Introduction
Incisional hernia is the most common complication
following abdominal surgery. The reported incidence after midline
laparotomy ranges from 3 to 26% and increases rapidly if the native
operation is complicated by wound infection or other conditions
(1,2). With the development of materials and
techniques, substantial attention is currently being paid to
hernioplasty. From the perspective of prevention, more work should
be performed before hernia occurs. The incision healing depends on
the balance of collagen synthesis, remodeling and degradation
(3). The linea alba consists of
transverse and oblique collagen fibers (4,5), and
cutting off these fibril bundles constitutes the anatomical basis
of midline incisional hernia. Collagen regeneration and remodeling,
especially the mature form of type I collagen (6), is the most important event in the
healing of the midline incision.
For collagen synthesis, mesenchymal stem cells
(MSCs) are widely used as seed cells in tissue regeneration
medicine because of their easy gaining, quick proliferation and
multiple differentiating ability. There have been few studies
focusing on MSCs for incision healing. Stoff et al (7) injected MSCs into intracutaneous
incisions, which significantly inhibited scar formation and
increased the tensile strength of the skin wounds. Heffner et
al (8) combined platelet-rich
plasma, collagen and MSCs to investigate the healing effect on
midline laparotomy incisions. The results indicated a marked
improvement in abdominal wall strength, and histological evaluation
confirmed increased vascularity and collagen abundance.
Bone morphogenetic proteins (BMPs) belong to the
family of transforming growth factor β, which can induce MSCs
differentiating into different tissues. Unlike other BMPs, BMP-12
has been reported to be less osteo-chondrogenic and to induce
tendon rather than bone formation in vivo, and has already
made some achievements in the field of tendon injuries (9,10). The
objective of this study is to investigate the curative effects of
MSCs' tenogenic differentiation and transplantation on incision
healing induced by BMP-12. To our knowledge, this study is a novel
exploration of MSCs' regenerative application in abdominal wall
surgery.
Materials and methods
Laboratory animals and groups
Fifty Sprague-Dawley rats (Silaike Inc., Shanghai,
China), weighing between 150 and 200 g, were randomly divided into
five groups: negative control (A), positive control (B), sham group
(C), native MSCs (D) and tenogenically differentiated MSCs (E). Ten
rats were included in each group. All experiments were conducted
with the approval of the Animal Care and Use Committee of Southeast
University (Nanjing, China).
Group A did not undergo any operation and nothing
was implanted. Group B only underwent operation, but did not
implant anything. Group C undergo operation, but only sponge
scaffold was implanted into the linea alba incision. Group D
undergo operation and sponge scaffold with native MSCs were
implanted. Group E undergo operation and sponge scaffold with
tenogenically differentiated MSCs were implanted.
Isolation and culture of MSCs
The rat bone marrow-derived MSCs were prepared as
described (11,12). Briefly, bone marrow was collected by
flushing the femur and tibia with medium, and MSCs were isolated by
gradient centrifugation for 30 min at room temperature, using
Ficoll-Paque PLUS (1.077 g/ml; GE Healthcare Life Sciences, Little
Chalfont, UK). After centrifugation, cell pellets were resuspended
in Dulbecco's modified Eagle's medium with low glucose (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), supplemented
with 10% fetal bovine serum and incubated at 37°C in a humidified
atmosphere containing 5% CO2 with regular replenishment
of medium every two to three days.
BMP-12 inducing tenogenic
differentiation of MSCs
MSCs at passage 3 were plated at a density of
5×102 cells/35 mm dish and treated with BMP-12
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) of 10 ng/ml for 48
h. Then, BMP-12 was removed and replaced by normal medium without
BMP-12 for another 5 days. reverse transcription-quantitative
polymerase chain reaction was used to evaluate the expression of
scleraxis (SCX), collagen I and collagen III at 48 h, 5 and 7 days.
The gene-specific primers are presented in Table I. The relative quantity of target
gene expression was analyzed using the comparative CT
(2−ΔΔCq) method (13).
GAPDH was used as an endogenous reference gene.
 | Table I.Primers for RT-qPCR analysis. |
Table I.
Primers for RT-qPCR analysis.
| Gene | Forward sequence | Reverse sequence |
|---|
| Scx |
5′-AGAGACGGCGGCGAGAAC-3′ |
5′-AATCGCCGTCTTTCTGTCACG-3′ |
| Col I |
5′-AATGGTGCTCCTGGTATTGC-3′ |
5′-GGTTCACCACTGTTGCCTTT-3′ |
| Col III |
5′-AGCTGGACCAAAAGGTTGATG-3′ |
5′-GACCTCGTGCTCCAGTTAGC-3′ |
| GAPDH |
5′-CCTGGCCAAGGTCATCCAT-3′ |
5′-GTCATGAGCCCTTCCACGAT-3′ |
In vivo implantation
The MSCs-sponge complex was built as previously
described (14). MSCs were suspended
in growth medium at 1×106 cells/ml, and 0.5 ml was
seeded onto sterilized 2×10 mm collagen sponge scaffolds.
