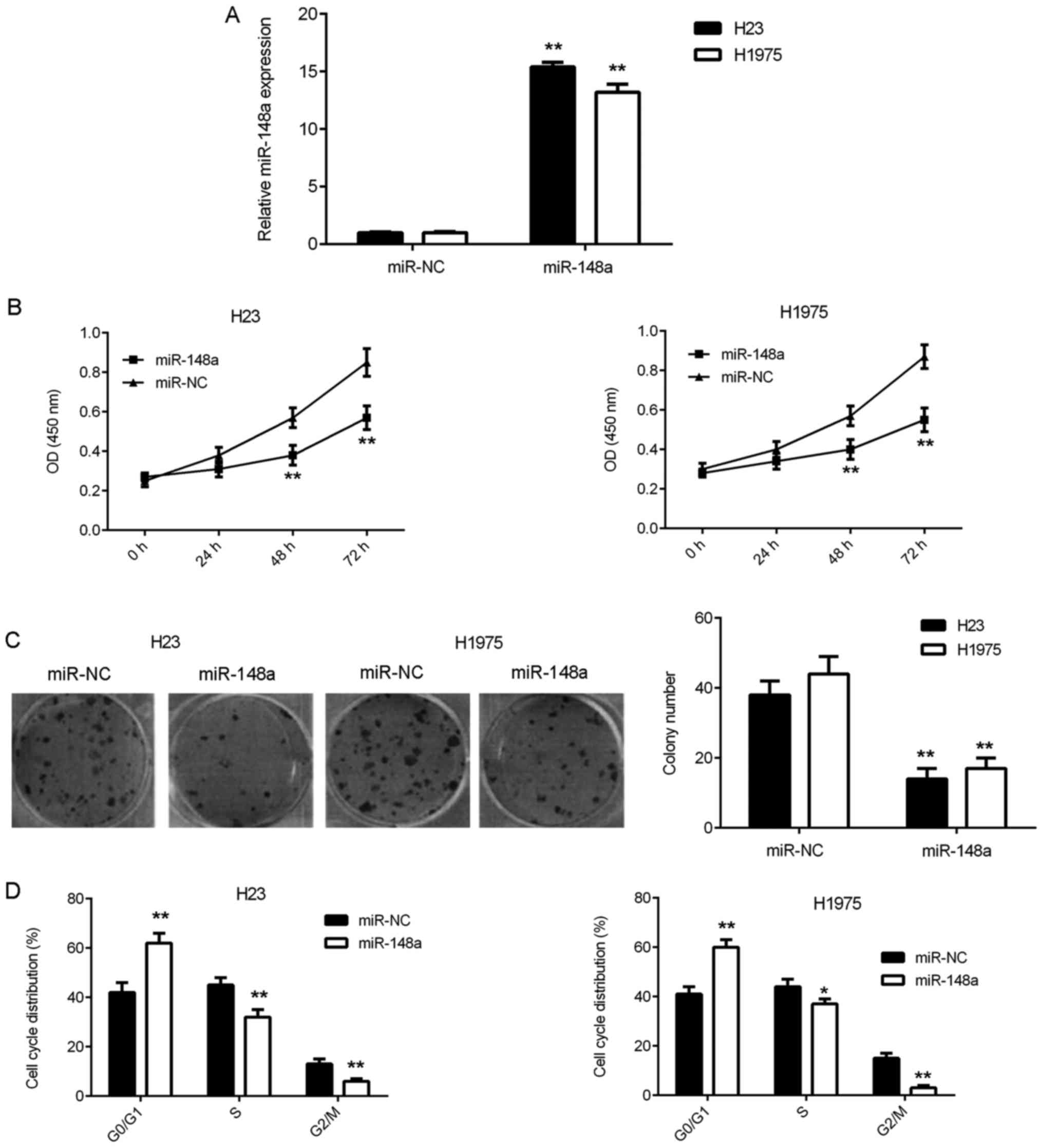
As miR-148a was downregulated in lung adenocarcinoma
cells, miR-148a mimics were transfected into H23 and H1975 cells to
increase its expression. Following the transfection, RT-qPCR was
conducted and the data revealed that miR-148a was significantly
upregulated in H23 and H1975 cells in the miR-148a group compared
with the control group (Fig. 2A).