Cell-seeded scaffolds were placed in culture dishes and incubated
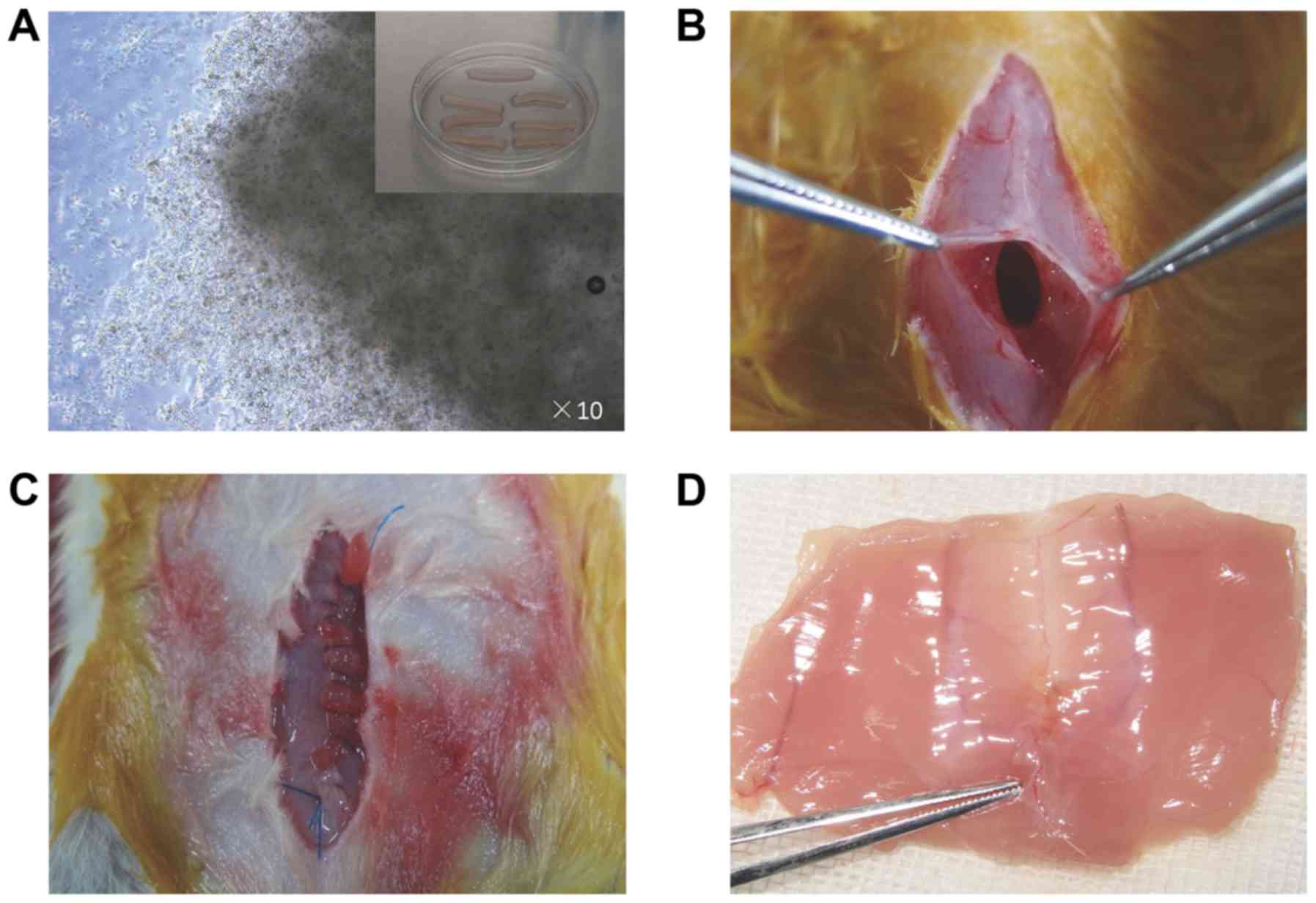
for 2 h in a minimum volume of normal medium (Fig. 1A), after which more medium was
applied to submerge the scaffolds for 24 h. Then, the MSCs-sponge
complex was cultured for an additional 7 days using 10 ng/ml of
BMP-12 or normal medium.
Animals were anesthetized with 0.3% pentobarbital
sodium, and the abdominal skin was sterilized with iodophor. A
longitudinal midline skin incision was made to expose the linea
alba, and a 10 mm long defect was created along the linea alba
(Fig. 1B), referring to a previous
study (15). Then, the linea alba
incision was closed using 5-0 prolene (Ethicon, Inc., Cincinnati,
OH, USA) with or without the MSCs-sponge complex (Fig. 1C). The suture/incision ratio was
4:1.
After the operation, the animals were raised for
another 4 weeks and then euthanized. The whole abdominal wall was
obtained for further analysis (Fig.
1D). The sample including the linea alba scar was pruned into
two parts with widths of 5 mm. One piece was preserved in saline
for tensiometric testing, and the other was fixed in 10% neutral
buffered zinc-formalin for histological analysis.
Tensiometric testing
Mechanical testing of the linea alba scar was
performed within 6 h of tissue harvest. According to the previous
study (16) and our pilot trial, the
sample for tensiometric testing requires special pruning because
the ventral muscles have a remarkably weaker anti-tensile capacity
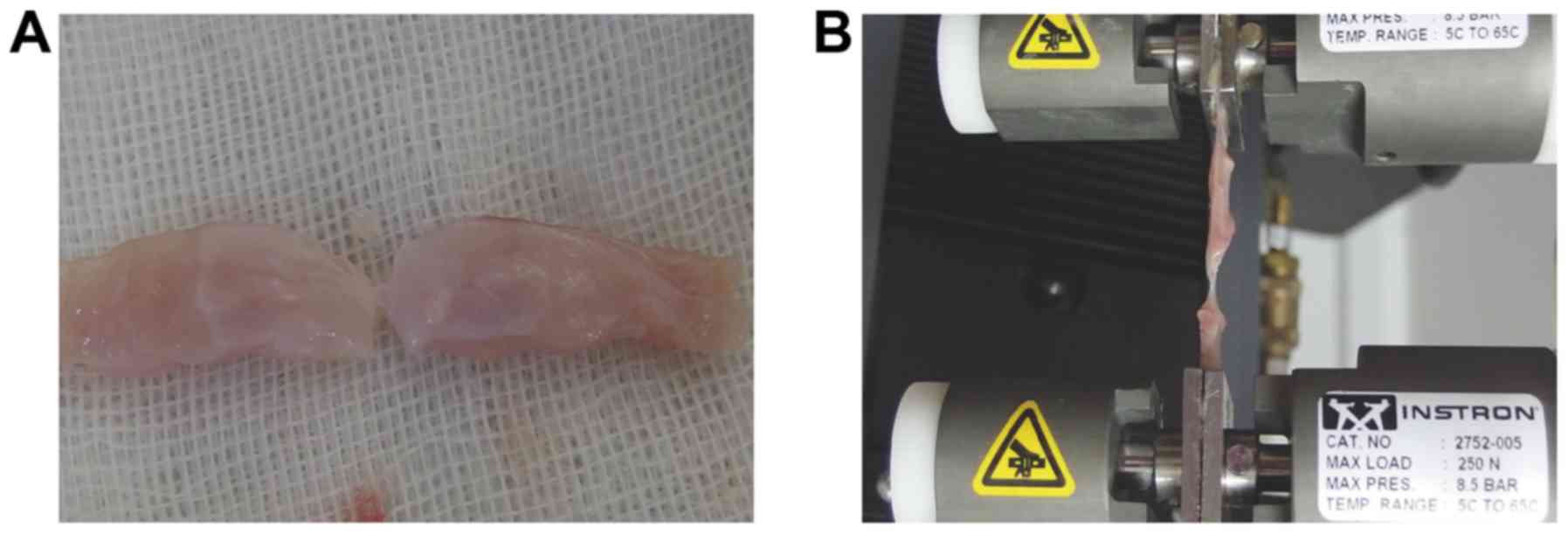
than the linea alba. As shown in Fig.
2A, only 2 mm of the midline scar was preserved compared to 5
mm of ventral muscles. It was loaded into the grips of an Instron
tensiometric machine (Instron, Norwood, MA, USA) equipped with a
250 N load cell. Tissue samples preloaded with 1 N force were then
loaded at a rate of 10 mm/min (17)
until complete failure (Fig. 2B).
The load and displacement data recorded were used to determine the
maximum force required for failure of the linea alba scar. Because
of the notable deformation of the tissues, the modulus of
elasticity and displacement index were not included in this study.
Data analysis was conducted using Instron's Bluehill software
package.
Histological analysis
The fixed tissues were cut transversely to present
the healing of the linea alba scar and stained with Masson's
Trichrome. The scale used in our study was a modified
semiquantitative histological analysis (Table II), which is analogous to that
described by Rice et al (17). Two blinded observers determined the
collagen amount and organization. Staining depth of the collagen
reflected the amount. Organization indicated as the orientation of
fibers. Each example was examined at four random sites to get an
average score.
 | Table II.Modified histological scoring system
for microscopic evaluation. |
Table II.