Additional experiments demonstrated that the overexpression of
miR-148a significantly decreased the proliferation and colony
formation of H23 and H1975 cells compared with the control group
(Fig. 2B and C). Flow cytometry was
then conducted to evaluate the cell cycle distribution and the data
revealed that the upregulation of miR-148a caused a significant
cell cycle arrest at the G1 stage compared with the control group
(Fig. 2D). These results suggest
that miR-148a overexpression inhibits the cell proliferation of the
lung adenocarcinoma via inducing cell cycle arrest.
E2F3 is a target of miR-148a in lung
adenocarcinoma cells
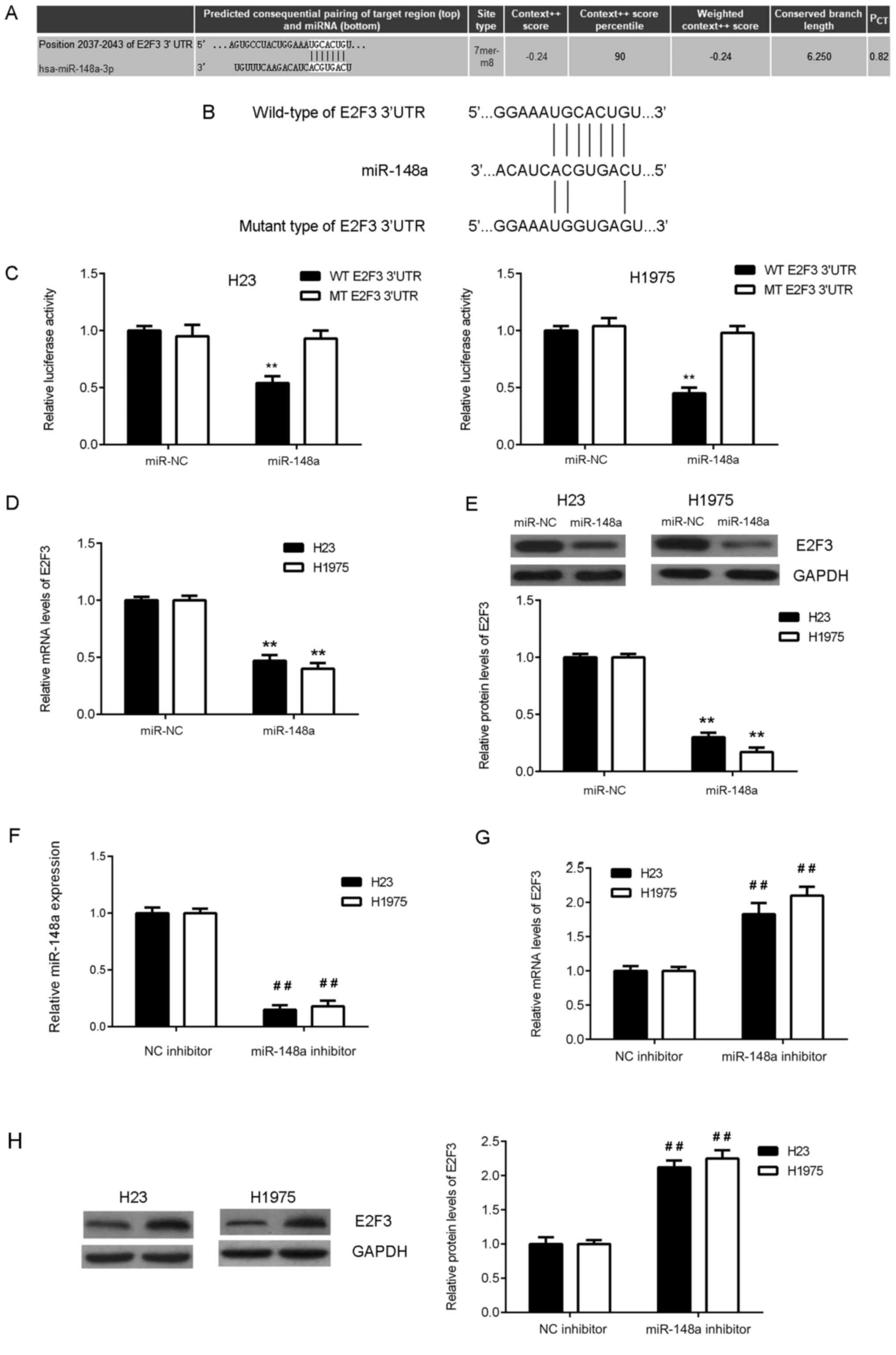
The underlying mechanisms of miR-148a in lung
adenocarcinoma cells were investigated. Bioinformatic analysis was
conducted to predict the putative target genes of miR-148a. As
presented in Fig. 3A, E2F3 was a
predicted target gene of miR-148a. To verify this prediction, a
dual-luciferase reporter gene assay was performed in H23 and H1975
cells. The data indicated that overexpression of miR-148a in the
miR-148a group significantly reduced the firefly luciferase
activity of the WT 3′-UTR of E2F3 compared with that of the control
group, but overexpression of miR-148a exhibited no significant
effect on the luciferase activity of the MT 3′-UTR of E2F3 in H23
and H1975 cells (Fig. 3B and C). It
was further observed that the overexpression of miR-148a
significantly reduced the mRNA and protein expression of E2F3 in
H23 and H1975 cells compared with the control group (Fig. 3D and E). Subsequently, H23 and H1975
cells were transfected with miR-148a inhibitor or NC inhibitor,
respectively. Following transfection, the expression of miR-148a
was significantly reduced in the miR-148a inhibitor group when
compared with the NC inhibitor group (Fig. 3F). In addition, it was demonstrated
that transfection with a miR-148a inhibitor significantly increased
the mRNA and protein expression of E2F3 in H23 and H1975 cells
compared with those in cells transfected with an NC inhibitor
(Fig. 3G and H). Therefore, E2F3 was
revealed to be a direct target of miR-148a and the expression of
E2F3 was negatively mediated by miR-148a in lung adenocarcinoma
cells.
E2F3 is upregulated in lung
adenocarcinoma
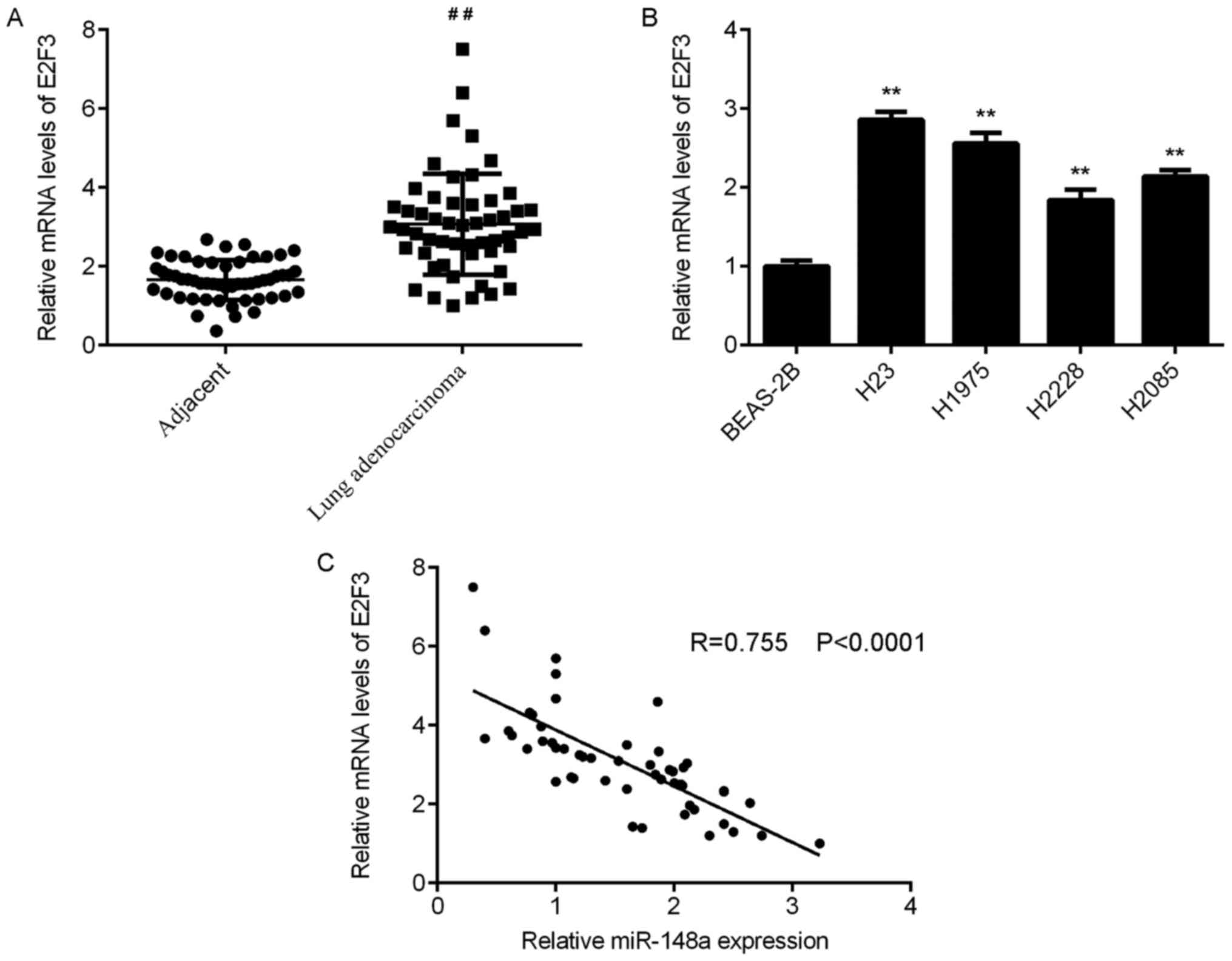
RT-qPCR analyses were conducted to examine E2F3 mRNA
expression in tissues, and the data indicated that E2F3 expression
was significantly upregulated in lung adenocarcinoma tissues
compared with that in adjacent non-tumour lung tissues (Fig. 4A). Then, the mRNA expression of E2F3
was examined in lung adenocarcinoma cell lines and BEAS-2B cells.
The data indicated that E2F3 was also upregulated in lung
adenocarcinoma cell lines compared with BEAS-2B cells (Fig. 4B). Notably, the expression of
miR-148a was significantly and inversely correlated with E2F3
expression in lung adenocarcinoma tissues (Fig. 4C). Therefore, the increased
expression of E2F3 may be caused by the reduced expression of
miR-148a in lung adenocarcinoma tissues.