Modified histological scoring system
for microscopic evaluation.
|
| Score |
|---|
|
|
|
|---|
| Collagen | 0 | 1 | 3 | 5 |
|---|
| Organization | Disorganized | Mildly organized | Moderately
organized | Well organized |
| Amount | None | Mild | Moderate | Abundant |
Staining depth was evaluated using ImageJ (Version
1.50g, National Institutes of Health, Bethesda, MD, USA) after the
graph was converted to black and white color. Group A was set as
the control with an average grayscale of 170. 0 (disorganized)
indicated the average grayscale was below 120, 1 (mildly organized)
indicated the average grayscale was between 121 and 130, 2
indicated the average grayscale was between 131 and 140, 3
(moderately organized) indicated the average grayscale was between
141 and 150, 4 indicated the average grayscale was between 151 and
160, and 5 (well organized) indicated the average grayscale was
between 161 and 170.
The four random sites' microphotographs of each
sample with a magnification ×200 was examined under light
microscopy to assess the fiber orientation. The collagen of group A
was set as the parallel fiber for quality control. If the fibers of
another group and group A tended to be parallel, the angle between
the two was small, otherwise the angle between the two increased
(18,19). 0 (none) indicated the angle was below
50°, 1 (mild) indicated the angle was between 50° and 59°, 2
indicated the angle was between 60° and 69°, 3 (moderate) indicated
the angle was between 70° and 79°, 4 indicated the angle was
between 80° and 89°, and 5 (abundant) indicated the angle was
between 90° and 100°.
Statistical analysis
Data are presented as the mean ± standard deviation
(SD), and analysis of variance (one-way ANOVA) was performed to
compare the differences among the 5 groups with post hoc LSD test.
Statistical analyses were performed using SPSS 13.0 statistics
software (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to
indicate statistical significance.
Results
BMP-12 induces MSCs tenocyte
differentiation
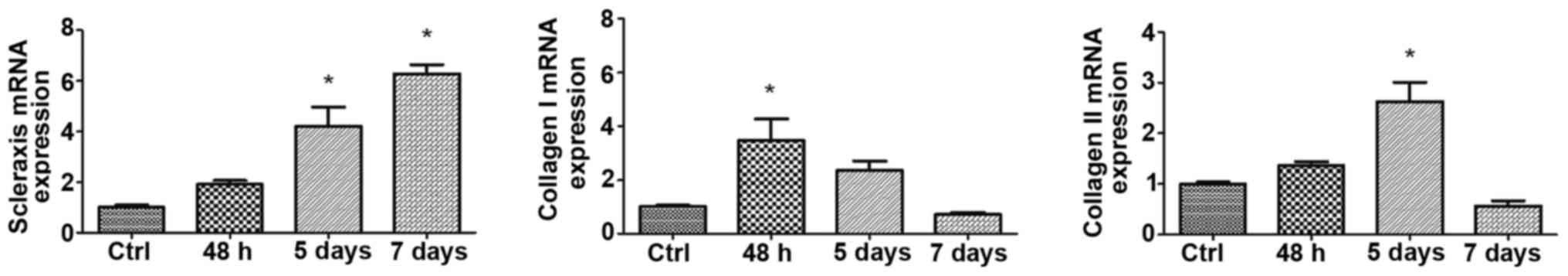
SCX is predominantly expressed in tendons and is
considered the most reliable phenotypic marker of the tenocytic
lineage. The expression levels of SCX, collagen I and collagen III
at 48 h, 5 and 7 days are shown in Fig.
3. Although BMP-12 was removed after 48 h, the expression of
SCX continued to increase until 7 days (P<0.01). Collagen I and
collagen III show a reversal of the pattern, i.e., their
expressions require persistent induction by BMP-12. The expression
of collagen I is more sensitive than the expression of collagen
III, with a rapid increase in collagen I at 48 h (P<0.01).
Induced MSCs enhance ultimate tensile
strength of linea alba incision
There was no death or hernia formation in the study.
Because of the tearing of the muscles during the tensiometric test,
one piece of the samples was wasted in groups A, B and D, and three
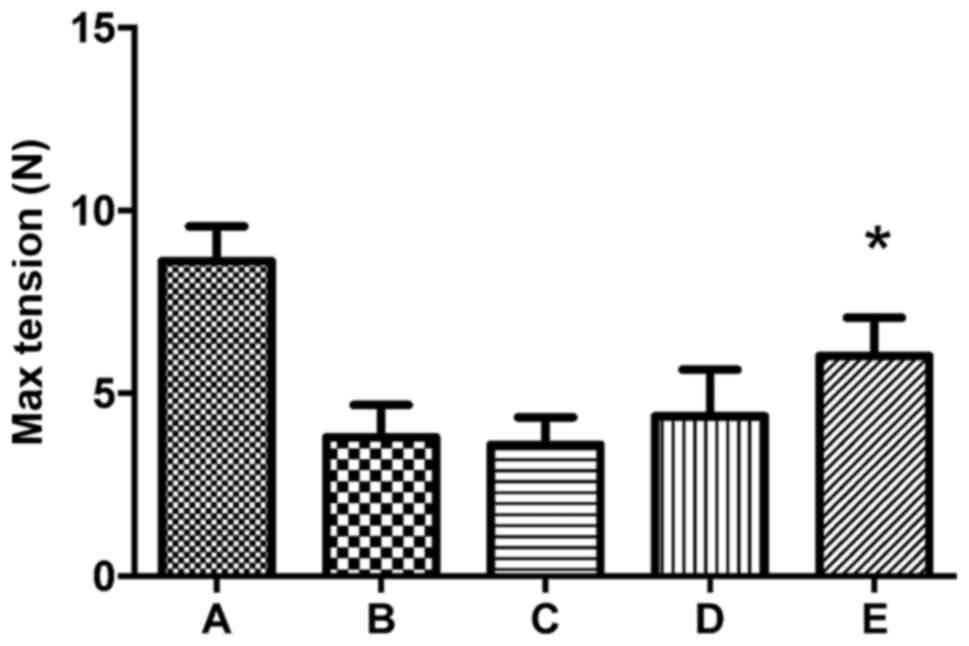
pieces in group E. The comparison of the 5 groups is presented in
Fig. 4. The max tension of the
normal linea alba is 8.62±0.94 N. The tension levels of groups B,
C, D and E are 44, 41.8, 51.6 and 69.7% compared to group A. The
difference is not significant between groups B, C and D
(P>0.05). Group E exhibits a greater increase in tension than
groups B, C and D, with increases of 58.6, 66.9 and 35.1%,
respectively (P<0.05).
Induced MSCs enhance collagen
remodeling of linea alba incision
There are 9 histological examples in Group A, B and
D, 10 histological examples in Group C and 7 examples in Group E.
Masson's histological analysis demonstrates that the tenogenic
differentiation of MSCs can obviously enhance the healing of the
linea alba incision (Table III),
as shown in histological sections (Fig.
5). Collagen organization and amount of Group E is better than
other three groups (P<0.05). Collagen organization of Group D is
better than group B and C, but worse than group E (P<0.05). By
contrast, the fibrous matrix are significantly disorderly organized
in Group B and C (Fig. 5Ba-Bb,
Fig. 5Ca-Cb).
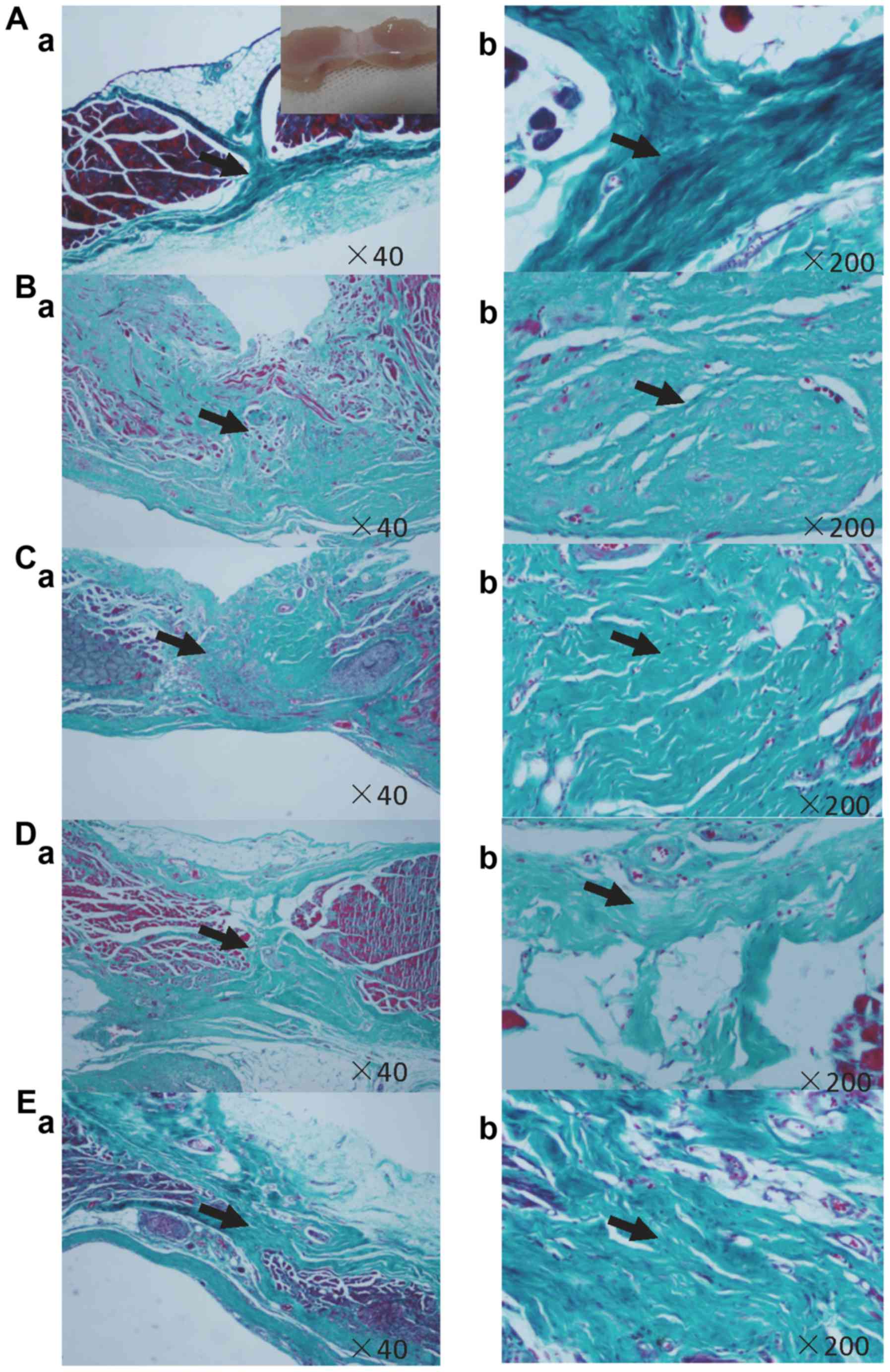 | Figure 5.Masson's Trichrome staining. (A)
Negative control, (B) positive control, (C) sham group, (D) native
MSCs and (E) tenogenically differentiated MSCs. Group E of
tenogenically differentiated MSCs demonstrates rich and well
aligned collagen fibrous matrix along the transversal (tensile)
axis of the incision. By contrast, the fibrous matrix in positive
control group B and sham group C are significantly disorderly in
organization. Organization and amount of collagen fibrous of native
MSCs group D are better than group B and C, but worse than group E,
which is corresponding to the semiquantitative histological
analysis (Table III). Aa, Ba, Ca,
Da, Ea ×40; Ab, Bb, Cb, Db, Eb ×200. MSCs, mesenchymal stem
cells. |
 | Table III.Histological scores of microscopic
examination. |
Table III.