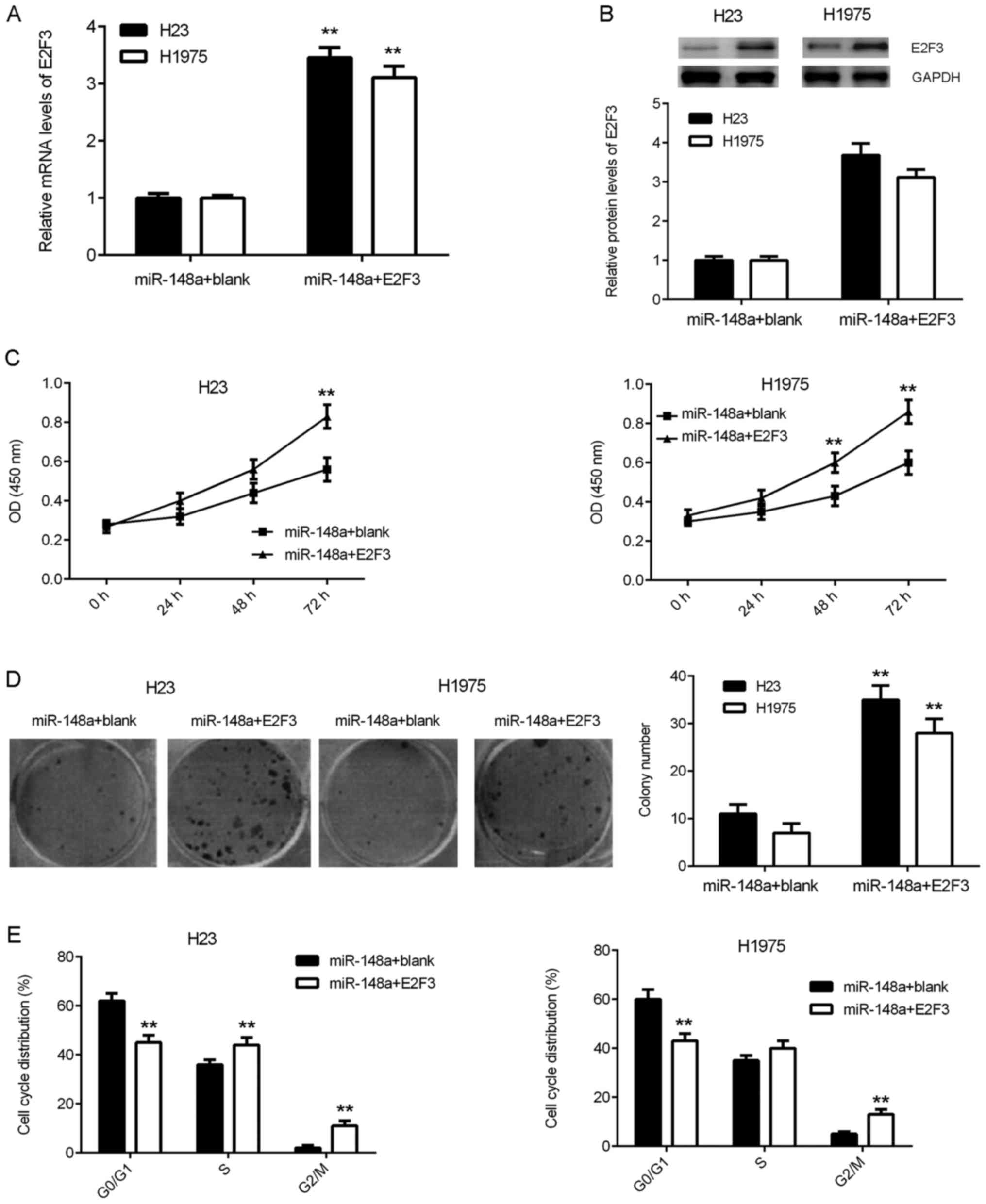
Restoration of E2F3 expression
counteracts the suppressive effects of miR-148a overexpression on
lung adenocarcinoma cells
The authors of the current study investigated
whether the suppressive effects of miR-148a on lung adenocarcinoma
cells were mediated by E2F3. H23 and H1975 cells were
co-transfected with miR-148a mimics and the E2F3 expression or
blank pcDNA3.1 plasmid. Following transfection, the E2F3 mRNA and
protein expression levels were significantly and markedly
increased, respectively, in the miR-148a+E2F3 group compared with
the miR-148a+blank group (Fig. 5A and
B). Subsequent functional assays revealed that the cell
proliferation, colony formation and cell cycle progression were
also significantly promoted in the miR-148a+E2F3 group compared
with the miR-148a+blank group (Fig.