Histological scores of microscopic
examination.
| Group | B | C | D | E |
|---|
| Organization | 1.6±0.7 | 1.5±0.53 | 2.7±0.83a | 3.5±0.98 |
| Amount | 1.2±0.42 | 1.6±0.7 | 1.3±0.48 | 2.7±0.82a |
Discussion
In recent years, great progress has been made in the
field of abdominal wall and hernia surgery, especially in terms of
materials science. Bioactive patch can remodel the abdominal wall
structure after implanted and inspires widespread interest of
researchers. Regarding MSCs in herniorrhaphy, few studies have
performed pilot explorations in this field. Gao et al
(20) tried to analyze the coating
ability of various meshes of MSCs, and four other articles
investigated the curative effects of hernia repair with surgical
mesh in combination with MSCs (21–24). In
short, the use of MSCs for regeneration is a novel field in
abdominal wall surgery. As Tez and Kiliç (25) indicated the stem cell origin theory
of the inguinal hernia, many further works are needed on MSCs,
fascia, prosthesis, heterogeneity and herniorrhaphy.
Many studies have demonstrated that exogenous BMP-12
can induce MSCs to undergo tenogenic differentiation with
increasing expression of special markers. However, this effect
seems time dependent and thus is more important for downstream
target genes. SCX is a tendon/ligament-specific transcription
factor that is required for tenogenic differentiation (26–28). Our
results reveal that BMP-12 dramatically increases SCX expression
when present, which is consistent with other studies (10,14).
However, the expression was previously reported to decrease when
BMP-12 was absent (14). Conversely,
expression continues to increase in our study, which may be
attributable to the longer inducing time. In short, gene
transfection of BMP-12 into MSCs seems to be a reliable method to
continually induce the tenogenic differentiation of MSCs.
We select the most two direct methods to evaluate
the healing results, including tensiometric testing and
histological analysis. Tensile strength corresponds to the
abdominal pressure, and the ultimate result means that the healing
incision can bear some violent activities, such as cough and jump.
Histological analysis presents that tenogenic differentiation of
MSCs in treating the linea alba incision demonstrates the
relatively rich and well aligned collagen fibrous matrix along the
transversal (tensile) axis of the incision. Native MSCs can only
improve the organization other than the amount of the regenerating
collagen fibers. Regrettably, there are some insignificances in the
semiquantitative histological analysis. Measuring diameters of the
regenerating collagen fibers using transmission electron microscopy
may be more convincing. However, we believe the results of our
study are also conclusive as that was described by Rice et
al (17).
In this study, we use a collagen sponge as an
implant scaffold. However, the collagen scaffold is an interference
factor for exploring the curative effect of MSCs. Thus, group C
transplants with only the collagen sponge served as the sham group.
There are two main reasons to involve the collagen sponge in this
trial. First, MSCs require continuing induction by BMP-12 to
undergo tenogenic differentiation, and collagen is a better carrier
than buffer or hyaluronan paste (29). At one, seven, and fourteen days,
approximately 80, 45, and 8%, respectively, of the initial dose of
BMP-12 is retained at the implant sites. Second, a collagen sponge
can provide a confined space scaffold for the MSCs. For the healing
of midline laparotomy incisions, we only want to increase the
production of collagen in the linea alba, and a sponge scaffold
provides solidity to prevent the diffusion of MSCs.
In our study, we confirm that BMP-12 induction of
tenogenic differentiation of MSCs enhances the healing of linea
alba incisions.
Acknowledgements
The authors would like to thank Dr Feng Cai
(Southeast University Medical School, Nanjing, China) for his
support in the isolation and culture of MSCs.
Funding
The present study was supported by Southeast
University Fundamental Research Fund (grant no. 2242017K40256).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DW isolated the MSCs, and performed tenogenic
differentiation and data analysis. J-MW performed the animal
experiments. Y-YT performed the tensiometric testing and
histological analysis. Z-LJ designed this study, analyzed and
interpreted some data, and revised the manuscript critically for
important intellectual content. As the corresponding author, he
also gave final approval of the version to be published. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were conducted with the approval of
the Animal Care and Use Committee of Southeast University (approval
no. 20140912).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sanders DL and Kingsnorth AN: The modern
management of incisional hernias. BMJ. 344:e28432012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gokani SA, Elmqvist KO, El-Koubani O, Ash
J, Biswas SK and Rigaudy M: A cost-utility analysis of small bite
sutures versus large bite sutures in the closure of midline
laparotomies in the United Kingdom National Health Service.
Clinicoecon Outcomes Res. 10:105–117. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Franz MG: The biology of hernia formation.
Surg Clin North Am. 88:1–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Axer H, Keyserlingk DG and Prescher A:
Collagen fibers in linea alba and rectus sheaths I. General scheme
and morphological aspects. J Surg Res. 96:127–134. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Axer H, von Keyserlingk DG and Prescher A:
Collagen fibers in linea alba and rectus sheaths II. Variability
and biomechanical aspects. J Surg Res. 96:239–245. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yildiz M, Yigit O, Sünter AV, Edizer DT,
Dursun N and Okcu O: Effects of intracordal estradiol and
dexamethasone injection on wound healing in vocal fold injuries. J
Voice pii. S0892–1997. 30557–X. 2018.
|
|
7
|
Stoff A, Rivera AA, Sanjib Banerjee N,
Moore ST, Michael Numnum T, Espinosa-de-Los-Monteros A, Richter DF,
Siegal GP, Chow LT, Feldman D, et al: Promotion of incisional wound
repair by human mesenchymal stem cell transplantation. Exp
Dermatol. 18:362–369. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heffner JJ, Holmes JW, Ferrari JP,
Krontiris-Litowitz J, Marie H, Fagan DL, Perko JC and Dorion HA:
Bone marrow-derived mesenchymal stromal cells and platelet-rich
plasma on a collagen matrix to improve fascial healing. Hernia.
16:677–687. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gelberman RH, Linderman SW, Jayaram R,
Dikina AD, Sakiyama-Elbert S, Alsberg E, Thomopoulos S and Shen H:
Combined administration of ASCs and BMP-12 promotes an M2
macrophage phenotype and enhances tendon healing. Clin Orthop Relat
Res. 475:2318–2331. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shen H, Gelberman RH, Silva MJ,
Sakiyama-Elbert SE and Thomopoulos S: BMP12 induces tenogenic
differentiation of adipose-derived stromal cells. PLoS One.
8:e776132013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lennon DP and Caplan AI: Isolation of rat
marrow-derived mesenchymal stem cells. Exp Hematol. 34:1606–1607.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cai F, Wu XT, Xie XH, Wang F, Hong X,
Zhuang SY, Zhu L, Rui YF and Shi R: Evaluation of intervertebral
disc regeneration with implantation of bone marrow mesenchymal stem
cells (BMSCs) using quantitative T2 mapping: A study in rabbits.