5C-E). These findings suggest that restoration of E2F3
expression counteracts the suppressive effects of miR-148a
overexpression on lung adenocarcinoma cells.
Discussion
The present study aimed to examine miR-148a
expression in lung adenocarcinoma and investigate the molecular
mechanisms of miR-148a underlying lung adenocarcinoma growth. It
was revealed that miR-148a was significantly downregulated in lung
adenocarcinoma tissues and cell lines, and low miR-148a expression
was significantly associated with advanced TNM stages and lymph
node metastasis, as well as a shorter survival time for patients.
Increasing miR-148a expression greatly decreased the cell
proliferation, colony formation and cell cycle progression of H23
and H1975 cells. E2F3 was identified as a target of miR-148a in H23
and H1975 cells. In addition, the expression of E2F3 was negatively
regulated by miR-148a in H23 and H1975 cells. E2F3 was greatly
upregulated in lung adenocarcinoma tissues and cell lines, and the
expression of miR-148a was inversely correlated with the E2F3
expression in lung adenocarcinoma tissues. Additional experiments
demonstrated that increasing E2F3 expression counteracted the
inhibitory effects on lung adenocarcinoma cells caused by miR-148a
overexpression. These findings suggest that the downregulation of
miR-148a may be implicated in the malignant progression of lung
adenocarcinoma.
Deregulations of miR-148a expression have been
observed in different cancer types (17–19). For
example, miR-148a expression was downregulated in gastric cancer
tissues compared with their corresponding non-tumour tissues, which
was significantly associated with advanced TNM stages and lymph
node metastasis, and induced the suppression of gastric cancer cell
migration and invasion (20). In
contrast, miR-148a is androgen-responsive and promotes prostate
cell growth by inhibiting cullin-associated NEDD8-dissociated
protein 1 expression (21). Reduced
miR-148a expression contributed to the suppression of osteosarcoma
cell death (22). These diverse
functions of miR-148a in different cancer types may be due to the
different tumour microenvironment.
Additionally, certain miR-148a targets have been
identified, including receptor tyrosine-protein kinase erbB-3
(23), rho-associated protein kinase
1 (ROCK1) (20), ubiquitin
carboxyl-terminal hydrolase 4 (19),
DNA (cytosine-5)-methyltransferase 1 (24), proto-oncogene Wnt-1 (25), collagenase 3 (26) and CCK-BR (9). Li et al (27) demonstrated that miR-148a suppressed
epithelial-mesenchymal transition by targeting ROCK1 in NSCLC
cells. He and Xue (15) reported
that miR-148a inhibited NSCLC cell proliferation and invasion via
inhibition of STAT3 expression. In the current study, E2F3 was
confirmed to be a direct target gene of miR-148a in lung
adenocarcinoma cells. E2F3 is a key regulator of the G1/S phase
transition, and the activation of E2F3 serves a role in promoting
the development and progression of different cancer types through
promoting the malignant phenotypes of cancer cells (28,29). In
addition, specific miRs have been identified as miRs that directly
target E2F3 to inhibit cancer progression. For instance, miR-503
inhibits cell proliferation and induces apoptosis in colorectal
cancer cells by targeting E2F3 (30). miR-200c inhibits the invasion,
migration and proliferation of bladder cancer cells through
inhibiting the expression of polycomb complex protein BMI-1 and
E2F3 (31). In the present, it was
revealed that E2F3 was significantly upregulated in lung
adenocarcinoma tissues and cell lines, and the increased expression
of E2F3 was inversely correlated with the reduced expression of
miR-148a in lung adenocarcinoma tissues. These findings suggest
that the downregulation of miR-148a contributes to the upregulation
of E2F3 in lung adenocarcinoma. As further experiments demonstrated
that the overexpression of E2F3 counteracted the inhibitory effects
of miR-148a on lung adenocarcinoma cells, the authors of the
present study suggest that targeting E2F3 may become a promising
therapeutic strategy for lung adenocarcinoma.