Int Orthop. 39:149–159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JY, Zhou Z, Taub PJ, Ramcharan M, Li
Y, Akinbiyi T, Maharam ER, Leong DJ, Laudier DM, Ruike T, et al:
BMP-12 treatment of adult mesenchymal stem cells in vitro augments
tendon-like tissue formation and defect repair in vivo. PLoS One.
6:e175312011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harth KC, Broome AM, Jacobs MR, Blatnik
JA, Zeinali F, Bajaksouzian S and Rosen MJ: Bacterial clearance of
biologic grafts used in hernia repair: An experimental study. Surg
Endosc. 25:2224–2229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tharappel JC, Ramineni SK, Reynolds D,
Puleo DA and Roth JS: Doxycycline impacts hernia repair outcomes. J
Surg Res. 184:699–704. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rice RD, Ayubi FS, Shaub ZJ, Parker DM,
Armstrong PJ and Tsai JW: Comparison of Surgisis, AlloDerm and
Vicryl woven mesh grafts for abdominal wall defect repair in an
animal model. Aesthetic Plast Surg. 34:290–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Zuijlen PP, de Vries HJ, Lamme EN,
Coppens JE, van Marle J, Kreis RW and Middelkoop E: Morphometry of
dermal collagen orientation by Fourier analysis is superior to
multi-observer assessment. J Pathol. 198:284–291. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marcos-Garcés V, Harvat M, Molina Aguilar
P, Ferrández Izquierdo A and Ruiz-Saurí A: Comparative measurement
of collagen bundle orientation by Fourier analysis and
semiquantitative evaluation: Reliability and agreement in Masson's
trichrome, Picrosirius red and confocal microscopy techniques. J
Microsc. 267:130–142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao Y, Liu LJ, Blatnik JA, Krpata DM,
Anderson JM, Criss CN, Posielski N and Novitsky YW: Methodology of
fibroblast and mesenchymal stem cell coating of surgical meshes: A
pilot analysis. J Biomed Mater Res B Appl Biomater. 102:797–805.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mestak O, Matouskova E, Spurkova Z,
Benkova K, Vesely P, Mestak J, Molitor M, Pombinho A and Sukop A:
Mesenchymal stem cells seeded on cross-linked and noncross-linked
acellular porcine dermal scaffolds for long-term full-thickness
hernia repair in a small animal model. Artif Organs. 38:572–579.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bogdan VG, Zafranskaya MM, Gain YM,
Demidchik YE, Bagatka SS and Ivanchik GI: Modification of collagen
formation by mesenchymal stem cells isolated from human adipose
tissue in culture and after autotransplantation for abdominal
hernia plasty. Bull Exp Biol Med. 156:152–155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Majumder A, Gao Y, Sadava EE, Anderson JM
and Novitsky YW: Cell-coating affects tissue integration of
synthetic and biologic meshes: Comparative analysis of the onlay
and underlay mesh positioning in rats. Surg Endosc. 30:4445–4453.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hoganson DM, Meppelink AM, Hinkel CJ,
Goldman SM, Liu XH, Nunley RM, Gaut JP and Vacanti JP:
Differentiation of human bone marrow mesenchymal stem cells on
decellularized extracellular matrix materials. J Biomed Mater Res
A. 102:2875–2883. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tez M and Kiliç YA: Stem cell origin
theory of the inguinal hernia. Med Hypotheses. 66:1042–1043. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shukunami C, Takimoto A, Nishizaki Y,
Yoshimoto Y, Tanaka S, Miura S, Watanabe H, Sakuma T, Yamamoto T,
Kondoh G and Hiraki Y: Scleraxis is a transcriptional activator
that regulates the expression of Tenomodulin, a marker of mature
tenocytes and ligamentocytes. Sci Rep. 8:31552018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Le W, Cheah AE and Yao J: Ex-vivo tendon
repair augmented with bone marrow derived mesenchymal stem cells
stimulated with myostatin for tenogenesis. J Hand Surg Asian Pac
Vol. 23:47–57. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Docheva D, Hunziker EB, Fässler R and
Brandau O: Tenomodulin is necessary for tenocyte proliferation and
tendon maturation. Mol Cell Biol. 25:699–705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Seeherman HJ, Archambault JM, Rodeo SA,
Turner AS, Zekas L, D'Augusta D, Li XJ, Smith E and Wozney JM:
rhBMP-12 accelerates healing of rotator cuff repairs in a sheep
model. J Bone Joint Surg Am. 90:2206–2219. 2008. View Article : Google Scholar : PubMed/NCBI
|