In summary, the present study demonstrated that
miR-148a has suppressive effects on the proliferation, colony
formation and cell cycle progression of lung adenocarcinoma cells,
at least in part, through directly targeting E2F3. Therefore, the
authors of the current study suggest that miR-148a may be used as a
potential candidate for the treatment of lung adenocarcinoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
JL collected clinical tissues and wrote the
manuscript. HT designed the study and revised the manuscript. LS
and JL performed all experiments.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of Affiliated Hospital of Binzhou Medical College. All
written informed consents have been obtained from patients involved
in the current study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M
and Croce CM: Human microRNA genes are frequently located at
fragile sites and genomic regions involved in cancers. Proc Natl
Acad Sci USA. 101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu Y, Zhao H, Rao M and Xu S:
MicroRNA-365 inhibits proliferation, migration and invasion of
glioma by targeting PIK3R3. Oncol Rep. 37:2185–2192. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng Y, Lv X, Wang X, Wang B, Shao X,
Huang Y, Shi L, Chen Z, Huang J and Huang P: MiR-181b promotes
chemoresistance in breast cancer by regulating Bim expression.
Oncol Rep. 35:683–690. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu S, Song L, Zeng S and Zhang L:
MALAT1-miR-124-RBG2 axis is involved in growth and invasion of
HR-HPV-positive cervical cancer cells. Tumour Biol. 37:633–640.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu B, Lv X, Su L, Wang B, Shao X, Huang Y,
Shi L, Chen Z, Huang J and Huang P: MiR-148a functions as a tumor
suppressor by targeting CCK-BR via inactivating STAT3 and Akt in
human gastric cancer. PLoS One. 11:e01589612016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu X, Zhang Y, Jasper J, Lykken E,
Alexander PB, Markowitz GJ, McDonnell DP, Li QJ and Wang XF:
MiR-148a functions to suppress metastasis and serves as a
prognostic indicator in triple-negative breast cancer. Oncotarget.
7:20381–20394. 2016.PubMed/NCBI
|
|
11
|
Feng H, Wang Y, Su J, Liang H, Zhang CY,
Chen X and Yao W: MicroRNA-148a suppresses the proliferation and
migration of pancreatic cancer cells by down-regulating ErbB3.
Pancreas. 45:1263–1271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li L, Chen YY, Li SQ, Huang C and Qin YZ:
Expression of miR-148/152 family as potential biomarkers in
non-small-cell lung cancer. Med Sci Monit. 21:1155–1161. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Joshi P, Jeon YJ, Lagana A, Middleton J,
Secchiero P, Garofalo M and Croce CM: MicroRNA-148a reduces
tumorigenesis and increases TRAIL-induced apoptosis in NSCLC. Proc
Natl Acad Sci USA. 112:8650–8655. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Yu T, Cao J, Liu L, Liu Y, Kong HW,
Zhu MX, Lin HC, Chu DD, Yao M and Yan MX: MicroRNA-148a suppresses
invasion and metastasis of human non-small-cell lung cancer. Cell
Physiol Biochem. 37:1847–1856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He M and Xue Y: MicroRNA-148a suppresses
proliferation and invasion potential of non-small cell lung
carcinomas via regulation of STAT3. Onco Targets Ther.
10:1353–1361. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gailhouste L, Gomez-Santos L, Hagiwara K,
Hatada I, Kitagawa N, Kawaharada K, Thirion M, Kosaka N, Takahashi
RU, Shibata T, et al: miR-148a plays a pivotal role in the liver by
promoting the hepatospecific phenotype and suppressing the
invasiveness of transformed cells. Hepatology. 58:1153–1165. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia J, Guo X, Yan J and Deng K: The role
of miR-148a in gastric cancer. J Cancer Res Clin Oncol.
140:1451–1456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heo MJ, Kim YM, Koo JH, Yang YM, An J, Lee
SK, Lee SJ, Kim KM, Park JW and Kim SG: microRNA-148a dysregulation
discriminates poor prognosis of hepatocellular carcinoma in
association with USP4 overexpression. Oncotarget. 5:2792–2806.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng B, Liang L, Wang C, Huang S, Cao X,
Zha R, Liu L, Jia D, Tian Q, Wu J, et al: MicroRNA-148a suppresses
tumor cell invasion and metastasis by downregulating ROCK1 in
gastric cancer. Clin Cancer Res. 17:7574–7583. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murata T, Takayama K, Katayama S, Urano T,
Horie-Inoue K, Ikeda K, Takahashi S, Kawazu C, Hasegawa A, Ouchi Y,
et al: miR-148a is an androgen-responsive microRNA that promotes
LNCaP prostate cell growth by repressing its target CAND1
expression. Prostate Cancer Prostatic Dis. 13:356–361. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bhattacharya S, Chalk AM, Ng AJ, Martin
TJ, Zannettino AC, Purton LE, Lu J, Baker EK and Walkley CR:
Increased miR-155-5p and reduced miR-148a-3p contribute to the
suppression of osteosarcoma cell death. Oncogene. 35:5282–5294.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu J, Li Q, Xu Q, Liu L and Jiang B:
MiR-148a inhibits angiogenesis by targeting ERBB3. J Biomed Res.
25:170–177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Long XR, He Y, Huang C and Li J:
MicroRNA-148a is silenced by hypermethylation and interacts with
DNA methyltransferase 1 in hepatocellular carcinogenesis. Int J
Oncol. 44:1915–1922. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang Q, He M, Ma MT, Wu HZ, Yu ZJ, Guan
S, Jiang LY, Wang Y, Zheng DD, Jin F and Wei MJ: MicroRNA-148a
inhibits breast cancer migration and invasion by directly targeting
WNT-1. Oncol Rep. 35:1425–1432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xue J, Chen Z, Gu X, Zhang Y and Zhang W:
MicroRNA-148a inhibits migration of breast cancer cells by
targeting MMP-13. Tumour Biol. 37:1581–1590. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J, Song Y, Wang Y, Luo J and Yu W:
MicroRNA-148a suppresses epithelial-to-mesenchymal transition by
targeting ROCK1 in non-small cell lung cancer cells. Mol Cell
Biochem. 380:277–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Noguchi S, Mori T, Otsuka Y, Yamada N,
Yasui Y, Iwasaki J, Kumazaki M, Maruo K and Akao Y: Anti-oncogenic
microRNA-203 induces senescence by targeting E2F3 protein in human
melanoma cells. J Biol Chem. 287:11769–11777. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiao F, Zhang W, Chen L, Chen F, Xie H,
Xing C, Yu X, Ding S, Chen K, Guo H, et al: MicroRNA-503 inhibits
the G1/S transition by downregulating cyclin D3 and E2F3 in
hepatocellular carcinoma. J Transl Med. 11:1952013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang SW, Yue J, Wang BC and Zhang XL:
miR-503 inhibits cell proliferation and induces apoptosis in
colorectal cancer cells by targeting E2F3. Int J Clin Exp Pathol.
8:12853–12860. 2015.PubMed/NCBI
|
|
31
|
Liu L, Qiu M, Tan G, Liang Z, Qin Y, Chen
L, Chen H and Liu J: miR-200c Inhibits invasion, migration and
proliferation of bladder cancer cells through down-regulation of
BMI-1 and E2F3. J Transl Med. 12:3052014. View Article : Google Scholar : PubMed/NCBI
|